Infections in the CNS During Childhood and the Risk of Subsequent Psychotic Illness: A Cohort Study of More Than One Million Swedish Subjects
Abstract
Objective: Infections during early life have been suggested to play a role in the etiology of schizophrenia. Most studies have focused on fetal life; few have explored risk associated with infection during childhood. The results of these have been inconsistent. The present study aims to investigate whether there is an increased risk of schizophrenia and other nonaffective psychoses associated with viral or bacterial CNS infections during childhood and, if so, which specific agents are involved. Method: A national cohort consisting of 1.2 million children born between 1973 and 1985 was followed up by using Swedish national registers to retrieve data on hospital admissions for CNS infections at 0–12 years of age (bacterial: N=2,435, viral: N=6,550) as well as admissions for nonaffective psychotic illnesses from the 14th birthday (N=2,269). Results: There was a slightly increased risk of nonaffective psychotic illness associated with viral CNS infections, as well as schizophrenia. There was no evidence of increased risk in relation to bacterial infections. When divided into specific agents, exposures to mumps virus or cytomegalovirus were associated with subsequent psychoses. Conclusions: Serious viral CNS infections during childhood appear to be associated with the later development of schizophrenia and nonaffective psychoses. The association with specific viruses suggests that the risk is related to infectious agents with a propensity to invade the brain parenchyma.
Schizophrenia has been suggested to be caused by disturbances in brain development (1 , 2) . Such disturbances may be of genetic and/or environmental origin. Previous reports have suggested that exposures to infections during early life can contribute to the development of schizophrenia in some cases. Although it is well known that early life viral infections in animals and humans have teratogenic effects (3) , it is far from clear if such infections in humans can cause psychiatric disease after a latency period of decades.
Infections During Fetal Life
A number of studies have previously associated the development of psychosis with exposure to infections during fetal life. Influenza A virus infection is the most extensively studied infectious agent in this respect, but the results are conflicting because of methodological shortcomings (4) . The majority of studies have used an ecological design; thus individual data on virus exposure have been lacking. There are only a few studies with data on an individual level. Brown and colleagues (5) reported on a sevenfold increased risk of schizophrenia in children born to mothers exposed to influenza during the first trimester as determined serologically, but because of a small number of cases, statistical evidence for this association was weak (p=0.08). Prenatal exposures to other viruses with affinity to the CNS, such as polio, rubella, and herpes simplex type 2 viruses, have also been proposed as risk factors for psychosis (6 – 8) , although no evidence for the involvement of the latter virus was found in a recent report (9) . In addition, the parasite T. gondii has attracted attention for its potential role in the etiology of schizophrenia (10 , 11) . Furthermore, studies of host responses to infections have found an association between psychotic illness in the offspring and increased levels of maternal inflammatory cytokines (12 , 13) .
Infections During Childhood
Although there has been a focus on risk exposures during the fetal period, there is a paucity of studies on the exposure to infections in the CNS during childhood as a potential risk factor for the future development of psychosis. This is somewhat surprising given the fact that the human brain continues to develop well into early adulthood (reviewed in reference 14 ). A Finnish follow-up of a birth cohort until 28 years of age reported no association between bacterial CNS infections during childhood and subsequent schizophrenia, whereas an increased risk was described for viral infections (odds ratio=4.8) (15) . In a later follow-up of the same cohort up to the age of 31 years, the risk associated with viral infections was, however, attenuated (odds ratio=2.5, 95% confidence interval [CI]=0.9–7.0) (16) . In another Finnish study, no increased risk for schizophrenia was found in a cohort consisting of 320 individuals who had suffered from viral CNS infections (the majority by enteroviruses) before their 15th birthday (17) . In addition to the small number of exposed individuals, this study lacked a proper control group and did not include data on bacterial infections. However, in a study from Sao Paulo of 173 individuals who had suffered from “epidemic meningitis” during childhood (mainly of bacterial origin), a fivefold increase in the prevalence of psychotic disorders occurred (18) , but the study suffers from a very high dropout rate. An association between nonspecified childhood meningitis (bacterial and viral) and adult schizophrenia has also been described (19) .
In conclusion, data concerning childhood exposures to both bacterial and viral CNS infections and the risk of subsequent psychosis are sparse and contradictory. The present study aims to investigate whether there is an increased risk of nonaffective psychotic illness associated with bacterial or viral infections in the CNS during childhood and, if so, which specific bacterial or viral agents are involved.
Materials and Methods
To address this question, we identified all children in Sweden born 1973–1985 who had contracted a CNS infection before their 12th birthday by using the Swedish National Inpatient Register, which contains data on all inpatient episodes since 1973. In addition, data on subsequent psychotic illness in the cohort until Dec. 31, 2002, was extracted. Because parental psychotic illness may be a confounder, information on parental inpatient care due to psychoses (nonaffective and/or affective) was linked from the inpatient register. By further record linkage to the medical birth register, other possible confounders were taken into account, namely urbanicity (born in a town with more than 250,000 inhabitants) and winter birth (December through March).
The following viral and bacterial CNS infections were defined as exposures with ICD-8 codes: viral infections—323 (encephalitis and encephalomyelitis), 045 (enterovirus meningitis: 04500 coxsackie, 04510 enterocytopathogenic human orphan virus, 04597 enterovirus meningitis suspecta, 04599 enterovirus not otherwise specified), 04699 (other enterovirus infections in the CNS), 05200 (varicellae meningoencephalitis), 05404 (herpes simplex meningoencephalitis), 062 (encephalitis caused by mosquitoes), 063 (encephalitis caused by ticks), 064 (virus encephalitis caused by arthropods), 065 (virus encephalitis not otherwise specified), 07201 (parotitis meningoencephalitis), 07502 (mononucleosis meningoencephalitis), 07920 (choriomeningitis lymphocytica), and 07950 (congenital cytomegalovirus, diagnosed after 1 month of age); bacterial infections—320 (purulent meningitis, 320,00 hemophilus influenzae, 320,10 penumococcum, 320,80 streptococcum, 320,88 other, 320,99 not otherwise specified), 09490 (syphilitic meningoencephalitis), 01300 (tuberculosis meningitis), and 03600 (meningococcal meningitis).
ICD-9 codes were the following: viral infections—321 (meningitis caused by viruses), 322 (nonpurulent meningitis not otherwise specified), 323 (virus encephalitis and encephalomyelitis), 046 (slow virus infection in the CNS), 047 (enterovirus meningitis: 047A coxsackie virus, 047B enterocytopathogenic human orphan virus, 047W other enterovirus, 047X enterovirus not otherwise specified), 048 (other illnesses in the CNS caused by enteroviruses), 049 (other viral CNS infections), 052W (varicellae meningitis), 053A (herpes zoster meningitis), 054D (herpes simplex encephalitis), 055A (morbilli encephalitis), 056A (rubella encephalomyelitis), 062 (encephalitis caused by mosquitoes), 063 (encephalitis caused by ticks), 064 (virus encephalitis caused by arthropods, 072B (parotitis meningitis), 072C (parotitis encephalitis), 771B (congenital cytomegalovirus, diagnosed after 1 month of age); bacterial infections—013 (tuberculosis meningitis), 036 (meningococcal meningitis and encephalitis), 094C (syphilitic meningoencephalitis), and 320 (bacterial meningitis: 320A hemophilus influenzae, 320B pneumococcum, 320C streptococcum, 320D staphylococcum, 320W other bacteria, 320X not otherwise specified).
The following diagnoses were defined as nonaffective psychotic illness: inpatient care for ICD-9 diagnoses—295 (schizophrenia), 297 (delusional syndrome), and 298 excluding A and B (reactive psychoses excluding depressive and manic psychoses); ICD-10 diagnoses—F20 (schizophrenia), F21 (pertubatio schizotypica), F22 (delusional syndrome), F23 (psychotic episode), F24 (induced delusional syndrome), F25 (schizoaffective syndrome), F28 (other psychoses), and F29 (psychosis not otherwise specified). The following diagnoses were defined as schizophrenia: inpatient care for ICD 9 diagnosis 295 (schizophrenia) and ICD-10 diagnosis F20 (schizophrenia). The national inpatient register was searched from Jan. 1, 1987 (or from the subjects’ 14th birthday), until Dec. 31, 2002 (until the persons were 17–29 years old).
Relative risk estimates and 95% CIs were calculated comparing exposed with unexposed children (children who had not had any CNS infection, whether of viral or bacterial origin) by using Mantel-Haenszel estimates. Analyses were performed crude and adjusted for age, sex, urbanicity, and a family history of psychosis. SAS software version 9.1 (SAS Institute, Cary, NC) was used in all statistical analyses. The study was approved by the research ethics committee at the Karolinska Institutet.
Results
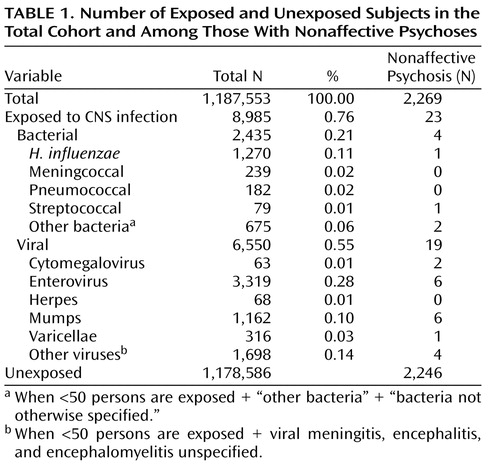
A total of 1,187,553 children were identified and followed up in the registers. Altogether, 2,435 children had been hospitalized for bacterial and 6,550 for viral CNS infections ( Table 1 ). Of the latter, 3,319 were caused by enteroviruses (50.7%) and 1,162 by the mumps virus (17.7%). Three hundred and sixteen children suffered from varicella zoster virus infections (4.8%), 63 from cytomegalovirus infections (1%) (18 children were diagnosed as having a congenital cytomegalovirus infection during their first month of life and were not included in the study), and 68 from herpes simplex virus infections (1%). Among the bacterial infections, H. influenzae was the most common agent (52.2%). Altogether, 2,269 individuals were diagnosed with a nonaffective psychosis. Among these, 23 individuals (including eight with schizophrenia) were diagnosed with a CNS infection before the age of 12 ( Table 1 ).
Covarying Risk Factors
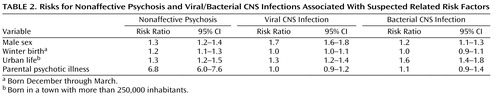
Suspected covarying risk factors are scrutinized in Table 2 , in which both the risk for psychoses and exposure to CNS infections are presented in association with each risk factor. Male sex and urban life are associated with increased risks of both psychoses and viral/bacterial CNS infections, whereas winter birth is not. Parental psychotic illness is weakly related to bacterial CNS infection. Thus, male sex, urbanicity and parental psychotic illness covary with both the outcome and the exposure and were added into the analysis ( Table 3 ). The rationale for this is to clarify whether these covarying risk factors are explained by infection. For example, if the main risk associated with urbanicity is mediated by infection, the risk associated with infection would decrease if urbanicity were added to the analysis.
Risk for Psychoses
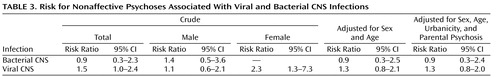
There was a slightly increased risk, at the limit of statistical significance (risk ratio=1.5, 95% CI=1.0–2.4), of developing a nonaffective psychosis (including schizophrenia) later in life if a child had been exposed to a viral CNS infection ( Table 3 ), and the risk was higher in females (risk ratio=2.3, 95% CI=1.3–7.3). Adjustment for sex and age did not affect the results substantially (risk ratio=1.3, 95% CI=0.8–2.1), nor did parental psychotic illness or urbanicity (risk ratio=1.3, 95% CI=0.8–2.0). There was no evidence of an increased risk associated with bacterial infections (risk ratio=0.9, 95% CI=0.3–2.3).
When the analyses were restricted to schizophrenia, the results were similar (data not shown). Viral CNS infection was associated with an increased risk of 1.6 (95% CI=1.0–2.5), whereas no support for an increased risk associated with bacterial infections (risk ratio=0.9, 95% CI=0.4–2.5) was found.
Specific Infectious Agents
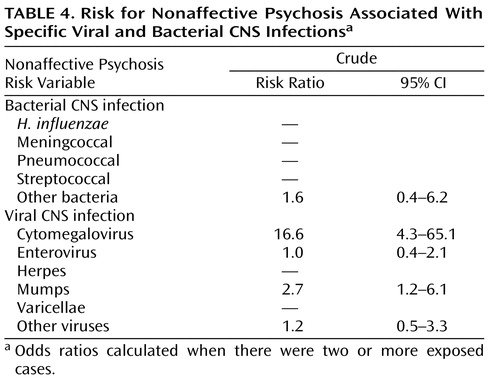
Concerning specific infectious agents ( Table 4 ), we found that the risk for nonaffective psychoses was related to CNS infections by the mumps virus (risk ratio=2.7, 95% CI=1.2–6.1) or cytomegalovirus (risk ratio=16.6, 95% CI=4.3–65.1). CNS infections caused by enteroviruses were not associated with the later development of nonaffective psychotic illness in this study (risk ratio=1.0, 95% CI=0.4–2.1).
Age of Onset and Exposure
The mean age of onset of psychoses did not differ between those exposed to viral infection and individuals not exposed (20.9 years for both groups). Among the exposed individuals, there was no difference in age at the time of infection between patients and comparison subjects (mean age=5.9 years for both groups). When categorized according to exposure to viral CNS infection before and after 6 years of age, the risk estimates did not differ significantly (1.54 and 1.49, respectively).
Description of Patients
Among the 23 individuals who were exposed to viral CNS infections during childhood and developed a psychotic illness, 14 were male (61%) and nine were female. This is in accordance with the general occurrence of CNS infections, which are more common among males (65% for viral and 57% for bacterial infections in this study).
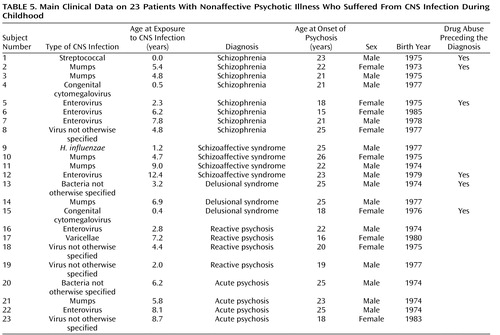
Clinical characteristics concerning exposures and outcomes are presented in Table 5 . The age at the time of exposure was evenly distributed during childhood. The most common age at onset of psychoses was between 18 and 26 years. Eight individuals had a diagnosis of schizophrenia, and four individuals had a diagnosis of schizoaffective syndrome. Two of those with a diagnosis of schizophrenia were exposed to mumps virus and three to enterovirus. Six of the 23 patients had a history of drug abuse, most often cannabis, before being diagnosed with psychotic illness.
Discussion
In this national cohort study, we found a weak association between viral CNS infections during childhood and the later development of nonaffective psychotic illnesses. On the other hand, there was no support for an increased risk associated with bacterial CNS infections. The power to detect a doubled risk for psychosis associated with exposure to CNS infections during childhood was fairly high in this study (0.9). The power to detect increased risks of 20%–50% (odds ratio=1.2–1.5) was much smaller (0.2–0.6). Nevertheless, this is the most robust and conclusive study to date. Thus, the results suggest that the risk associated with viral CNS infections is relatively weak: less than doubled, but a null effect cannot be ruled out.
Exposure to infections was not apparently associated with any specific age of onset of psychoses nor did the age at the time of exposure seem to matter substantially. One would have expected that infections earlier in life would be more harmful than later on. However, this result is in accordance with a study from Sao Paolo (20) .
Adding parental psychotic illness and urbanicity to the analysis had no substantial influence on the results, suggesting that these established risk factors are not mediated by infection (increased susceptibility to infections or exposures to infections). However, from Table 2 , it is evident that living in an urban area increases the risk of having CNS infections during childhood. Thus, we had expected an effect. The small numbers may be one explanation. The other is that infections are truly not related to urban risk.
Of the different viral infections, mumps and cytomegalovirus infections were associated with increased risk for psychoses in this study. It should, however, be noted that the numbers are small, and the results concerning specific infectious agents should therefore be interpreted with caution. Nevertheless, it is of interest to note that mumps before vaccination was the single most common cause of aseptic meningitis or mild encephalitis, 15% of all cases (21) . This figure is similar to the 17% observed in the present material from Sweden, where general vaccination against measles, mumps, and rubella viruses was first introduced in 1982 (22) .
Mumps virus can be genotyped according to sequence variations in the gene encoding the small hydrophobic protein of the virus, and currently, 12 different genotypes, designated A–L, with varying degrees of neurovirulence have been identified (23) . During the years 1973–1985, strains of genotypes C and D were circulating in Sweden, and these appear to be more neurovirulent than those of genotype A (24) . In a study of 18 patients infected with mumps strains of genotypes C or D, Tecle and coworkers (24) reported that nine patients suffered from meningitis only and nine had a combination of parotitis and meningitis, whereas none suffered from parotitis only. This contrasts markedly with infections with the genotype A, which caused parotitis only in the vast majority of the patients (24) . From earlier reports, it is also well known that mumps virus can be highly neurovirulent. During certain epidemics, more than 50% of patients with parotitis showed pleocytosis in the CSF, whereas approximately half of the number of patients with mumps, meningitis, or encephalitis may show no signs of parotitis (reviewed in reference 3 ). Thus, different risk estimates for mumps virus can potentially be obtained for different circulating strains.
Mumps virus probably invades the brain across the choroid plexus, from which the virus spreads through the ventricles to cause infection of ependymal cells (reviewed in reference 25 ). In experimental models, neurovirulent genotypes of mumps virus may cause hydrocephalus (20) . In such models, neurovirulent genotypes can also cause persistent infections of neurons and induce developmental disturbances (for a review, see reference 26 ). According to the report by Julkunen and coworkers (27) , the frequency or severity of sequelae following mumps encephalitis can be high. In their Finnish cohort, 23 of 47 patients examined 1–15 years after the disease experienced difficulties in memory and learning, focal motor or sensory signs, or loss of hearing and visual acuity. Chronic mumps virus CNS infections in humans have also occasionally been described (28 , 29) . In addition to these effects of mumps on the nervous system, we suggest that mumps infections during childhood can be associated with an increased risk for the future development of nonaffective psychosis. Although mumps virus antigens have never been found in brains from patients with schizophrenia postmortem, abnormal antibody titers to the virus in CSF have been reported (reviewed in reference 30 ).
Cytomegalovirus infection was also found to confer an increased risk for the future development of nonaffective psychotic illness (risk ratio=16.6, 95% CI=4.3–65.1), although only two patients were identified in the present material. It should be noted that of the 81 individuals having a diagnosis of congenital cytomegalovirus infection, only 18 were diagnosed during their first month of life. Since these children were presumed to be infected prenatally, they were excluded from the current study. In a prospective study conducted in Malmö, Sweden, during the late 1970s and early 1980s, 35% of the children who tested negative at birth shed virus in the urine already at 6 months of age (31) , indicating a high rate of infection during infancy. Consequently, the majority of the 63 children diagnosed at older ages were probably infected postnatally. The two children who developed nonaffective psychosis in the present material were diagnosed at 5 and 6 months of age, respectively. With regard to the long-term outcome of cytomegalovirus infections, the age at the time of infection is a major determinant. Although congenital cytomegalovirus infections are considered the leading infectious cause of brain damage in children (32) , postnatally acquired cytomegalovirus infection is believed to be far more harmless (33 , 34) . However, studies on the long-term consequences of postnatally acquired cytomegalovirus infections are missing (34) . The present study suggests that delayed psychiatric symptoms should be considered in future studies. Although cytomegalovirus genomes have not been found in postmortem brains from schizophrenic patients (35 , 36) , an association between recent onset and deficit schizophrenia and cytomegalovirus antibody seropositivity has been described (37 , 38) . Like mumps virus, cytomegalovirus is thought to infect cells in periventricular regions followed by invasion of the brain parenchyma (reviewed in reference 39 ).
Methodological Concerns
The strength of the current study is the longitudinal design with data collected prospectively, which minimize the risk for bias. On the other hand, a potential weakness of the study is that the diagnoses are register based, although in a validation study, the register diagnoses of schizophrenia proved to be in accordance to DSM-IV in 82% of the cases (40) . Furthermore, the Swedish National Discharge Register was not complete during the first years of the study; therefore, data from seven of 26 county councils are missing for 1973, but we have no reason to believe that this lack of data is selective. In this study, exposure is defined as inpatient care for CNS infections. It is, therefore, likely that many cases of mild meningitis/encephalitis pass without detection. On the other hand, the different diagnoses of CNS infections appear to be accurate in the Swedish National Discharge Register (41) . Consequently, this study describes only the risk associated with register-based CNS infections requiring hospital treatment. The same applies to the patients with a diagnosis of psychiatric disease, which encompass only hospital-treated cases and therefore most likely include patients with more severe symptoms than those observed in outpatients, which were not included in the present study.
Finally, although the study population consisted of 1.2 million children, our power was limited to detect increased risks of a risk ratio less than 1.5. Nevertheless, very strong associations may be ruled out.
In conclusion, this study shows that serious viral CNS infections during childhood may be associated with the later development of schizophrenia and nonaffective psychosis. Our finding that an elevated risk is associated with mumps virus and cytomegalovirus infections indicates that the risk relates to infectious agents with a propensity to invade the brain parenchyma rather than to CNS infections in general.
1. Murray RM, Lewis SW: Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987; 295:681–682Google Scholar
2. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Google Scholar
3. Johnson RT: Viral Infections of the Nervous System. Philadelphia, Lippincott-Raven Publishers, 1998Google Scholar
4. Brown AS, Susser ES: In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev 2002; 8:51–57Google Scholar
5. Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES: Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry 2004; 61:774–780Google Scholar
6. Suvisaari J, Haukka J, Tanskanen A, Hovi T, Lönnqvist J: Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry 1999; 156:1100–1102Google Scholar
7. Brown AS, Cohen P, Greenwald S, Susser E: Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry 2000; 157:438–443Google Scholar
8. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH: Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001; 58:1032–1037Google Scholar
9. Brown AS, Schaefer CA, Quesenberry CP Jr, Shen L, Susser ES: No evidence of relation between maternal exposure to herpes simplex virus type 2 and risk of schizophrenia? Am J Psychiatry 2006; 163:2178–2180Google Scholar
10. Brown AS, Schaefer CA, Quesenberry CP Jr, Liu L, Babulas VP, Susser ES: Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry 2005; 162:767–773Google Scholar
11. Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Torrey EF, Yolken RH: Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry 2007; 61:688–693Google Scholar
12. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH: Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun 2001; 15:411–420Google Scholar
13. Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES: Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry 2004; 161:889–895Google Scholar
14. de Graaf-Peters VB, Hadders-Algra M: Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 2006; 82:257–266Google Scholar
15. Rantakallio P, Jones P, Moring J, Von Wendt L: Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol 1997; 26:837–843Google Scholar
16. Koponen H, Rantakallio P, Veijola J, Jones P, Jokelainen J, Isohanni M: Childhood central nervous system infections and risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci 2004; 254:9–13Google Scholar
17. Suvisaari J, Mautemps N, Haukka J, Hovi T, Lönnqvist J: Childhood central nervous system viral infections and adult schizophrenia. Am J Psychiatry 2003; 160:1183–1185Google Scholar
18. Gattaz WF, Abrahao AL, Foccacia R: Childhood meningitis, brain maturation and the risk of psychosis. Eur Arch Psychiatry Clin Neurosci 2004; 254:23–26Google Scholar
19. Leask SJ, Done DJ, Crow TJ: Adult psychosis, common childhood infections and neurological soft signs in a national birth cohort. Br J Psychiatry 2002; 181:387–392Google Scholar
20. Rubin SA, Afzal MA, Powell CL, Bentley ML, Auda GR, Taffs RE, Carbone KM: The rat-based neurovirulence safety test for the assessment of mumps virus neurovirulence in humans: an international collaborative study. J Infect Dis 2005; 191:1123–1128Google Scholar
21. Meyer HM Jr, Johnson RT, Crawford IP, Dascomb HE, Rogers NG: Central nervous system syndromes of “viral” etiology: a study of 173 cases. Am J Med 1960; 29:334–347Google Scholar
22. Broliden K, Abreu ER, Arneborn M, Bottiger M: Immunity to mumps before and after MMR vaccination at 12 years of age in the first generation offered the two-dose immunization programme. Vaccine 1998; 16:323–327Google Scholar
23. Rafiefard F, Johansson B, Tecle T, Orvell C: Characterization of mumps virus strains with varying neurovirulence. Scand J Infect Dis 2005; 37:330–337Google Scholar
24. Tecle T, Johansson B, Jejcic A, Forsgren M, Orvell C: Characterization of three co-circulating genotypes of the small hydrophobic protein gene of mumps virus. J Gen Virol 1998; 79(part 12):2929–2937Google Scholar
25. Carbone KM, Wolinsky JS: Mumps virus, in Fields Virology, vol 1. Edited by Knipe DM, Howley PM. Philadelphia, Lippincott Williams & Wilkins, 2001, pp 1381–1400Google Scholar
26. Kristensson K, Löve A, Norrby E: Experimental mumps virus infections in the developing nervous system, in Virus Infections and the Developing Nervous System. Edited by Johnson RT, Lyon G. Dordrecht, the Netherlands, Kluwer Academic, 1988, pp 143–150Google Scholar
27. Julkunen I, Koskiniemi M, Lehtokoski-Lehtiniemi E, Sainio K, Vaheri A: Chronic mumps virus encephalitis: mumps antibody levels in cerebrospinal fluid. J Neuroimmunol 1985; 8:167–175Google Scholar
28. Vaheri A, Julkunen I, Koskiniemi ML: Chronic encephalomyelitis with specific increase in intrathecal mumps antibodies. Lancet 1982; 2:685–688Google Scholar
29. Nojd J, Tecle T, Samuelsson A, Orvell C: Mumps virus neutralizing antibodies do not protect against reinfection with a heterologous mumps virus genotype. Vaccine 2001; 19:1727–1731Google Scholar
30. Yolken RH, Torrey EF: Viruses, schizophrenia and bipolar disorder. Clin Microbiol Rev 1995; 8:131–145Google Scholar
31. Ahlfors K, Ivarsson S-A, Harris S: Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden: review of prospective studies available in the literature. Scand J Infect Dis 1999; 31:443–457Google Scholar
32. Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF: Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr 1997; 130:624–630Google Scholar
33. Kumar ML, Nankervis GA, Jacobs IB, Ernhart CB, Glasson CE, McMillan PM, Gold E: Congenital and postnatally acquired cytomegalovirus infections: long-term follow-up. J Pediatr 1984; 104:674–679Google Scholar
34. Vollmer B, Seibold-Weiger K, Schmitz-Salue C, Hamprecht K, Goelz R, Krageloh-Mann I, Speer CP: Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis 2004; 23:322–327Google Scholar
35. Alexander RC, Spector SA, Casanova M, Kleinman J, Wyatt RJ, Kirch DG: Search for cytomegalovirus in the postmortem brains of schizophrenic patients using the polymerase chain reaction. Arch Gen Psychiatry 1992; 49:47–53Google Scholar
36. Taller AM, Asher DM, Pomeroy KL, Eldadah BA, Godec MS, Falkai PG, Bogert B, Kleinman JE, Stevens JR, Torrey EF: Search for viral nucleic acid sequences in brain tissues of patients with schizophrenia using nested polymerase chain reaction. Arch Gen Psychiatry 1996; 53:32–40Google Scholar
37. Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH: Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci 2004; 254:4–8Google Scholar
38. Dickerson F, Kirkpatrick B, Boronow J, Stallings C, Origoni A, Yolken R: Deficit schizophrenia: association with serum antibodies to cytomegalovirus. Schizophr Bull 2006; 32:396–400Google Scholar
39. Tsutsui Y, Kosugi I, Kawasaki H: Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol 2005; 15:327–345Google Scholar
40. Dalman C, Broms J, Cullberg J, Allebeck P: Young cases of schizophrenia identified in a national inpatient register: are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol 2002; 37:527–531Google Scholar
41. Gedeborg R, Furebring M, Michaelsson K: Diagnosis-dependent misclassification of infections using administrative data variably affected incidence and mortality estimates in ICU patients. J Clin Epidemiol 2007; 60:155–162Google Scholar