Increased Error-Related Brain Activity in Pediatric Obsessive-Compulsive Disorder Before and After Treatment
Abstract
Objective: The error-related negativity is a negative deflection in the event-related potential maximal approximately 50 msec after the commission of errors. The error-related negativity is generated in the anterior cingulate cortex, and both anterior cingulate cortex hyperactivity and increased error-related brain activity have been reported in adults with obsessive-compulsive disorder (OCD). However, no study to date, to the authors’ knowledge, has examined error-related brain activity in pediatric patients with OCD, and no study has examined error-related brain activity in OCD both before and after treatment. Method: The error-related negativity was measured in 18 treatment-seeking pediatric patients with OCD and 18 age-matched comparison subjects. Of these patients, 10 returned for a second testing session after cognitive behavior therapy; 13 comparison children participated a second time after a comparable interval. Results: In the pretreatment group, the error-related negativity was reliably larger in pediatric patients with OCD in relation to comparison subjects. This difference was also evident after treatment. There was no relationship between error-related negativity and symptom severity or changes in symptom severity. Conclusions: Consistent with studies in adult patients, increased error-related brain activity is evident in pediatric patients with OCD. Furthermore, increased error-related brain activity does not appear to change as a function of symptom reduction after therapy. These results suggest that an increased error-related negativity may be a trait-like marker for psychopathology and might be a useful endophenotype.
Obsessive-compulsive disorder (OCD) is an anxiety disorder defined by the presence of recurrent obsessions and/or compulsions that cause significant impairment (1) . OCD is fairly common, affecting approximately 2% of the population (2) ; it commonly begins in adolescence and is associated with a chronic course if untreated (3) . OCD adversely influences social, work, and family functioning and compromises general quality of life across the developmental spectrum (4) .
In terms of the neural substrates of OCD, neuroimaging studies have consistently reported abnormalities in frontostriatal circuits, including the anterior cingulate cortex (5 – 8) . The anterior cingulate cortex is part of the medial prefrontal cortex implicated in modulating both emotional and cognitive processing (cf., reference 9 ). In recent years, the anterior cingulate cortex has been increasingly the focus of studies on emotion-cognition interactions involved in error detection.
Specifically, the error-related negativity is a frontally maximal negative deflection in the response-locked event-related potential that begins around the time of an incorrect response and peaks approximately 50 msec later (10 , 11) . Functionally, the error-related negativity is thought to reflect the mismatch or coactivation of the actual and intended responses on error trials (10 , 12) . Studies that use source localization suggest that the error-related negativity is generated in the anterior cingulate cortex (13) , which is consistent with human magneto-encephalographic (14) , functional neuroimaging (15) , and intracerebral recording data (16) . Because the error-related negativity has been observed across multiple stimulus and response modalities (13) , it appears to reflect the activity of a generic action-monitoring system (11 , 12) .
To date, to our knowledge, all studies on error-related brain activity and OCD have been conducted with adults. The first study to establish an association between error-related brain activity and OCD was reported by Gehring et al. (17) , who measured error-related negativity in a group of adult patients with OCD. Gehring et al. found an enhanced error-related negativity in the OCD group relative to matched comparison subjects; additionally, the magnitude of the error-related negativity was correlated with OCD symptom severity. Subsequent studies also reported hyperactive error-related brain activity in relation to clinical OCD in adults (18 , 19) and elevated self-reported obsessive-compulsive symptoms (20) .
The relationship between increased error-related brain activity and OCD has also been supported by studies employing functional magnetic resonance imaging (fMRI): Ursu and colleagues (21) reported that patients with OCD had increased error-related brain activity localized to the anterior cingulate cortex and that this hyperactivity correlated with symptom severity. More recently, Fitzgerald et al. (22) and Maltby et al. (23) have also reported hyperactive error-related anterior cingulate cortex activity in adult patients with OCD. Collectively, these results have been taken as evidence for the proposal that the neurobiological basis of OCD may involve abnormalities of an action-monitoring or error-detection system, which could give rise to repeated doubts about actions and concern over potential mistakes (24) .
Despite consistent evidence indicating hyperactive error processing in adult OCD, no studies to date, to our knowledge, have examined this issue in pediatric OCD populations. One study reported increased error-related negativity magnitudes in children as a function of parent-reported obsessive-compulsive behaviors (25) , suggesting that children with OCD may also be characterized by hyperactive error processing. In light of the fact that OCD is often reported to begin in childhood or adolescence, evaluating the neural circuitry of error processing in pediatric patients is a necessary step toward understanding the developmental course of OCD pathophysiology. Accordingly, the primary goal of the present study was to determine whether children with OCD, like adults with OCD, have enhanced brain activity related to error detection. Based on adult studies, we hypothesized that pediatric patients with OCD will evince enhanced error-related brain activity when compared to age-matched comparison subjects.
Additionally, error-related negativity might be used to assess treatment-related changes and their relationship to brain function. In support of this possibility, the magnitude of error-related brain activity in OCD patients has been related to OCD symptom severity in three studies using both fMRI and event-related potential methods (17 , 21 , 22) . However, two studies did not find a relationship between error-related negativity amplitude and OCD severity (18 , 19) , and increasing state levels of anxiety do not appear to affect the amplitude of the error-related negativity (26) , suggesting that the error-related negativity may reflect a trait-like vulnerability factor. In light of these issues, a second goal of this study was to examine whether treatment-related changes in pediatric OCD are related to changes in error-related brain activity. If the error-related negativity relates directly to symptom severity in OCD, then reductions in OCD symptoms should be accompanied by a reduction in error-related negativity; however, if an increased error-related negativity reflects a trait-like vulnerability factor, then the error-related negativity should remain enhanced after treatment.
Overall, then, the purpose of the present study was to evaluate error-related brain activity in pediatric OCD patients both before and after therapy. To this end, we measured the error-related negativity in 18 treatment-seeking pediatric patients with OCD and 18 age-matched healthy comparison children. All of the OCD children were assessed before beginning exposure and response prevention therapy, an efficacious form of cognitive behavior therapy for OCD in youth (27) . All participants were asked to return for a second testing session—OCD patients after therapy and comparison participants after a comparable delay.
Method for Pretreatment Group
Participants
Pediatric patients were recruited through the Center for the Treatment and Study of Anxiety within the University of Pennsylvania’s Department of Psychiatry. All pediatric OCD patients were treatment-seeking and were being treated through the fee-for-service clinic or as part of NIMH-funded treatment trials. Pediatric comparison subjects were recruited from the surrounding community. Patients were asked to participate in the current experiment both before and after psychotherapy. Comparison subjects were asked to participate in the experiment at two time points; the second testing was scheduled so that it corresponded to the pre-to-post patient timeframe. All subjects were paid $10 per session for their participation in the study. After complete description of the study to the subjects, written informed consent was obtained. All subjects and their parents completed consent forms approved by the University of Pennsylvania’s internal review board. OCD patients were assessed by a clinician at the Center for the Treatment and Study of Anxiety in Philadelphia.
DSM-IV criteria for OCD were met by all participants in the OCD group; the severity of OCD was assessed with the Children’s Yale-Brown Obsessive Compulsive Scale (28) . Patients were excluded if they met DSM-IV criteria for bipolar disorder, were primarily depressed, or evidenced psychotic symptoms. All comparison participants were free of axis I diagnosis based on a clinical interview with the Mini International Neuropsychiatric Interview (29) . These measures were obtained from the child; no other measures were given to all participants. Consistent with previous adult OCD studies on the error-related negativity, medication was uncontrolled. Medications being taken (and number of patients taking the medication) were the following: clomipramine (two), sertraline (three), escitalopram (four), fluoxetine (one), bupropion (two), and fluvoxamine (one). All patients with OCD presented with multiple domains of obsessions and compulsions.
The OCD group was composed of 18 pediatric patients with OCD who were age-matched to 18 comparison subjects; the average age was 13.3 years (SD=2.8, range=8–17) and 11.9 years (SD=2.6, range=8–16), respectively (t=1.67, df=34, p>0.10). Consistent with the relatively higher prevalence of OCD in boys than girls in pediatric samples (30) , the OCD group was composed of 13 boys, whereas the comparison group was composed of eight boys (χ 2 =2.86, df=1, p>0.05). The average pretreatment Children’s Yale-Brown Obsessive Compulsive Scale score in the OCD group was 25.6 (SD=5.1), indicating moderate to severe OCD.
Task
A modified Simon task (unpublished work by Simon, 1990) was administered on a computer with Presentation software (Neurobehavioral Systems, Inc., Albany, Calif.) to control the presentation and timing of all stimuli, the determination of response accuracy, and the measurement of reaction times.
Throughout the task, subjects were shown arrows oriented either to the right, to the left, or to the top of a 17-inch monitor. The arrows were positioned in the center of the screen in red or green fronted against a black background. A fixation mark (+) was presented before the onset of each stimulus. The subjects were instructed to press the left or right control key with their left or right hands, respectively, in response to the color of the arrow and to disregard its orientation. At a viewing distance of roughly 65 cm, each arrow subtended approximately 3° of visual angle (width of arrow) by 10°of visual angle (length of arrow).
Procedure
Each participant was seated 0.65 meters directly in front of the computer monitor and given two blocks of 18 practice trials. In one condition, the subjects were told to press the left control key when the arrow was red and the right control key when the arrow was green. In the other condition, the correspondence between the keys and arrow color was reversed. These conditions were counterbalanced across subjects and across testing sessions such that participants with both pre- and posttreatment data performed both versions of the task. The subjects were told to place equal emphasis on speed and accuracy in their responses. After practice, all subjects received 12 blocks of 48 trials (576 total trials), with each block initiated by the subject. Arrow stimuli were presented for 200 msec at random intervals between 2000 and 2400 msec.
Psychophysiological Recording, Data Reduction, and Analysis
The EEG was recorded with a Quik-Cap (Neuromedical Supplies, El Paso, Tex.). Recordings were taken from three locations along the midline: frontal (Fz), central (Cz), and parietal (Pz). In addition, tin electrodes (Med Associates, St. Albans, Vt.) were placed on the left and right mastoids (A1 and A2, respectively). During the recording, all activity was referenced to Cz. The electro-oculogram generated from blinks and vertical eye movements was also recorded with miniature electrodes placed approximately 1 cm above and below the participant’s right eye. The right earlobe served as a ground site. All EEG/electro-oculogram electrode impedances were below 10 KW, and the data from all channels were recorded by a Grass model 7D polygraph with Grass model 7P1F preamplifiers (Grass Technologies, West Warwick, R.I.) (bandpass=0.05–35.00 Hz).
All bioelectric signals were digitized on a laboratory microcomputer using VPM software (31) . The EEG was sampled at 200 Hz. Data collection began with the onset of the imperative stimuli and continued for 1500 msec. Offline, the EEG for each trial was corrected for vertical electro-oculogram artifacts with the method developed by Gratton et al. (32) and then rereferenced to the average activity of the mastoid electrodes. Trials were rejected and not counted in subsequent analyses if there were excessive physiological artifacts (i.e., 25 msec of invariant analogue data on any channel or A/D values on any channel that equaled that converter’s minimum or maximum values). Single-trial EEG data were low-pass filtered at 20 Hz with a digital filter. Finally, the EEG for each trial was time-locked to its respective reaction time and averaged across error and correct trials. Because reaction time tends to be faster for error compared to correct trials (17 , 18 , 20) , event-related potential data are presented for error trials and a subset of correct trials matched to error trials on the basis of reaction time.
To quantify brain activity, each data point after response onset was subtracted from a baseline equal to the average activity in a 100 msec window –150 to –50 msec before the response. The error-related negativity and the error-related negativity-like response on correct trials—the correct response negativity—were quantified at Fz, where they were maximal, with a base-to-peak measure: the error-related negativity and correct response negativity were scored as the most negative peak in a –50 to 100 msec postresponse window, relative to the most positive value in the 100 msec preceding the negative peak. Error-related negativity and correct response negativity amplitude were evaluated in the overall group with a two- (trial) by-three (location) repeated-measures analysis of variance (ANOVA). Based on a significant interaction between trial type and location (F=19.07, df=2, 70, p<0.001), we determined that the difference between the error-related negativity and correct response negativity was larger at Fz than both Cz (t=2.67, df=35, p<0.01) and Pz (t=4.98, df=35, p<0.001) and larger at Cz than Pz (t=4.26, df=35, p<0.001). Behavioral measures included the number of erroneous and correct trials for each subject, as well as accuracy expressed as a percentage of valid trials. Average reaction times on errors and correct trials were calculated separately. Finally, reaction time and accuracy after errors were evaluated to determine if there are group differences in post-error behavioral adjustments. All behavioral and event-related potential measures were statistically evaluated with SPSS (Version 10.1) General Linear Model software (SPSS, Chicago).
Results
Error-Related Potential Data
Figure 1 presents response-locked event-related potential data for error and correct trials from OCD (left) and comparison (right) participants. Consistent with previous studies, a two- (group) by-two (trial type) repeated measures ANOVA on error-related negativity and correct response negativity magnitude confirmed that the error-related negativity was larger than the correct response negativity (F=11.72, df=1, 34, p<0.01). OCD patients were characterized by larger error-related negativities—but not correct response negativities—than comparison participants, which was confirmed by a significant interaction between group and trial type (F=5.61, df=1, 34, p<0.05). However, there was not an overall difference between OCD and comparison participants (F=2.31, df=1, 34, p>0.10). Error-related negativity magnitude was significantly correlated with age, becoming larger with increasing age (r=–0.62, p<0.001); additionally, the correlation between age and event-related negativity was somewhat larger in the comparison (r=–0.74, p<0.001) than the OCD group (r=–0.46, p<0.05), although this difference was not reliable (p>0.20). Correct response negativity amplitude was uncorrelated with age in all comparisons (p>0.60). Thus, older children were characterized by larger error-related negativities in both comparison and OCD groups, consistent with previous developmental work on the error-related negativity (33) . Additionally, we evaluated the effect of gender in the pretreatment comparison group, which had a relatively equal split between boys and girls; neither error-related negativity (t=0.49, df=16, p>0.60) nor correct response negativity (t=0.37, df=16, p>0.70) amplitude differed as a function of gender. Finally, we compared the correct response negativity and error-related negativity between OCD patients who were taking medications and those who were not in the pretreatment group; medication status did not interact with the difference between the error-related negativity and correct response negativity (F<1.0, df=1, 16), and medication did not influence the magnitude of the error-related negativity and correct response negativity overall (F<1.0, df=1, 16). Finally, in the pre- to posttreatment group, medication status was unrelated to change in error-related negativity and correct response negativity and unrelated to change in symptom severity (all p>0.20).
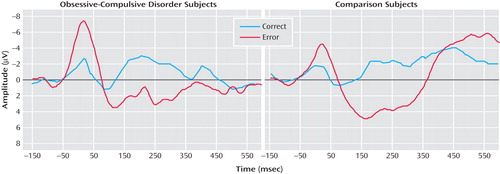
a Responses occurred at 0 msec.
The magnitude of the error-related negativity and correct response negativity were uncorrelated with symptom severity (r=–0.09, df=18, p>0.70; and r=–0.13, df=18, p>0.55, respectively) in the OCD group. Similar nonsignificant results were obtained with nonparametric correlation coefficients (all p>0.30).
Behavioral Results
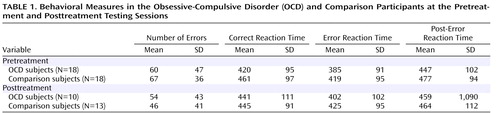
Performance data are presented in Table 1 . The OCD and comparison groups had comparable performance data (t=0.57, df=34, p>0.55). Although the participants had faster reaction times on error trials than correct trials (F=65.36, df=1, 34, p<0.001), the OCD group did not differ from the comparison group (F<1.0, df=1, 34), and the interaction between trial type and group was not significant (F=1.48, df=1, 34, p>0.20). Additionally, we examined reaction times on correct trials that follow errors and compared this post-error slowing to the overall reaction time on correct trials. Consistent with previous studies, all subjects were reliably slower after errors (F=8.04, df=1, 34, p<0.01); the amount of post-error slowing, however, did not differ as a function of group status (t=0.83, df=34, p>0.40). Thus, the OCD and comparison groups had comparable behavioral data in terms of performance accuracy, reaction time, and post-error reaction time slowing.
Discussion
In a group of 18 pediatric patients with OCD and 18 age-matched healthy comparison children, the magnitude of the error-related negativity was reliably larger in the OCD group; this difference was not apparent on correct trials, suggesting a specific enhancement of error-related brain activity in children with OCD. These results are generally consistent with previous error-processing studies in adult OCD samples, with both event-related potential (17 – 20) and fMRI (21 – 23) . The present results indicate that hyperactive error-related brain activity also characterizes children with OCD.
It is important to note that the increased error-related brain activity in pediatric OCD patients cannot simply reflect performance differences: the OCD patients and healthy comparison participants made a comparable number of errors and, had similar reaction times and post-error reaction time slowing. Unlike Gehring et al. (17) , we did not find correlations between OCD symptom severity and event-related negativity magnitude. Of interest, both Johannes et al. (19) and Ruchsow et al. (18) also failed to find a relationship between OCD severity and error-related negativity amplitude. One possibility is that an increased error-related negativity reflects a vulnerability to developing OCD and does not change with fluctuations in symptom severity. To further examine this possibility, we examined error-related negativity both before and after therapy.
Method for Pre- to Posttreatment Changes
Participants
OCD patients were recruited before beginning cognitive behavior therapy at the Center for the Treatment and Study of Anxiety. Cognitive-behavior therapy for OCD involves approximately 15 1-hour individual sessions. Although treatment is typically delivered weekly, some patients opt for a more intensive therapeutic regimen with comparable efficacy (27) . Comparison participants were asked to return for the second testing with a schedule similar to that of OCD patients.
Just over half of the initial group returned for the second testing session; the group of subjects with both time 1 and time 2 event-related potential data consisted of 10 pediatric OCD patients (three boys) and 13 pediatric comparison subjects (nine boys); the average ages were 12.5 years (SD=3.2) and 12.3 years (SD=2.7), respectively (t=0.16, df=21, p>0.85); the groups did not differ reliably in terms of gender (χ 2 =3.49, df=1, p>0.05).
For the subjects with both pre- and posttreatment data, the average pretreatment score on the Children’s Yale-Brown Obsessive Compulsive Scale was 24.3 (SD=6.1), indicating moderate to severe OCD; of importance, this subset of OCD patients was equivalent in severity to the patients without posttreatment data (mean=27.1, SD=3.1; t=1.17, df=16, p>0.25). Pre- and posttreatment Children’s Yale-Brown Obsessive Compulsive Scale scores were available for eight of the 10 patients who participated in the event-related study at both time points; for these patients, posttreatment Children’s Yale-Brown Obsessive Compulsive Scale scores (mean=11.5, SD=4.1) differed significantly from pretreatment scores (mean=23.4, SD=6.5; t=3.79, df=7, p<0.01); notably, no subject had an increase in Children’s Yale-Brown Obsessive Compulsive Scale score after therapy, and a score of 12 is actually below the cutoff for clinical OCD.
Task and Procedure
The task, procedures, and data analysis strategies were identical to those described above.
Results
Error-Related Potential Data
Figure 2 presents response-locked event-related potential data from the pretreatment (left) and posttreatment (right) testing sessions for OCD (top) and comparison (bottom) participants. The error-related negativity and correct response negativity were analyzed with a two- (group) by-two- (testing session) by-two (trial type) repeated measures ANOVA. The effect of trial type was significant, indicating that the event-related negativity was reliably larger than the correct response negativity (F=6.88, df=1, 21, p<0.05). As in the pretreatment analyses, there was no overall difference between groups (F<1.0, df=1, 21), but there was a significant interaction between group and trial type (F=7.48, df=1, 21, p<0.05). There was no main effect of testing session (F<1.0, df=1, 21), and testing session did not interact with group (F=2.69, df=1, 21, p>0.10) or trial type (F<1.0, df=1, 21); the three-way interaction did not reach significance (F<1.0, df=1, 21). Thus, OCD patients differed from comparison participants in terms of error-related negativity but not correct response negativity at both the pre- and posttreatment testing sessions. Change in symptom severity was uncorrelated with change in error-related negativity (r=–0.03, p>0.90) and correct response negativity (r=0.17, p>0.65); additionally, magnitude of the pretreatment event-related negativity did not predict change in symptom severity (r=–0.09, p>0.80).
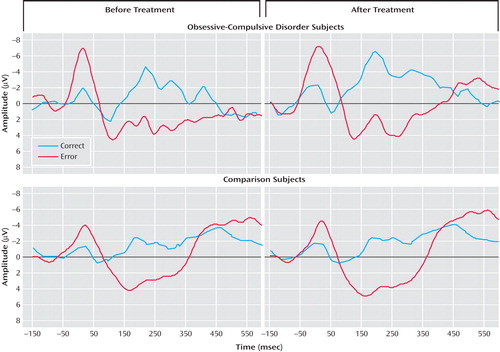
a Responses occurred at 0 msec.
Behavioral Results
Behavioral data for participants with both pre- and posttreatment data are presented in Table 1 . Although the participants made reliably fewer errors at the posttreatment than the pretreatment session (F=4.58, df=1, 21, p<0.05), this did not vary as a function of group status (F<1.0, df=1, 21), and the two groups did not differ overall in terms of the number of errors committed (F<1.0, df=1, 21). In terms of reaction time, the participants were faster on error than correct trials (F=62.34, df=1, 21, p<0.001), but all other main effects and interactions did not reach significance (p>0.05). Compared to the mean correct trial reaction time, all subjects had slower reaction times on correct trials that followed errors (F=5.18, df=1, 21, p<0.05). However, post-error slowing did not change as a function of testing session and did not vary by group status; all interactions were not significant (p>0.50). Consistent with the pretreatment analyses, all subjects demonstrated faster reaction times on error than correct trials, and post-error trials were characterized by relatively slower reaction times; however, OCD and comparison subjects did not differ on any behavioral measures.
General Discussion
Existing neurodevelopmental models of OCD highlight functional and structural abnormalities of the anterior cingulate cortex (8) , the precise region in the medial prefrontal cortex where the error-related negativity is generated (14 , 17) . The anterior cingulate cortex continues to mature into early adulthood (33) , and the available data suggest that the error-related negativity follows a similar developmental trajectory, becoming larger throughout adolescence (33) . In the present study, too, the magnitude of error-related negativity was related to age. In this developmental context, pediatric OCD patients were characterized by an increased error-related negativity, indicating increased error-related anterior cingulate cortex activity in children with OCD.
Both the error-detection (11) and conflict-monitoring (12) accounts of the error-related negativity propose that anterior cingulate cortex activity during response monitoring signals the need to increase cognitive control; in fact, anterior cingulate cortex activity during response monitoring has been coupled with subsequent activity in the prefrontal cortex (34) . These data, then, are consistent with the notion that exaggerated error-related brain activity in OCD may signal an increased response to errors, which may prompt increased cognitive, behavioral, and affective processing (35) . Consistent with this notion, patients with OCD doubt their actions, are concerned about mistakes, and often repeat actions until they are performed “correctly.” Thus, the notion that OCD is characterized by hyperactive error-related brain activity is consistent with the phenomenology of the disorder. In fact, Pitman (24) suggested that rituals, symptomatic of OCD, are performed to reduce abnormal error signals. Consistent with this suggestion, several studies have found increased error-related anterior cingulate cortex activity in adult patients with OCD (17 – 20) .
In the present study, however, increased error-related brain activity in OCD was maintained over the course of successful therapy and, therefore, was unrelated to clinical status. The present data, then, are inconsistent with Pitman’s suggestion; hyperactive anterior cingulate cortex activity during response monitoring does not appear to maintain OCD symptoms because if this were true, the error-related negativity would be reduced after successful therapy. Rather, these data suggest that hyperactive error-related brain activity in OCD reflects a trait-like marker that is unrelated to changes in symptom severity.
It is unclear whether the present results characterize all patients with OCD, patients with certain subtypes of OCD, or patients with anxiety disorders more broadly. Along these lines, however, there is growing evidence that abnormalities of error-processing generalize beyond OCD and even beyond the anxiety disorders. For instance, Hajcak et al. (35) found that participants prone to generalized anxiety disorder had increased error-related brain activity, and a recent study found that children with anxiety disorders other than OCD were also characterized by increased error-related brain activity (36) . In addition, Chiu and Deldin (37) observed enhanced error-related negativity in patients with current major depressive disorder. These data suggest that abnormal error-related negativities may relate to overlapping features of anxiety and depression and are consistent with other another study of ours in which we report increased error-related negativities in relation to high trait levels of negative affect (38) .
Error-related negativity magnitude is sensitive to manipulations of error value and importance (39) . An increased error-related negativity, therefore, might indicate that errors are processed as maladaptively significant in high-negative-affect individuals (cf. reference 37 ). The size of the error-related negativity has also been shown to predict the degree to which participants learn about negative, but not positive, consequences of their actions. Within this framework, an increased error-related negativity may reflect neural correlates of avoidance learning and the underlying propensity for individuals at risk for affective psychopathology to avoid negative outcomes (40) . We are examining the error-related negativity in at-risk populations to further evaluate this possibility.
1. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Press, 1994Google Scholar
2. Karno M, Golding JM, Sorenson SB, Burnam MA: The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry 1988; 45:1094–1099Google Scholar
3. Rasmussen SA, Eisen JL: The epidemiology and differential diagnosis of obsessive compulsive disorder. J Clin Psychiatry 1992; 53(suppl):4–10Google Scholar
4. Piacentini J, Bergman RL, Keller M, McCracken J: Functional impairment in children and adolescents with obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2003; 13(suppl 1):S61–S69Google Scholar
5. Breiter HC, Rauch SL: Functional MRI and the study of OCD: from symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage 1996; 4(suppl 3):S127–S138Google Scholar
6. Saxena S, Rauch SL: Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000; 23:563–586Google Scholar
7. Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM: fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 2000; 34:317–324Google Scholar
8. Rosenberg DR, Keshavan MS: AE Bennett Research Award: toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry 1998; 43:623–640Google Scholar
9. Bush G, Luu P, Posner MI: Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000; 4:215–222Google Scholar
10. Falkenstein M, Hohnsbein J, Hoormann J, Blanke L: Effects of crossmodal divided attention on late ERP components, II: error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 1991; 78:447–455Google Scholar
11. Holroyd CB, Coles MG: The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 2002; 109:679–709Google Scholar
12. Yeung N, Cohen JD, Botvinick MM: The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 2004; 111:931–959Google Scholar
13. Holroyd CB, Dien J, Coles MG: Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci Lett 1998; 242:65–68Google Scholar
14. Miltner WH, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MG: Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol 2003; 64:157–166Google Scholar
15. Kiehl KA, Liddle PF, Hopfinger JB: Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 2000; 37:216–223Google Scholar
16. Brázdil M, Roman R, Daniel P, Rektor I: Intracerebral error-related negativity in a simple go/no-go task. J Psychophysiol 2005; 19:244–255Google Scholar
17. Gehring WJ, Himle J, Nisenson LG: Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci 2000; 11:1–6Google Scholar
18. Ruchsow M, Grön G, Reuter K, Spitzer M, Hermle L, Kiefer M: Error-related brain activity in patients with obsessive-compulsive disorder and in healthy controls. J Psychophysiol 2005; 19:298–304Google Scholar
19. Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, Munte TF, Dietrich DE: Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res 2001; 108:101–110Google Scholar
20. Hajcak G, Simons RF: Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res 2002; 110:63–72Google Scholar
21. Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS: Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci 2003; 14:347–353Google Scholar
22. Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF: Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry 2005; 57:287–294Google Scholar
23. Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA: Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage 2005; 24:495–503Google Scholar
24. Pitman RK: A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry 1987; 28:334–343Google Scholar
25. Santesso DL, Segalowitz SJ, Schmidt LA: Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol 2006; 29:431–445Google Scholar
26. Moser JS, Hajcak G, Simons RF: The effects of fear on performance monitoring and attentional allocation. Psychophysiology 2005; 42:261–268Google Scholar
27. Storch EA, Geffken GR, Merlo LJ, Mann G, Duke D, Munson M, Adkins J, Grabill KM, Murphy TK, Goodman WK: Family-based cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: comparison of intensive and weekly approaches. J Am Acad Child Adolesc Psychiatry 2007; 46:469–478Google Scholar
28. Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF: Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852Google Scholar
29. Sheehan D, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC: The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 1997; 12:232–241Google Scholar
30. Swedo SE, Rapoport JL, Leonard H, Lenane M, Cheslow D: Obsessive-compulsive disorder in children and adolescents: clinical phenomenology of 70 consecutive cases. Arch Gen Psychiatry 1989; 46:335–341Google Scholar
31. Cook EW III: VPM Reference Manual. Birmingham, University of Alabama, 1995Google Scholar
32. Gratton G, Coles MG, Donchin E: A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 1983; 55:468–484Google Scholar
33. Davies PL, Segalowitz SJ, Gavin WJ: Development of response-monitoring ERPs in 7- to 25-year-olds. Dev Neuropsychol 2004; 25:355–376Google Scholar
34. Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS: Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303:1023–1026Google Scholar
35. Hajcak G, McDonald N, Simons RF: Anxiety and error-related brain activity. Biol Psychol 2003; 64:77–90Google Scholar
36. Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND: Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry 2006; 47:1073–1082Google Scholar
37. Chiu PH, Deldin PJ: Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry 2007; 164:608–616Google Scholar
38. Hajcak G, McDonald N, Simons RF: Error-related psychophysiology and negative affect. Brain Cogn 2004; 56:189–197Google Scholar
39. Hajcak G, Moser JS, Yeung N, Simons RF: On the ERN and the significance of errors. Psychophysiology 2005; 42:151–160Google Scholar
40. Frank MJ, Woroch BS, Curran T: Error-related negativity predicts reinforcement learning and conflict biases. Neuron 2005; 47:495–501Google Scholar