The Serotonin Transporter Genotype and Social Support and Moderation of Posttraumatic Stress Disorder and Depression in Hurricane-Exposed Adults
Abstract
Objective: Disasters are associated with increased risk of posttraumatic stress disorder (PTSD) and major depression, but no study, to the authors’ knowledge, has determined whether genotype interacts with disaster exposure and social support to moderate risk of these phenotypes. The authors tested the hypothesis that a polymorphism in the serotonin transporter gene (locus, SLC6A4 ; variant, serotonin 5-HTTLPR) moderates risk of posthurricane PTSD and major depression given high hurricane exposure and low social support. Method: The authors interviewed a household probability sample of adults 6–9 months after the 2004 hurricanes about hurricane exposure, social support, and posthurricane PTSD and major depression. DNA was collected from a subset of participants. Participants were 589 adults ages 18 and older from 38 Florida counties who provided valid DNA samples. Outcome measures were DSM-IV diagnoses of posthurricane PTSD and major depression derived from structured interviews. Results: The low-expression variant of the 5-HTTLPR polymorphism increased risk of posthurricane PTSD and major depression but only under the conditions of high hurricane exposure and low social support after adjustment for sex, ancestry (as determined by Bayesian clustering of genotypes), and age. Similar effects were found for major depression. High-risk individuals (high hurricane exposure, the low-expression 5-HTTLPR variant, low social support) were at 4.5 times the risk of developing PTSD and major depression of low-risk individuals. Conclusions: The low-expression variant of the 5-HTTLPR polymorphism modifies risk of postdisaster PTSD and major depression under conditions of high hurricane exposure and low social support, confirming and extending previous research.
Exposure to disaster is a prevalent environmental stressor that increases the risk of posttraumatic stress disorder (PTSD) and major depression, particularly when disasters cause widespread loss of life or property destruction (1 – 3) . Hurricanes have been found to produce these effects in numerous population-based samples (3 , 4) , and their potential for widespread devastation was dramatically demonstrated by Hurricanes Katrina and Rita in the Gulf Coast regions of the United States in 2005. However, not enough is known about factors that influence the psychological effects of disasters. The majority of exposed individuals experience transitory distress but do not develop PTSD or major depression (1 – 3) . The environmental stressor condition most likely to produce these phenotypes is high disaster exposure and low social support (3 , 5 , 6) . However, there is considerable variation in the risk of these phenotypes even among such high-risk individuals. This article examines the role of social support and serotonin transporter protein ( SLC6A4 ) genotype (serotonin 5-HTTLPR) in moderating PTSD and major depression phenotypes after hurricane exposure among adults.
Genetic influences explain a substantial proportion of the variance in both PTSD and major depression phenotypes and may moderate susceptibility to the adverse mental health effects of disaster (7 – 9) . Caspi and colleagues (9) found that a common variable number of tandem repeats polymorphism in the promoter region of the serotonin transporter gene ( SLC6A4 ), designated as 5-HTTLPR, moderated the relation between stressful life events and depression. Specifically, individuals possessing one or two copies of the short 5-HTTLPR allele, which is less transcriptionally efficient than the long allele (10) , had higher levels of depression and suicidality in the context of recent life stressors. Six studies have replicated the Caspi et al. study (11 – 16) ; two yielded partial replications (17 , 18) , and two other published studies failed to replicate this genotype-by-environment interaction (19 , 20) . Extending the findings of Caspi et al., Kaufman et al. (12) found that high social support was a protective factor for depression, even given history of traumatic stress exposure and the short 5-HTTLPR allele. Notably, although two studies have reported significant genotype-by-environment interaction relative to potentially traumatic events (9 , 12) , several others have not distinguished between traumatic (e.g., rape, physical assault, large-scale disasters) and nontraumatic (e.g., financial strain, relationship problems, unemployment) stressors. Furthermore, none of these studies reported on PTSD, which is highly comorbid with depression (21) , is more conceptually relevant to extreme stressor exposure, and has been associated with 5-HTTLPR allele frequencies in a Korean patient population (22) .
According to the steps outlined by Moffitt et al. (23) , postdisaster population-based research appears to be a particularly good candidate for a genotype-by-environment approach. First, disasters are a proximal environmental pathogen for the mental disorders in question. Second, there is marked variability in phenotypic response among people exposed to disaster. Third, SLC6A4 is a plausible candidate susceptibility gene that is consistently related to individual differences in physiological and psychological responses to stressors (9 , 11 – 16) . Fourth, studying the mental health consequences of disasters addresses a key challenge to genotype-by-environment research: gene-environment correlation, or the fact that some risk factors commonly considered to be “environmental” are actually influenced by genetic factors (24) . Disasters are extremely stressful events that, by definition, are outside of the individual victim’s control. Thus, the issue of gene-environment correlation is less of a concern methodologically in disaster mental health studies.
The present study examined genotype-by-environment interaction considering the 5-HTTLPR polymorphism, high hurricane exposure and low social support, and posthurricane PTSD and major depression, extending previous research in three ways. First, we studied an epidemiologic sample of Florida adults who were assessed for posthurricane PTSD and major depression with structured diagnostic interviews. Ours is the first study of which we are aware to examine the role of genotype by environment in mental disorder phenotypes after an acute potentially traumatic stressor. Second, prior reports of genotype by environment have not examined PTSD as an outcome, despite its conceptual relevance to extreme stressors. Third, we assessed an additional single nucleotide polymorphism that occurs on the background of 5-HTTLPR and allows “triallelic” genotyping with more accurate functional classification (25 , 26) . Most previous studies have used a diallelic 5-HTTLPR genotype classification in which alleles are classified as either short or long; however, the “G” variant of the A/G single nucleotide polymorphism on a long allele background (l G ) has low expression levels similar to those of the short allele.
We hypothesized that the low-expression variant (short or l G ) of the 5-HTTLPR polymorphism would increase the risk of PTSD and major depression only under the most extreme environmental stressor condition of high hurricane exposure and low social support.
Method
Data Collection and Sample
This article focuses on 589 participants in the Florida Hurricane Study who completed structured telephone interviews and who provided saliva samples through the mail that yielded genotype data for the 5-HTTLPR polymorphism. More details about the sampling procedure and methodology for the Florida Hurricane Study are provided elsewhere (1) . Telephone interviews were conducted with a probability sample of 1,543 English- and Spanish-speaking adults from telephone households in 38 counties in Florida within 6–9 months of the 2004 Florida hurricane season, between April 5 and June 12, 2005. Older adults (ages 60 and older) were oversampled to address research questions specific to this age group (1) .
Demographic characteristics of the 589 sample participants were as follows: 36.5% men, 63.5% women; 22.6% ≤59 years of age, 76.6% ≥60 years of age, 0.8% missing age data; 90% white, 3.9% black, 3.9% Hispanic, 1.7% other, 0.5% missing self-report race/ethnicity data.
Verbal consent was obtained from the participants; the participants were sent a letter documenting the elements of verbal consent and providing them with contact information for the principal investigator. Participants who completed the diagnostic interview and returned saliva samples were paid $20. The institutional review board of the Medical University of South Carolina approved all procedures.
Assessment Procedure
Part 1. Diagnostic interviews
Sample selection and telephone interviewing was performed by Schulman, Ronca, Bucuvalas, Inc., a national survey research firm with considerable experience conducting interviews by telephone about exposure to traumatic stressors such as disasters (2 , 4 , 27) . Random-digit-dial procedures were used to locate households within the sampling frame. Respondents were randomly selected by using the most recent birthday method when multiple eligible adults were present within a household. A highly structured interview using computer-assisted telephone interview procedures was constructed and utilized. The computer-assisted telephone interview format uses a computer program that projects instructions and questions on a computer screen that are read verbatim by interviewers to participants over the telephone. Interviewers then recorded participants’ responses on a computer with closed-ended response options. Supervisors randomly monitored interviews in progress, thereby providing considerable quality control over data collection. Interviews were conducted in either English or Spanish, depending on the participant’s preference, and averaged 26.5 minutes in length.
Hurricane exposure was assessed with five important indicators identified in previous research on Hurricanes Hugo and Andrew on the basis of their relation to posthurricane mental health functioning (4 , 27) : 1) present during hurricane-force winds or major flooding; 2) lack of adequate access to food, water, electricity, telephone, or clothing for a week or longer; 3) two or more hurricane-related losses of furniture, sentimental possessions, automobile, pets, crops, trees, or garden; 4) displacement from home for 1 week or longer; and 5) out-of-pocket losses of $1,000 or more that were not reimbursed by insurance or other sources. Based on previous research (4 , 27) , high hurricane exposure was defined as having experienced two or more of these five indicators; 45.8% of the participants had high hurricane exposure.
Social support during the 6 months before the hurricanes was assessed with a modified five-item version of the Medical Outcomes Study module (28) . Our prior work using the same measure showed that social support moderated the risk of PTSD and major depression after the 2001 terrorist attacks on the World Trade Center (2) . We measured three aspects of social support. The participants were asked about emotional (e.g., “someone available to love you and make you feel wanted”), instrumental (e.g., “someone available to help you if you were confined to bed”), and appraisal (e.g., “someone available to give you good advice in a crisis”) social support and responded to items on a four-point scale from “none of the time” to “all of the time” (sample range=0–20; mean=15.9, SD=4.8). Low social support was operationalized as a score of 15 or less (36.4% of the sample) based on the cutoff score that was derived in our study of the September 11th terrorist attacks (2) . This scale had good reliability (alpha=0.86).
PTSD since the hurricanes was assessed with the National Women’s Study PTSD module, a widely used measure in population-based epidemiological research originally modified from the Diagnostic Interview Schedule. Research on the National Women’s Study PTSD module has provided support for concurrent validity and several forms of reliability (e.g., temporal stability, internal consistency, diagnostic reliability [29] ). The National Women’s Study PTSD module was validated in the DSM-IV PTSD field trial against the Structured Clinical Interview for DSM (SCID), in which the interrater kappa coefficient was 0.85 for the diagnosis of PTSD and comparisons between scores on the National Women’s Study PTSD module and the SCID yielded a kappa coefficient of 0.71 for current and 0.77 for lifetime PTSD (30) . Research also has found high correspondence between telephone and in-person administration of the PTSD module as well as the depression module described below (31) . We operationalized PTSD diagnosis based on DSM-IV symptom requirements, including functional impairment.
Major depression since the hurricanes was assessed with modified questions from the SCID-IV. Following DSM-IV criteria, the respondents met criteria for major depression if they had five or more depressive symptoms for at least 2 weeks, including endorsement of one or both of the symptoms relating to depressed mood or loss of interest or pleasure. Past-year major depression identified by this measure is associated with lower reported work quality and mental health treatment-seeking after the addition of control for demographic characteristics, assault history, and PTSD diagnoses (32) .
Part 2. Collection of DNA samples
Saliva samples were obtained for DNA extraction and analyses. At the end of the interviews, the participants were told that a second part of the study involved collecting genetic material to determine whether genetic characteristics influenced reactions to stressful events such as hurricanes. They were informed that the genetic information would be kept strictly confidential, that their names would never be attached to the genetic test results, and that participation involved providing a saliva sample. The participants were mailed a kit that included instructions, a 50-ml test tube, a small bottle of mouthwash, and a return mailer. The participants were instructed to swish the mouthwash for 20–30 seconds, expectorate the contents into the test tube, place the test tube into a plastic foam container, and mail the container with the test tube to the Yale University laboratory. The return kit had an ID number but no personal identifiers in order to protect the participant’s confidentiality. The Yale laboratory was blind to the participants’ interview responses.
Saliva samples were provided by 651 participants (42.2% response rate). Of these, valid ancestry data were available for 623 cases (95.7%), and valid genotype data were available for 589 cases (90.6%). The likelihood of submitting a saliva sample did not differ in relation to sex, level of hurricane exposure, level of social support, or PTSD and major depression status. Additional details on response rate and correlates of participation are summarized elsewhere (33) .
Genotyping
DNA was extracted from saliva using PUREGENE (Gentra Systems, Minneapolis) kits. We examined the functional variable number of tandem repeats polymorphism in the 5′ flanking regulatory (promoter) region of the gene ( SLC6A4 ) coding for the serotonin transporter protein. This polymorphism (5-HTTLPR) has two common alleles that have been characterized according to their length as long (16 repeats) and short (14 repeats). Genotyping was performed with polymerase chain reaction followed by size fractionation as described elsewhere (34) with prior Msp l restriction endonuclease digestion for triallelic classification (26) , which allowed classification of long alleles into l A and l G (l G has lower reuptake efficiency, similar to the short allele) variants. Accuracy of short/liter genotyping was confirmed through reanalysis of 100% of the specimens. The uncommon l G alleles were classified as short for analysis, as was done in previous research (26) . In addition, 36 markers were genotyped to provide ancestry information (35 , 36) . We added one additional highly informative single nucleotide polymorphism marker, SLC24A5(37) , to the panel described previously.
Ancestry Proportion Scores
Ancestry proportion scores were generated to avoid spurious associations that can result from variation in allele frequency and prevalence of trait by population; such differences are known to occur for the SLC6A4 locus (34) . The participants’ ancestries were estimated by Bayesian cluster analysis with the marker panel described above on the procedures and STRUCTURE software developed by Pritchard and colleagues (38 , 39) . For the STRUCTURE analysis, we specified the “admixture” and “allele frequencies correlated” models and used 100,000 burn-in and 100,000 Markov chain Monte Carlo iterations.
Statistical Analyses
Logistic regression was used to predict diagnostic outcomes. Sex, age, and ancestral proportion scores were entered as covariates in all models. The main effects of level of hurricane exposure, social support, and 5-HTTLPR genotype and all possible higher-order interactions with genotype were tested. For these analyses, triallelic genotypes were reclassified as a diallelic model according to their level of gene expression: l A /liter A as long/liter, l A /liter G and l A /short as long/short and l G /liter G , l G /short, and short/short as short/short (23 , 25) .
Results
PTSD and Major Depression in Hurricane-Exposed Adults
Among adults with genotype data for 5-HTTLPR (N=589), the prevalence of posthurricane PTSD was 3.2% (N=19) and the prevalence of posthurricane major depression was 5.6% (N=33). Low social support was associated with both PTSD and major depression (PTSD: χ 2 =7.82, df=1, p=0.005; major depression: χ 2 =15.11, df=1, p<0.001). High hurricane exposure was associated with PTSD (χ 2 =6.37, df=1, p<0.02) but not major depression (χ 2 =0.12, df=1, p=0.73).
Genotype and Ancestry
Triallelic genotype frequencies were as follows: long/liter=26.1% (N=154), long/short=53.5% (N=315), short/short=20.4% (N=120). The frequency of the long/liter genotype was higher among AA adults (long/liter=34.8%, short/liter=47.8%, short/short=17.4%) than among EA adults (long/liter=25.1%, short/liter=54.9%, short/short=20.0%). However, this difference was not statistically significant (χ 2 =1.09, df=2, p=0.58).
Genotype-Environment Correlation
There was no association between the 5-HTTLPR genotype and high hurricane exposure (χ 2 =0.28, df=2, p=0.78) or low social support (χ 2 =2.00, df=2, p=0.37).
Ancestral Proportion Scores, PTSD, and Major Depression
There was no difference between those with (mean=0.12, SD=0.27) and without PTSD (mean=0.06, SD=0.20; t=–0.98, df=18.63, p=0.34) and those with (mean=0.05, SD=0.14) and without depression (mean=0.06, SD=0.20; t=0.45, df=40.08, p=0.66) in ancestral proportion scores.
PTSD and Major Depression Diagnoses
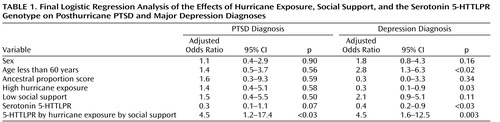
Table 1 reports the results of the final logistic regression model for posthurricane PTSD and major depression diagnoses. The two-way interaction terms for genotype by hurricane exposure and for genotype by social support were not significant for either PTSD or major depression. The three-way-interaction term for 5-HTTLPR genotype, social support, and hurricane exposure was significant in the adjusted logistic regression model for posthurricane PTSD diagnosis (Wald χ 2 =4.76, df=1, p<0.03; odds ratio=4.5, 95% confidence interval [CI]=1.2–17.4) and posthurricane major depression diagnosis (Wald χ 2 =8.55, df=1, p=0.003; odds ratio=4.5, 95% CI=1.6–12.6).
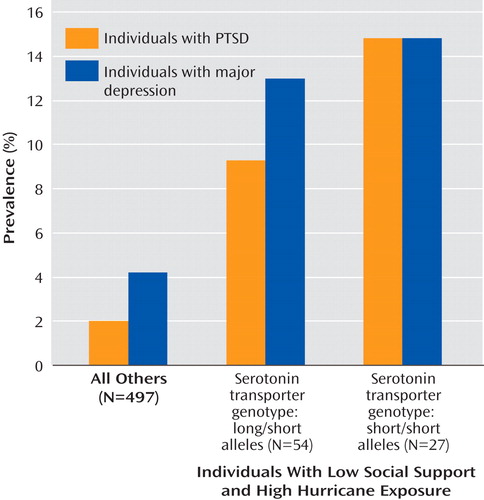
For post hoc analyses, the participants were divided into three groups: 1) high risk (N=27) were those with the short/short genotype, low social support, and high hurricane exposure; 2) medium risk (N=54) were those with the long/short genotype, low social support, and high hurricane exposure; and (3) low risk (N=498) were all others. Figure 1 shows the prevalences of posthurricane PTSD and major depression diagnoses for the three risk groups. For PTSD diagnosis, the prevalences were 14.8%, 9.3%, and 2.0%, respectively (χ 2 =19.94, df=2, p<0.001). For the major depression diagnosis, the prevalences were 14.8%, 13.0%, and 4.2%, respectively (χ 2 =11.82, df=2, p=0.003). Specifically, adults in the high-risk group were significantly more likely to have posthurricane PTSD than those in the low-risk group (odds ratio=20.1, 95% CI=1.4–296.6, p=0.03). The high-risk group showed a tendency toward being more likely to develop PTSD than the medium-risk group (odds ratio=5.0, 95% CI=0.8–30.8, p=0.09). The medium-risk group was not significantly more likely to have PTSD than the low-risk group (odds ratio=4.1, 95% CI=0.6–26.3, p=0.14). Adults in the high risk group (odds ratio=21.5, 95% CI=2.6–179.6, p=0.005) and medium-risk group (odds ratio=7.6, 95% CI=1.5–38.9) were significantly more likely to have major depression than those in the low-risk group. The high-risk group was not significantly more likely to have major depression than the medium-risk group (odds ratio=2.8, 95% CI=0.6–13.3, p=0.19).
Secondary analyses were conducted with European Americans only (N=530). The results paralleled those for the entire cohort. Specifically, the three-way interaction term for 5-HTTLPR genotype, social support, and hurricane exposure remained significant for major depression diagnosis (odds ratio=3.7, 95% CI=1.2–1.2, p=0.02) and showed a strong tendency for PTSD diagnosis (odds ratio=4.1, 95% CI=0.9–19.5, p=0.07).
Discussion
Our findings supported the hypothesis that the serotonin transporter gene moderates risk of the phenotypes of PTSD and major depression under the high environmental stressor conditions of high hurricane exposure and low social support. Specifically, the 5-HTTLPR genotype was unrelated to hurricane exposure, social support, or either of the two phenotypes at the main effect level, but the low-expression variant of 5-HTTLPR increased the risk of each phenotype under the high stressor condition. This genotype-by-environment finding for the major depression phenotype was found for the combined sample of male and female participants, and it replicates previous findings (9 , 11 – 16) , extending the type of environmental stressor to disasters. Our findings also confirmed the important role of social support in either buffering or aggravating the effects of other major environmental stressors.
We identified five potential limitations of the data presented in this article. First, individuals who relocated outside of the 38 hurricane-affected counties or lacked telephone service 6–9 months after the hurricanes were excluded from the study. However, telephone service was restored to the vast majority of Florida households several months before the study, and these hurricanes did not result in the type of massive evacuation outside the affected area that occurred following Hurricane Katrina. Second, the return rates of saliva samples were lower than optimal, but attrition was not significantly related to major study variables of hurricane exposure, social support, PTSD and major depression phenotypes, or sex. Third, diagnostic and PTSD and major depression data were obtained from highly structured interviews administered by lay interviewers over the telephone rather than from in-person clinical interviews. However, these interview measures of the PTSD and major depression phenotypes have been used in epidemiological telephone surveys after several major disasters (2 , 29) , and there is substantial evidence supporting the reliability and validity of both measures (29 , 30 , 32) . Furthermore, prior research comparing telephone and in-person assessment with these measures found no significant differences between the two methods (31) . Fourth, measurement of social support could have been affected by retrospective recall biases. Fifth, the study was cross-sectional, and therefore, we are unable to determine with certainty whether posthurricane major depression and PTSD represent new onsets of the disorders or recurrence among individuals who might have had prior episodes. From an epidemiologic perspective, predisaster psychopathology could be a confounder in studies concerned with relations between risk factors and postdisaster outcomes. However, in the context of an analysis that centers around genetic determinants in which we show a relation among postdisaster psychopathology, social support, and genetic influences, we suggest that it is unlikely that predisaster psychopathology could substantially influence the results documented here.
Although the 2004 Florida hurricanes were destructive and had a major impact on many Floridians, other recent natural disasters such as Hurricanes Katrina and Rita and the 2004 tsunami in southeast Asia produced substantially greater loss of life, property damage, and community disruption. Thus, these disasters produced high disaster exposure for a greater proportion of the population than was experienced by our sample. Also, the widespread and persistent dispersion of individuals following these recent disasters could be expected to have disrupted existing family and community social support networks. Therefore, these recent disasters likely produced much higher “doses” than our study of the twin environmental stressors of high exposure and low social support. If high exposure and low social support increase the risk of postdisaster PTSD and major depression, we would expect these phenotypes to be more prevalent among Katrina, Rita, and 2004 tsunami survivors than in the current study. How would we expect genotype by environment to operate for the PTSD and major depression phenotypes in the context of such high environment stressor disasters? One possibility is that the environment would carry greater weight in postdisaster phenotypes, and genotype-by-environment disaster effects might be reduced. However, another possibility is that genotype-by-disaster effects are diluted in the absence of a strong environment, suggesting that an even stronger genotype-by-environment relation might be found in more destructive, disruptive disasters than in the type of disaster we studied. Additional research is needed to clarify this question.
To the best of our knowledge, this is the first study to collect genetic samples by mail in the context of a large epidemiological telephone survey. Other studies have collected saliva samples for DNA analyses by mail (25 , 40) , but this has been in the context of studies in which investigators had ongoing research relationships with participants. Additional research is needed to replicate our methodology and findings, but our results suggest that it is both feasible and useful to add genetic components to studies with telephone interviews to collect data about exposure to disasters and other traumatic events as well as the phenotypes of PTSD and major depression. Such studies can add to our knowledge of how genetic and environmental risk and protective factors interact to foster resilience or increase psychopathology after disasters. Our findings also confirm the importance of assessing the degree of hurricane exposure and social support among disaster survivors.
1. Acierno R, Ruggiero KJ, Kilpatrick DG, Resnick HS, Galea S: Risk and protective factors for psychopathology among older versus younger adults following the 2004 Florida hurricanes. Am J Geriatr Psychiatry 2006; 14:1051–1059Google Scholar
2. Galea S, Ahern J, Resnick H, Kilpatrick D, Bucuvalas M, Gold J, Vlahov D: Psychological sequelae of the Sept 11 terrorist attacks in New York City. N Engl J Med 2002; 346:982–987Google Scholar
3. Norris FH, Elrod CL: Psychosocial consequences of disaster: a review of past research, in Methods for Disaster Mental Health Research. Edited by Norris FH, Galea S, Friedman MJ, Watson PJ. New York, Guilford, 2006, pp 20–42Google Scholar
4. Freedy J, Saladin M, Kilpatrick D, Resnick H, Saunders B: Understanding acute psychological distress following natural disaster. J Trauma Stress 1994; 7:257–273Google Scholar
5. Brewin CR, Andrews B, Valentine JD: Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000; 68:748–766Google Scholar
6. Galea S, Nandi A, Vlahov D: The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev 2005; 27:78–91Google Scholar
7. Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ: Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry 2002; 159:1675–1681Google Scholar
8. Sullivan PF, Neale MC, Kendler KS: Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157:1552–1562Google Scholar
9. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389Google Scholar
10. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Banjamin J, Muller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Google Scholar
11. Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J: Stress-related negative affectivity and genetically altered serotonin transporter function. Arch Gen Psychiatry 2006; 63:989–996Google Scholar
12. Kaufman J, Yang B, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J: Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci 2004; 101:17316–17321Google Scholar
13. Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J: Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry 2006; 59:673–680Google Scholar
14. Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B: The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry 2005; 62:529–535Google Scholar
15. Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, Blair IP, Parker G, Schofield PR: Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry 2006; 188:210–215Google Scholar
16. Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ: Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry 2006; 163:1588–1593Google Scholar
17. Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW: Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry 2004; 9:908–915Google Scholar
18. Grabe HJ, Lange M, Wolff B, Völzke H, Lucht M, Freyberger HJ, John U, Cascorbi I: Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry 2005; 10:220–224Google Scholar
19. Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG: The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med 2005; 35:101–111Google Scholar
20. Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J: Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry 2006; 59:224–229Google Scholar
21. Kessler RC, Chiu WT, Demler O, Walters EE: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey replication. Arch Gen Psychiatry 2005; 62:617–627Google Scholar
22. Lee H, Lee M, Kang R, Kim H, Kim S, Kee B, Kim YH, Kim Y, Kim J, Yeon BK, Ohio KS, Ohio B, Yoon J, Lee C, Jung HY, Chee I, Paik IH: Influence of the serotonin transporter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety 2005; 21:135–139Google Scholar
23. Moffitt TE, Caspi A, Rutter M: Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry 2005; 62:473–481Google Scholar
24. Kendler KS, Baker JH: Genetic influences on measures of the environment: a systematic review. Psychol Med 2006; 36:1–12Google Scholar
25. Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA: An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res 2005; 29:8–16Google Scholar
26. Stein MB, Seedat S, Gelernter J: Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology (Berl) 2006; 187:68–72Google Scholar
27. Freedy J, Resnick H, Kilpatrick D: Conceptual framework for evaluating disaster impact: implications for clinical intervention, in Clinical Response to Trauma in the Community. Edited by Austin LS. Washington, DC, American Psychiatric Press, 1993, pp 3–23Google Scholar
28. Sherbourne CD, Stewart AL: The MOS Social Support Survey. Soc Sci Med 1991; 32:705–714Google Scholar
29. Ruggiero KJ, Rheingold AA, Resnick HS, Kilpatrick DG, Galea S: Comparison of two widely used PTSD-screening instruments: implications for public mental health planning. J Trauma Stress 2006; 19:699–707Google Scholar
30. Kilpatrick DG, Resnick HS, Freedy JR, Pelcovitz D, Resick PA, Roth S, van der Kolk B: The posttraumatic stress disorder field trial: evaluation of the PTSD construct—criteria A through E, in DSM-IV Sourcebook. Edited by Widiger T, Pincus HA, First MB, Ross R, Davis W. Washington, DC, American Psychiatric Press, 1998, pp 803–844Google Scholar
31. Acierno R, Resnick H, Kilpatrick D, Stark-Riemer W: Assessing elder victimization: demonstration of a methodology. Soc Psychiatry Psychiatr Epidemiol 2003; 38:644–653Google Scholar
32. Boscarino JA, Galea S, Adams RE, Ahern J, Resnick H, Vlahov D: Mental health service and medication use in New York City after the Sept 11, 2001, terrorist attack. Psychiatr Serv 2004; 55:274–283Google Scholar
33. Galea S, Acierno R, Ruggiero KJ, Resnick H, Tracy M, Kilpatrick DG: Social context and the psychobiology of post-traumatic stress. Ann N Y Acad Sci 2006; 1071:231–241Google Scholar
34. Gelernter J, Kranzler H, Cubells JF: Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet 1997; 101:243–246Google Scholar
35. Stein MB, Schork NJ, Gelernter J: A polymorphism of the b 1 -adrenergic receptor is associated with low extraversion. Biol Psychiatry 2004; 56:217–224 Google Scholar
36. Yang N, Li H, Criswell LA, Gregersen PK, Alarcon-Riquelme ME, Kittles R, Shigeta R, Silva G, Patel PI, Belmont JW, Selden MF: Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum Gene 2005; 118:382–392Google Scholar
37. Lamason RL, Mohideen MA, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, Sinha S, Moore JL, Jagadeeswaran P, Zhao W, Ning G, Makalowska I, McKeigue PM, O’Donnell D, Kittles R, Parra EJ, Mangini NJ, Grunwald DJ, Shriver MD, Canfield VA, Cheng KC: SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005; 310:1782–1786Google Scholar
38. Falush D, Stephens M, Pritchard JK: Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Am J Hum Genet 2003; 164:1567–1587Google Scholar
39. Pritchard JK, Rosenberg NA: Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet 1999; 65:220–228Google Scholar
40. Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R: DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet 1997; 27:251–257Google Scholar