Insulin and Insulin-Like Growth Factor-1 Abnormalities in Antipsychotic-Naive Schizophrenia
Abstract
Objective: The purpose of this study was to examine the evidence for the insulin-like growth factor-1 (IGF-1) deficiency hypothesis in the pathogenesis of schizophrenia. Method: The authors examined the fasting plasma levels of glucose, insulin, IGF-1, and cortisol in antipsychotic-naive schizophrenia patients (N=44) relative to age- and sex-matched healthy comparison subjects (N=44). Patients and comparison subjects were also matched for anthropometric measures and physical activity. Results: Schizophrenia patients had a significantly higher mean plasma insulin level as well as a significantly higher mean insulin resistance score relative to healthy comparison subjects. The mean plasma IGF-1 level was significantly lower in patients. IGF-1 levels had a significant negative correlation with plasma insulin levels. The total positive symptoms score as well as the hallucinations subscore had a significant inverse relationship with IGF-1 levels. Conclusions: Deficient IGF-1 might underlie insulin resistance in schizophrenia. The IGF-1 deficit in antipsychotic-naive schizophrenia patients and its significant correlation with psychopathology scores suggest that IGF-1 might be potentially involved in the pathogenesis of schizophrenia.
Schizophrenia is associated with an increased risk to develop impaired glucose tolerance, insulin resistance, and type II diabetes mellitus (1) . It has been suggested that these metabolic abnormalities could be secondary to the use of antipsychotics in treating schizophrenia (2) . However, insulin resistance has been reported in schizophrenia patients even during the preneuroleptic era (3) . In addition, increased rates of type II diabetes mellitus have been observed in family members of schizophrenia patients (4) . Moreover, first-episode, antipsychotic-naive schizophrenia patients were shown to have significantly increased impaired glucose tolerance and higher insulin resistance relative to healthy comparison subjects (5) . Hence, it is possible that insulin resistance might be intrinsic to the pathogenesis of schizophrenia.
Insulin-like growth factor-1 (IGF-1) is essential for optimal insulin sensitivity, and deficient IGF-1 can lead to insulin resistance (6) . Interestingly, IGF-1 deficit also has been hypothesized to be involved in the pathogenesis of schizophrenia (7) . This potential association between IGF-1 deficit and schizophrenia is strengthened by evidence from multiple perspectives (7) . Indeed, lower IGF-1 levels and higher insulin levels were reported in schizophrenia patients receiving clozapine (8) .
In the present study, we examined fasting plasma levels of glucose, insulin, IGF-1, and cortisol in antipsychotic-naive schizophrenia patients (N=44) relative to healthy comparison subjects (N=44). Based on the IGF-1 deficiency hypothesis (7) , we predicted that schizophrenia patients would have significantly higher insulin resistance and lower IGF-1 levels than healthy comparison subjects.
Method
Patients attending the clinical services of the National Institute of Mental Health and Neuro Sciences (NIMHANS) (India) who fulfilled DSM-IV criteria for schizophrenia, were never treated with any psychotropic medications (including antipsychotics), and were not substance abusing (N=51) were referred by the screening clinical psychiatrist for the study. Of these, 44 patients (mean age=33.0 years [SD=7.7]; 23 men) fulfilled all study criteria and were included in the study. Of the remaining seven patients, four did not give consent and three were excluded because of obesity or family history of diabetes mellitus. The diagnosis of schizophrenia was established by using the Mini-International Neuropsychiatric Interview (9) , with results confirmed by another psychiatrist through an independent clinical interview. The details related to illness onset and antipsychotic-naive status were carefully ascertained by reliable information obtained from at least two adult relatives. The mean age at onset of psychosis was 29.9 years [SD=7.9], and the mean duration of untreated psychosis was 39.2 months [SD=40.8]. Psychotic symptoms were assessed using the Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS).
Healthy comparison subjects (N=44) (mean age=32.5 years [SD=7.6]; 23 men), who volunteered for the study, were recruited from among hospital staff and their friends. The social class score, as determined by the monthly income of the family, did not differ significantly between patients (mean=2.7 [SD=0.9]) and comparison subjects (mean=2.9 [SD=1.2], p>0.2). Subjects were screened to rule out psychiatric disorder by using the General Health Questionnaire and a comprehensive mental status examination. None of the healthy comparison subjects had a family history of psychiatric disorder among their first-degree relatives.
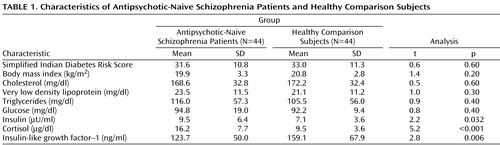
For all subjects, weight (kg), height (m), and waist circumference (cm) were measured, and body mass index (kg/m 2 ) was calculated. The propensity to develop diabetes mellitus was assessed in all subjects using the Simplified Indian Diabetes Risk Score (10) . The Simplified Indian Diabetes Risk Score is calculated based on the following parameters that are recommended by the American Diabetes Association: age, waist circumference, family history of diabetes mellitus, and physical activity. The Simplified Indian Diabetes Risk Score is a validated tool to assess the risk for developing diabetes mellitus in Indian populations. Although this instrument has yet to be validated in people with mental illness, the simplicity of the questions as well as the scoring system enabled us to use it with ease in schizophrenia patients. In addition, the information used to calculate the Simplified Indian Diabetes Risk Score was obtained from at least two adult relatives of each patient to ensure reliability. None of the subjects (patients or comparison subjects) had a family history of diabetes in any of their first-degree relatives. All subjects had a Simplified Indian Diabetes Risk Score ≤50.
Patients and comparison subjects did not score positive for alcohol use as examined by the CAGE questionnaire. None used stimulants or opiate drugs; none had a history or clinical feature suggestive of a neurological/medical disorder; and none had abnormal movements as assessed by the Abnormal Involuntary Movements Scale. Clinical assessments and blood sample collection were performed on the same day before starting treatment with antipsychotics. After complete description of the study to the subjects, written informed consent was obtained. The NIMHANS Ethics Committee approved the study.
Blood samples were collected from all subjects between 0800 and 0900 hours after a 12-hour overnight fast. Blood was drawn from an antecubital vein into Vacutainer tubes (Becton and Dickinson, U.S.A.). After centrifugation of blood, specimens were aliquoted in cryogenic vials for storage at –80°C until analysis. Glucose levels, liver and renal function tests, and serum lipid profiles were analyzed using the Olympus AU400 analyzer. Cortisol, insulin, and IGF-1 levels were quantified utilizing commercially available kits (Immulite, Diagnostics Product Corporation, Los Angeles, Calif.) based on enzyme-amplified immuno-chemiluminescence. The analytical range of methods for cortisol, insulin, and IGF-1 was found to be within 0.2–50 mg/dl, 2.0–300 mU/ml, and 20–1600 ng/ml, respectively, with an intra- and interassay precision within the range of 5%–10%. The insulin resistance was calculated based on the updated Homeostasis Model Assessment (11) using the computerized Homeostasis Model Assessment calculator (version 2.2) (http://www.dtu.ox.ac.uk/). Data analysis was performed using the SPSS-11.0 with the following statistics: Student’s t test (two-tailed), analysis of covariance (ANCOVA), and Spearman’s correlation.
Results
As a group, patients and healthy comparison subjects did not differ significantly in age and sex ratio (p>0.6). The characteristics of the study subjects are given in Table 1 . Total Simplified Indian Diabetes Risk Score did not differ significantly between patients and comparison subjects. Patients and comparison subjects were not significantly different in waist circumference and physical activity as measured by the Simplified Indian Diabetes Risk Score. The body mass index of patients and comparison subjects did not differ significantly. Fasting lipid profile was not significantly different between patients and comparison subjects. In all subjects, liver and renal functions were within normal limits.
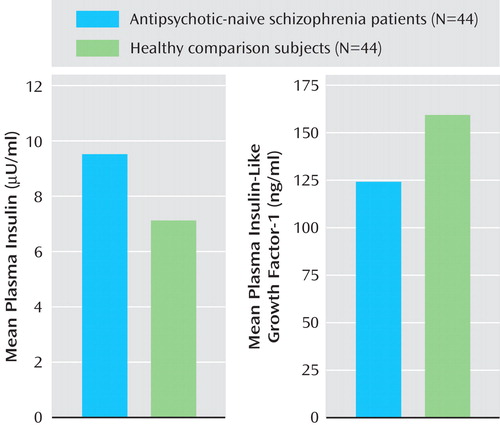
The mean fasting plasma levels of glucose for patients and healthy comparison subjects did not differ significantly ( Table 1 ). Patients had significantly higher mean plasma insulin levels relative to healthy comparison subjects ( Figure 1 ). In addition, the insulin resistance score, which was measured by the Homeostasis Model Assessment index, was significantly higher in patients (1.2 [SD=0.8]) than in healthy comparison subjects (0.9 [SD=0.4]) (t=2.3, df=86, p=0.039). Patients had a significantly higher mean cortisol level than healthy subjects. Mean plasma insulin (F=4.4, p=0.039) continued to be significantly higher in schizophrenia patients even after we controlled for the potential effects of cortisol differences between patients and healthy comparison subjects (one-way ANCOVA test using cortisol level as a covariate). The mean plasma IGF-1 level was significantly lower in patients than in healthy comparison subjects ( Figure 1 ). IGF-1 levels had a significant negative correlation with plasma insulin levels (r s =–0.24, p=0.026).
The SAPS and SANS mean total scores were 30.7 [SD=17.4] and 63.1 [SD=33.3], respectively. The psychopathology subscores were as follows: 1) SAPS subscores: hallucinations, 9.8 (SD=10.6); delusions, 12.5 (SD=9.8); bizarre behavior, 5.8 (SD=4.4); positive formal thought disorder, 2.7 (SD=6.6); 2) SANS subscores: blunting, 15.3 (SD=12.1); alogia, 7.9 (SD=8.2); avolition-apathy, 13.2 (SD=5.6); anhedonia-asociality, 18.0 (SD=7.2); and attention, 8.7 (SD=6.2). SAPS total score (r s =–0.33, p=0.028) as well as the hallucinations subscore (r s =–0.39, p=0.008) had a significant negative correlation with IGF-1 levels. However, after application of Bonferroni correction to control for multiple correlations, the correlation between the IGF-1 levels and hallucinations subscore alone remained significant (p<0.01). IGF-1 levels did not correlate significantly with SANS scores, age at onset, or duration of untreated psychosis. Plasma insulin as well as cortisol levels did not correlate significantly with age at onset, duration of untreated psychosis, or psychopathology scores.
Discussion
This is the first study to examine antipsychotic-naive schizophrenia patients for plasma IGF-1, along with insulin and cortisol levels. In this study, plasma insulin and insulin resistance were significantly higher in patients than in healthy comparison subjects. Schizophrenia patients had significantly lower IGF-1 levels relative to comparison subjects. Plasma IGF-1 levels had a significant negative correlation with plasma insulin levels. IGF-1 levels had negative significant correlations with the SAPS total score as well as hallucinations subscore.
Our study observation of significantly increased insulin resistance in antipsychotic-naive schizophrenia replicates a previous report (5) . The persistence of hyperinsulinemia even after controlling for cortisol levels suggests that this is independent of hypercortisolemia. In addition, significantly lower IGF-1 levels as well as inverse correlation between IGF-1 and insulin are in tune with a previous study on clozapine-treated schizophrenia patients (8) . Since IGF-1 is essential for insulin sensitivity (6) , IGF-1 deficit might be one of the factors underlying insulin resistance in schizophrenia.
Several lines of evidence suggest that IGF-1 might play a significant role in the pathogenesis of schizophrenia (see [7] for review). Low birth weight, leanness, and short stature (which are linked to decreased IGF-1 levels) are shown to be associated with increased risk for developing schizophrenia (7) . Additionally, the negative association between schizophrenia and cancer (12) has been hypothesized to be secondary to low IGF-1 levels (7) . Moreover, genome-wide scans have identified regions on chromosome 12p13-q24, close to those for the IGF-I gene, to be linked to schizophrenia (13) . While all this indirect evidence suggests that IGF-1 abnormalities might be associated with schizophrenia, our study adds to the direct evidence supporting this link.
IGF-1 has neuroprotective, antiapoptotic properties that are crucial for optimal neurodevelopment of the brain (14) . It is possible that low IGF-1 levels might render the brain more vulnerable to neurodevelopmental insults potentially culminating in schizophrenia. Since IGF-1 receptors are concentrated in the hippocampus, this brain region is likely to be more affected because of IGF-1 deficits (14) . Interestingly, hippocampal dysfunction has been proposed to potentially underlie the genesis of auditory hallucinations in schizophrenia (15) . Hence, the significant correlation between low IGF-1 levels and the severity of hallucinations, as observed in this study, might further support the potential role of IGF-1 in the pathogenesis of schizophrenia.
While examination of antipsychotic-naive schizophrenia patients with well-matched healthy comparison subjects avoided many potential confounding factors, the study findings have to be considered in the context of the following limitations. Although the lack of substance abuse in the study population avoided the potential confounding effects of substance on biochemical assessments, this might restrict the generalizability of the study findings. Although the patients were comparable with healthy comparison subjects in body mass index and waist circumference, we did not apply structured assessments for dietary intake. It is possible that patients may have had a period of undernutrition prior to presentation that could have resulted in decreased IGF-1 levels. However, we carefully assessed the historical information to exclude significant malnutrition. In addition, it has been reported that malnutrition leads to reduction in both insulin as well as IGF-1 levels (16) . Hence, our findings of significantly lower IGF-1 levels with concurrent higher insulin levels in schizophrenia patients suggest that the observed IGF-1 deficits are less likely because of malnutrition.
Our study did not measure IGF binding proteins (IGFBP-1 and IGFBP-3), which might play a potential role in regulating IGF-1 bioactivity in relation to food intake. This might be considered as a potential limitation. However, since all the blood samples were collected after an overnight fast, the issue pertaining to postmeal variability of IGFBP-1 may not be relevant for this study. Furthermore, unlike IGFBP-1, IGFBP-3 (which binds a major portion of the serum IGF-1) shows very little change following meals (17) . Another limitation of our study is that the growth hormone secretion was not assessed, which would have helped in examining the possibility of hypothalamopituitary-axis dysfunction.
In conclusion, IGF-1 deficit in antipsychotic-naive schizophrenia patients and its significant correlation with psychopathology suggest that IGF-1 might be potentially involved in the pathogenesis of schizophrenia. However, the cross-sectional nature of this study makes one cautious to interpret definitive causality. Future studies should replicate and extend these novel findings, especially by examining follow-up analyses of patients after antipsychotic treatment as well as assessment of subjects at high risk for schizophrenia.
1. Ryan MC, Thakore JH: Physical consequences of schizophrenia and its treatment: the metabolic syndrome. Life Sci 2002; 71:239–257Google Scholar
2. Lindenmayer JP, Nathan AM, Smith RC: Hyperglycemia associated with the use of atypical antipsychotics. J Clin Psychiatry 2001; 62:30–38Google Scholar
3. Freeman H: Resistance to insulin in mentally disturbed soldiers. Arch Neurol Psychiatry 1946; 56:74–78Google Scholar
4. Mukherjee S, Schnur DB, Reddy R: Family history of type 2 diabetes in schizophrenic patients. Lancet 1989; 1:495Google Scholar
5. Ryan MC, Collins P, Thakore JH: Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry 2003; 160:284–289Google Scholar
6. Holt RI, Simpson HL, Sonksen PH: The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet Med 2003; 20:3–15Google Scholar
7. Gunnell D, Holly JM: Do insulin-like growth factors underlie associations of birth complications, fetal and pre-adult growth with schizophrenia? Schizophr Res 2004; 67:309–311Google Scholar
8. Melkersson KI, Hulting AL, Brismar KE: Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J Clin Psychiatry 1999; 60:783–791Google Scholar
9. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33Google Scholar
10. Mohan V, Deepa R, Deepa M, Somannavar S, Datta M: A Simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J Assoc Physicians India 2005; 53:759–763Google Scholar
11. Levy JC, Matthews DR, Hermans MP: Correct Homeostasis Model Assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998; 21:2191–2192Google Scholar
12. Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J: Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001; 58:573–578Google Scholar
13. DeLisi LE, Shaw SH, Crow TJ, Shields G, Smith AB, Larach VW, Wellman N, Loftus J, Nanthakumar B, Razi K, Stewart J, Comazzi M, Vita A, Heffner T, Sherrington R: A genome-wide scan for linkage to chromosomal regions in 382 sibling pairs with schizophrenia or schizoaffective disorder. Am J Psychiatry 2002; 159:803–812Google Scholar
14. Dore S, Kar S, Quirion R: Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A 1997; 94:4772–4777Google Scholar
15. Gisabella B, Bolshakov VY, Benes FM: Regulation of synaptic plasticity in a schizophrenia model. Proc Natl Acad Sci U S A 2005; 102:13301–13306Google Scholar
16. Haspolat K, Ece A, Gurkan F, Atamer Y, Tutanc M, Yolbas I: Relationships between leptin, insulin, IGF-1 and IGFBP-3 in children with energy malnutrition. Clin Biochem 2007; 40:201–205Google Scholar
17. Bereket A, Wilson TA, Blethen SL, Fan J, Frost RA, Gelato MC, Lang CH: Effect of short-term fasting on free/dissociable insulin-like growth factor I concentrations in normal human serum. J Clin Endocrinol Metab 1996; 81:4379–4384Google Scholar