Acute Effect of Methadone Maintenance Dose on Brain fMRI Response to Heroin-Related Cues
Abstract
Objective: Environmental drug-related cues have been implicated as a cause of illicit heroin use during methadone maintenance treatment of heroin dependence. The authors sought to identify the functional neuroanatomy of the brain response to visual heroin-related stimuli in methadone maintenance patients. Method: Event-related functional magnetic resonance imaging was used to compare brain responses to heroin-related stimuli and matched neutral stimuli in 25 patients in methadone maintenance treatment. Patients were studied before and after administration of their regular daily methadone dose. Results: The heightened responses to heroin-related stimuli in the insula, amygdala, and hippocampal complex, but not the orbitofrontal and ventral anterior cingulate cortices, were acutely reduced after administration of the daily methadone dose. Conclusions: The medial prefrontal cortex and the extended limbic system in methadone maintenance patients with a history of heroin dependence remains responsive to salient drug cues, which suggests a continued vulnerability to relapse. Vulnerability may be highest at the end of the 24-hour interdose interval.
Methadone maintenance is the standard agonist substitution therapy for heroin dependence. While methadone is efficient in reducing withdrawal and euphoria, illicit opiate use remains a significant problem during methadone maintenance treatment of heroin addiction. Recent advances in neuroscience suggest that drug-related cues, acting through learning and conditioning mechanisms, may play an important role in relapse to seeking and consuming heroin (1 , 2) . Clinical experience suggests that methadone decreases craving (3) , but this effect may be attenuated toward the end of the 24-hour interdose interval (4) . Methadone can also prime heroin cue response and craving (5 , 6) . Identifying the brain correlates of drug cue response in formerly heroin-dependent methadone maintenance patients at different phases of the dosing cycle could identify potential markers of vulnerability. The orbitofrontal and ventral anterior cingulate cortices, insula, amygdala, and hippocampal complex are the brain regions that mediate motivation and memory and respond to drug cues (2 , 7 , 8) . We used functional magnetic resonance imaging (fMRI) and visual heroin-related stimuli to test the hypotheses that 1) methadone maintenance patients would exhibit cue-induced brain activations in these a priori regions of interest and 2) these activations would be acutely reduced after the daily methadone dosing.
Method
Participants
The participants were 25 patients receiving a daily methadone dose (mean=115 mg, SD=61) who had been enrolled in a methadone maintenance program for a mean of 54 months (SD=33); participants’ last heroin use was 4 days to 54 months ago (mean=16 months, SD=13). Thirteen participants were men; 18 were Caucasian, five were African American, and one was Asian; their mean age was 36 years (SD=11). Heroin was the lifetime “drug of choice” for all participants. All were current tobacco users with a history of polysubstance drug use and no other current DSM axis I diagnoses or past diagnoses of schizophrenia spectrum or bipolar disorders. Abstinence from illicit drug use and compliance with methadone maintenance was confirmed by urine toxicology testing at enrollment and before each imaging session.
Study Design
The study protocol was approved by the University of Pennsylvania institutional review board. Participants were studied on two separate occasions 3 to 4 weeks apart: once before and once after they received their regular daily methadone dose at a methadone maintenance clinic. The order of the predose and postdose sessions was randomized and counterbalanced. The predose session was 90 minutes before the scheduled dosing, and the postdose session was 90 minutes after the dosing; these time points are near the peak and trough of methadone plasma levels with daily dosing (4) . Blood was drawn to determine plasma concentrations of methadone 30–60 minutes before each imaging session. Fifteen participants took part in both sessions, six only in a predose session, and four only in a postdose session.
Task
Each stimulus set had two stimulus classes: heroin-related and neutral. The 24 heroin-related stimuli were images of heroin injection, preparation, and paraphernalia (Cityvision, Inc., Boston). The 24 neutral stimuli were images of household objects and chores, graphically and contextually matched to the heroin-related stimuli. Each stimulus contained a 500-msec heroin-related or neutral stimulus, followed by a 1,500-msec crosshair. Stimuli were separated by a variable interval (0–18 sec) during which the crosshair was displayed. Stimuli were rear-projected to the center of the visual field through a mirror mounted on the scanner head coil, in a random, fast, event-related fashion (9) , using the E-Prime software package (Psychology Software Tools, Inc., Pittsburgh).
Subjective heroin craving was assessed on a 0–9 scale before and after each imaging session. Paired t tests were used to test changes in subjective craving score for significance. Stimuli sets were not repeated within subjects.
Image Acquisition
Imaging was performed on a 3-T Siemens Trio scanner (Siemens, Iselin, N.J.). A magnetization-prepared gradient echo image (MPRAGE), repetition time=1620 msec, echo time=3.87 msec, field of view=250 mm, matrix=192×256, effective voxel resolution of 1×1×1 mm) was acquired for anatomic overlays of functional data and spatial normalization. Blood-oxygen-level-dependent (BOLD) functional imaging was performed in the axial plane using a 33-slice, single-shot gradient-echo echo-planar sequence (repetition time=2000 msec, echo time=30 msec, field of view=192 mm, matrix=64×64, slice thickness=3 mm, gap=0 mm). This sequence delivered a nominal voxel resolution of 3×3×3 mm.
Data Analysis
Imaging data were subjected to quality control, preprocessing, and statistical analysis procedures using the fMRI Expert Analysis Tool, version 5.63, from the FMRIB Software Library (www. fmrib.ox.ac.uk/analysis/research). Individual-level preprocessing included slice-time correction using Fourier-space time-series phase shifting, motion correction to the median image using trilinear interpolation, high-pass temporal filtering, and spatial smoothing with a 9-mm full width at half maximum isotropic kernel and scaling using mean-based intensity normalization. The median functional image was coregistered to the corresponding high-resolution T 1 -weighted structural image and transformed into standard anatomical space (T 1 Montreal Neurological Institute template) using trilinear interpolation; transformation parameters were later applied to statistical images. The first-level (single-subject) statistical analysis was carried out using FMRIB"s improved general linear model with local autocorrelation correction. Regressors were modeled for conditions of interest (heroin-related stimuli and neutral stimuli) using a canonical hemodynamic response function with a temporal derivative. Six rigid body movement parameters from the motion correction were modeled as nuisance covariates. Second-level (group) analyses were carried out using FMRIB"s local analysis of mixed effects. Parameter estimates for each contrast of interest (i.e., heroin-related stimuli versus neutral stimuli) were entered into one-sample t tests for each predose and postdose session, where the mean estimate across subjects was tested against zero. A random-effects paired t test analysis was carried out between pre- and postdose sessions to compare the mean estimates from the two samples and test for statistical significance. In accordance with our hypothesis, the a priori regions of interest included the orbitofrontal cortex (comprising the rectus, olfactory, and medial frontal gyri and the medial and orbital aspects of the inferior, middle, and superior frontal gyri) (10) , the anterior cingulate cortex (limited to the ventral anterior cingulate cortex), the hippocampal complex (comprising the hippocampus and the parahippocampal gyrus), the amygdala, and the insula. The region-of-interest masks were imported from the Automated Anatomical Labeling atlas toolbox (11) . Activations within the a priori regions of interest were identified using an uncorrected threshold of p<0.05 and cluster extent of 20 voxels. Pharmacological and behavioral data were summarized as means with standard deviations and analyzed with two-tailed paired t tests.
Results
Pharmacological and Behavioral
The mean methadone plasma concentration was 388 ng/ml (SD=279) in the predose sessions and 545 ng/ml (SD=361) in the postdose sessions (t=2.2, df=18, p<0.02). The baseline subjective craving scores before the predose (mean=0.6, SD=1.3) and postdose (mean=0.1, SD=0.4) sessions were not significantly different. After the cues session, the subjective craving scores were significantly higher in both predose (mean=2.4, SD=2.9; t=3.2, df=24, p<0.002) and postdose (mean=1.2, SD=2.0; t=1.9, df=19, p<0.035) conditions. The subjective craving scores after the predose session were also significantly higher than the subjective craving scores after the postdose session (t=2.0, df=20, p<0.028).
Imaging
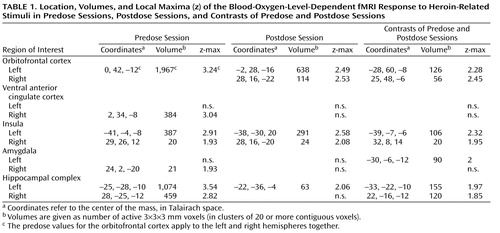
Twenty-one predose and 16 postdose datasets, 15 of which were paired, survived quality control procedures and were included in the analysis ( Figure 1 and Table 1 ). In the predose condition (df=20), brain response to heroin-related stimuli was significantly higher than to neutral stimuli (p<0.05, one-sample t test) in the left and right orbitofrontal cortex, anterior cingulate cortex, hippocampal complex, and insulae and in the right amygdala. No brain regions were more active in response to neutral stimuli than to heroin-related stimuli. In the postdose condition (df=15), the response to heroin-related stimuli was significantly higher than to neutral stimuli in the left and right orbitofrontal cortex, the insulae, and the left hippocampal complex, but not in the anterior cingulate cortex or amygdala. A direct comparison between predose and postdose responses to heroin-related stimuli (df=14) showed that the response to heroin-related stimuli in the predose condition was significantly larger than in the postdose condition in the left and right orbitofrontal cortex, insulae, and hippocampal complex, and the left amygdala.
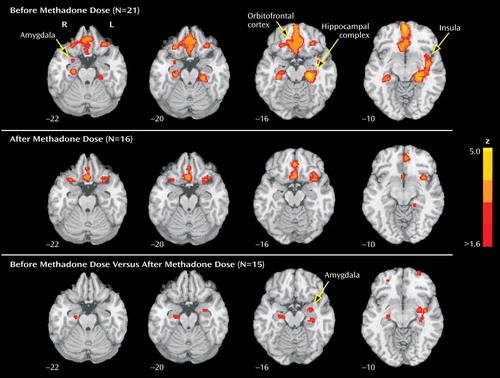
a Top row: Heightened response to heroin-related stimuli compared with neutral stimuli, before the daily methadone dose, in the left and right orbitofrontal cortex, ventral anterior cingulate cortex, and hippocampal complex, the left insula, and the right amygdala. Middle row: After the daily methadone dose, heightened response to heroin-related stimuli in the left and right orbitofrontal cortex and hippocampal complex and the left insula. Bottom row: Significant differences between the response to heroin-related stimuli before the methadone dose compared with after the methadone dose, in the left amygdala, the left and right hippocampal complex, and the left insula. Images are displayed over a Talairach-normalized template in radiological convention (left hemisphere to the viewer’s right) and thresholded at z ≥1.64, with spatial extent threshold of >20 voxels, uncorrected for multiple comparisons.
Discussion
This is the first report of brain fMRI response to relevant visual drug cues in the medial prefrontal and extended limbic systems in stable patients in methadone maintenance treatment. Our findings show that in this population, the learned response to drug cues may persist despite long-term agonist treatment. The brain regions we identified are similar to those identified in neuroimaging studies of cue reactivity in untreated heroin dependence (8) and of opiate administration in healthy volunteers (12) , which suggests an overlap between the systems mediating learned and pharmacological effects of opiates. The reduced response to heroin-related stimuli in the postdose state (relative to the predose state) in the amygdala, the hippocampal complex, and the insulae could be caused by the combined effects of reduced drug expectancy (13) and higher methadone plasma levels in the postdose session. A placebo-controlled study could help dissociate these factors.
Insula activation during heroin-related stimuli is in agreement with a recent study highlighting the importance of this region in addictive behavior (14) . Despite the insula’s role in interoception (15) , the reduction in both subjective craving and insula fMRI response to heroin-related stimuli after methadone administration renders interoceptive action a less likely predominant mechanism of methadone effects. The orbitofrontal cortex and ventral anterior cingulate cortex differ from the amygdala in their role in the processing of affective valence and intensity dimensions of cues (16) . Thus, a minimal orbitofrontal cortex effect, an absent anterior cingulate cortex effect, and a prominent amygdala effect of methadone administration suggests that heroin-related stimuli maintain their motivational value even when their perceived intensity is acutely reduced (10) . Alternatively, the combined effects of prolonged heroin addiction and methadone maintenance could alter the relative sensitivity of these regions to the acute effects of methadone (17) . Our results call for research to determine whether prefrontal deactivation after methadone administration predicts a lower risk of relapse to heroin after some period of abstinence. Research is also needed to determine whether the fMRI paradigm we employed could be used for treatment optimization of relapse-prone methadone maintenance patients who may benefit from a higher dose or frequency of methadone or from longer-acting opioid agents, such as buprenorphine.
In conclusion, patients in methadone maintenance treatment maintain an enhanced brain response to salient visual drug cues in the extended limbic system. This response is acutely blunted after the daily methadone dose for a period shorter than the 24-hour interdose interval. These findings suggest that stable methadone maintenance patients remain vulnerable to drug cues, and their vulnerability may be highest just before the expected dosing time. As a whole, our findings could help elucidate predictors of relapse to illicit opioid use in methadone maintenance patients.
1. O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B: Conditioned narcotic withdrawal in humans. Science 1977; 195:1000–1002Google Scholar
2. Hyman SE, Malenka RC, Nestler EJ: Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 2006; 29:565–598Google Scholar
3. Greenwald MK: Early impact of methadone induction for heroin dependence: differential effects of two dose sequences in a randomized controlled study. Exp Clin Psychopharmacol 2006; 14:52–67Google Scholar
4. Dyer KR, White JM: Patterns of symptom complaints in methadone maintenance patients. Addiction 1997; 92:1445–1455Google Scholar
5. Curran HV, Bolton J, Wanigaratne S, Smyth C: Additional methadone increases craving for heroin: a double-blind, placebo-controlled study of chronic opiate users receiving methadone substitution treatment. Addiction 1999; 94:665–674Google Scholar
6. Shaham Y, Rajabi H, Stewart J: Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci 1996; 16:1957–1963Google Scholar
7. Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL: Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 2001; 292:1175–1178Google Scholar
8. Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ: Functional connectivity analysis of the neural circuits of opiate craving: “more” rather than “different”? Neuroimage 2003; 20:1964–1970Google Scholar
9. Dale AM: Optimal experimental design for event-related fMRI. Hum Brain Mapp 1999; 8:109–114Google Scholar
10. Kringelbach ML, Rolls ET: The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72:341–372Google Scholar
11. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH: An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Google Scholar
12. Becerra L, Harter K, Gonzalez RG, Borsook D: Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 2006; 103:208–216Google Scholar
13. Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM: Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage 2006; 32:1782–1792Google Scholar
14. Naqvi NH, Rudrauf D, Damasio H, Bechara A: Damage to the insula disrupts addiction to cigarette smoking. Science 2007; 315:531–534Google Scholar
15. Gray MA, Critchley HD: Interoceptive basis to craving. Neuron 2007; 54:183–186Google Scholar
16. Lewis PA, Critchley HD, Rotshtein P, Dolan RJ: Neural correlates of processing valence and arousal in affective words. Cereb Cortex 2007; 17:742–748Google Scholar
17. Martin TJ, Kahn WR, Xiao R, Childers SR: Differential regional effects of methadone maintenance compared to heroin dependence on mu-opioid receptor desensitization in rat brain. Synapse 2007; 61:176–184Google Scholar