A Meta-Analytic Review of Psychosocial Interventions for Substance Use Disorders
Abstract
Objective: Despite significant advances in psychosocial treatments for substance use disorders, the relative success of these approaches has not been well documented. In this meta-analysis, the authors provide effect sizes for various types of psychosocial treatments, as well as abstinence and treatment-retention rates for cannabis, cocaine, opiate, and polysubstance abuse and dependence treatment trials. Method: With a comprehensive series of literature searches, the authors identified a total of 34 well-controlled treatment conditions—five for cannabis, nine for cocaine, seven for opiate, and 13 for polysubstance users—representing the treatment of 2,340 patients. Psychosocial treatments evaluated included contingency management, relapse prevention, general cognitive behavior therapy, and treatments combining cognitive behavior therapy and contingency management. Results: Overall, controlled trial data suggest that psychosocial treatments provide benefits reflecting a moderate effect size according to Cohen’s standards. These interventions were most efficacious for cannabis use and least efficacious for polysubstance use. The strongest effect was found for contingency management interventions. Approximately one-third of participants across all psychosocial treatments dropped out before treatment completion compared to 44.6% for the control conditions. Conclusions: Effect sizes for psychosocial treatments for illicit drugs ranged from the low-moderate to high-moderate range, depending on the substance disorder and treatment under study. Given the long-term social, emotional, and cognitive impairments associated with substance use disorders, these effect sizes are noteworthy and comparable to those for other efficacious treatments in psychiatry.
In the last 30 years, there has been significant progress in the development and validation of psychosocial treatments for substance abuse and dependence, with a predominant focus on the validation of cognitive behavioral treatments (1) . Prominent among these approaches have been contingency management interventions and interventions emphasizing functional analyses and strategies for changing higher-risk situations for drug use in either relapse prevention or other cognitive behavioral formats.
Based on principles of operant conditioning, contingency management interventions offer incentives or rewards to encourage specific behavioral goals. In the case of treatments for substance dependence, monetary and nonmonetary rewards typically have been made contingent on negative toxicology screens, indicating abstinence from drug use. The approaches have shown consistent success, with drug use disorders ranging from opiate and cocaine dependence to nicotine dependence (2 – 7) . An alternative approach, relapse prevention, focuses on identifying and intervening with higher-risk situations or events for drug use by helping individuals either avoid or manage these situations by rehearsing alternative (nondrug) responses. Similar to contingency management, relapse prevention approaches have shown benefit in a wide range of trials for illicit drug and alcohol use disorders (8 , 9) . A similar focus on completing a functional analysis of cues for use and rehearsing behavioral and cognitive nondrug responses characterizes a variety of other cognitive behavioral approaches (10) .
Although a number of smaller meta-analyses have been conducted for pharmacologic interventions for substance use disorders (11 – 14) , few intervention-specific (15) and, to our knowledge, no comprehensive meta-analyses have been conducted for psychosocial treatments for illicit substance use disorders. Indeed, little is known about how the broad range of psychosocial treatments compare to one another across different outcome variables (e.g., abstinence, dropout rate, etc.), and even less is known about the overall strength of psychosocial treatments across different drugs of abuse (1) .
The current study uses a meta-analysis to systematically investigate the efficacy of psychosocial treatments for substance use disorders and provides indices of the strength of findings for specific interventions and specific drug use disorders. Because meta-analyses have been conducted for psychosocial treatments of alcohol and nicotine use disorders (16 – 20) , we focused only on illicit substance use disorders. As existing meta-analyses have focused on motivational interviewing interventions (21 – 24) , which tend to be employed in single-session formats, we chose to target more comprehensive interventions. Additionally, because 12-step interventions are distinct from traditional psychotherapies, these interventions were used as control conditions only. We provide a comprehensive account of the strength of cognitive behavioral treatments for cocaine, opiates, cannabis, and polysubstance abuse and dependence. In addition, we provide an analysis of various outcome variables (including abstinence, treatment retention, and treatment dropout). Finally, we provide evidence for potential moderating factors in treatment outcome across studies.
Method
Study Selection
We selected randomized, controlled clinical trials for inclusion in this meta-analysis by performing a comprehensive search strategy. First, we conducted a computer-based PsycINFO search of available articles published between 1840 and March of 2005 using the following key terms to conduct title searches (asterisks denote that any characters/letters can follow the last character in the terms): cocaine, crack, opi*, heroin, amphetamine*, methamphetamine*, MDMA, ecstasy, methylenedioxymethamphetamine, cannabis, marijuana, psychedelic, mushroom, glue, inhalant, poly*, substance* abuse, substance* use, addict*, and dependen* singularly and in combination with the following secondary descriptors: treatment*, trial*, outcome*, therapy, random*, intervention*, medication*, psychopharm*, pharm*, buprenorphine, behavior*, counseling, cognitive, meta analys*, contingency, and voucher*. Second, we performed a computer-based MEDLINE search of articles available between 1966 and March 2005, combined with a Cochrane Central Register of Controlled Trials search for the first quarter of 2005 with the following search terms: substance abuse and randomized, or drug abuse and randomized, or drug dependence and randomized, or cocaine and randomized, or heroin and randomized, or opioid and randomized, or opiates and randomized, or methamphetamine and randomized, or amphetamine and randomized, or marijuana and randomized, or polydrug and randomized. These terms were searched as key, title, abstract, name of substance, and MeSH subject heading terms. This search was combined with a MeSH subject heading search in the same database with the following terms: amphetamine-related disorders, cocaine-related disorders, marijuana abuse, opioid-related disorders, phencyclidine abuse, substance abuse, and intravenous. We limited both the PsycINFO and the MEDLINE searches to studies conducted with human participants and published in the English language. All titles or abstracts for the citations produced were screened, and articles were collected for any citation that appeared to meet inclusion criteria.
Studies meeting all of the following inclusion criteria were included in the meta-analysis:
Investigations of the efficacy of any individual psychosocial treatments for substance abuse/dependence, with the exception of alcohol abuse/dependence and nicotine abuse/dependence
Randomized, controlled trials including a comparison group that could consist of inactive (e.g., waitlisted) or active (e.g., treatment as usual) treatments
Studies including adult (18 years and older) participants only
Studies including one or more of our posttreatment outcome measures (described below) to allow for comparable outcome data across studies
Investigations of the efficacy of nonintensive outpatient treatments, which we defined as consisting of no more than three 2-hour treatment sessions per week
Studies with medication as a backdrop condition were included only if the medication dosage did not vary between the active treatment and control conditions. Controlled trials comparing the efficacy of various treatment intensities (e.g., once per week versus twice per week individual therapy of the same therapy type) were excluded if a clear control group (e.g., one receiving inactive treatment or treatment as usual) was unavailable (seven studies). When multiple control conditions were included in these trials, we employed the “most intensive” treatment condition as the active treatment to compare to the control condition, and any other conditions of varying treatment intensity were excluded. Studies containing follow-up data beyond 1 month posttreatment but not presenting data at posttreatment were excluded (four studies). Likewise, we excluded studies employing control conditions known to be efficacious for substance abuse treatment (e.g., cognitive behavior therapy, five studies) and studies using trials of wrap-around service treatment (one study), therapeutic workplaces/work therapies (one study), hypnotherapy (one study), telecommunication networking (one study), brief motivational enhancement (two studies), supportive/expressive therapy (one study), and acupuncture (seven studies). In total, 30 studies were excluded from consideration.
Procedure
When available, data on a number of descriptive variables were collected for each study, including the following: sex (data from 91.2% of the studies available), ethnicity (76.5% available), employment status (64.7% available), marital status (61.8% available), average length of substance use (55.9% available), comorbid alcohol abuse/dependence diagnoses (20.6% available), numbers of weeks treatment was administered (97.1% available), number of treatment sessions per week (58.8%), number of participants entered per condition (100% available), treatment retention rates (47.1% available), and numerous outcome variables, as described below.
Treatments were categorized into the following treatment condition “types”: contingency management/vouchers (14 studies), general cognitive behavior therapy interventions (13 studies), relapse prevention (five studies), and cognitive behavior therapy plus contingency management combined (two studies). Descriptions of treatment conditions were used to categorize each condition into its best-fit treatment type.
Given the lack of “gold standard” outcome measures in the substance abuse treatment literature, we reviewed all controlled trials meeting our inclusion criteria, as well as all available substance abuse treatment reviews and meta-analyses, to develop a set of variables that would sufficiently allow for outcome comparison across studies. These variables included a combination of self-report data (data from 58.8% of the studies available) and measures employing toxicology screening procedures (76.5% available). Self-report outcome variables were the following:
Mean maximum number of days or weeks abstinent throughout treatment
Mean percent of days abstinent throughout treatment
Percent of sample abstinent for 3 or more weeks throughout treatment
Percent of sample demonstrating posttreatment and/or clinically significant abstinence
Posttreatment scores on the drug scale of the Addiction Severity Index (25)
Toxicology screen variables were 1) mean number of negative screens throughout treatment, 2) mean percent of negative screens throughout treatment, and 3) percent of sample demonstrating clinically significant abstinence.
With respect to the percent of the sample demonstrating posttreatment and/or clinically significant abstinence, for both self-report and toxicology screen data, studies varied widely in defining this variable. These definitions of posttreatment and/or clinically significant abstinence varied from 4 to 24 weeks of abstinence (or abstinence for the entire length of treatment), as measured by means of the participants’ self-report or toxicology screen results. The 3-week abstinence cutoff appeared to be the most widely employed measure of clinically significant change, as these data were presented by 11 (30%) of the studies included in this meta-analysis. The Addiction Severity Index (used by 16 studies) (25) and the Time Line Follow-Back interview method (used by four studies) (26) were the most widely used interviews for collecting self-report data across studies.
These eight outcome variables were used for the purposes of calculating treatment versus control condition effect size estimates. Effect size was calculated using Cohen’s d (27) . Owing to inconsistency in the outcome variables employed by each study, when studies presented data on two or more outcome variables, we aggregated (averaged) across these variables to yield an aggregate mean effect size for each study. In addition, to determine the differential effect of self-report versus toxicology screen outcome data, we aggregated outcome variables within each study to yield 1) an aggregate mean overall effect size, 2) an aggregate mean self-report effect size, and 3) an aggregate mean toxicology screen effect size. Finally, as a general measure of abstinence rates, we provide the percent of the sample demonstrating posttreatment and/or clinically significant abstinence variable (these data were reported by 16 of the 37 studies), or, if this variable was not reported for a particular study, we calculated this data from the information provided (data calculated for six studies).
Results
Study and Sample Characteristics
Effect sizes were calculated for a total of 34 studies. Across all studies, 2,340 entered the treatment and identified control conditions. The mean age of the participants across all studies was 34.9 years (range of means=20.6 to 43.0, SD=4.5). On average, samples were 62.2% male and 61.0% Caucasian. The majority of the participants were single/unmarried (67.7%), and less than half were employed part-time or full-time (42.5%). The participants reported an average of 10.1 years of substance use (SD=5.1). Of the seven studies reporting specific diagnostic criteria, approximately 50.7% of the participants met criteria for comorbid alcohol abuse or dependence, although alcohol was not the targeted substance in the treatment study.
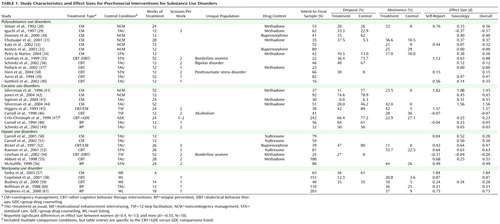
Table 1 presents a detailed overview of these treatments, including 14 identified as contingency management, two as cognitive behavior therapy/contingency management combination conditions, 13 as general cognitive behavior therapy interventions, and five as relapse prevention. Of these, 13 of the treatments were for polysubstance use, nine for cocaine use, seven for opiate use, and five for cannabis use disorders. Treatment types were not significantly associated with (confounded with) the targeted drug use disorders according to chi-square analyses (contingency management versus all other treatments).
Overall, 43.6% of the studies included samples in which the participants received medication maintenance (e.g., methadone maintenance) in conjunction with both the experimental treatment and control conditions. The mean length of treatment across all conditions was 21 weeks (range=4–52 weeks, SD=14), and the average number of sessions per week averaged 1.8 sessions (range=1–3, SD=0.8). The mean intent-to-treat sample size per treatment condition was 38.23 (SD=32.00), ranging from five to 135 participants across all conditions.
Treatment Retention
Approximately one-third of the participants across all conditions dropped out before treatment completion (35.4%). Mean dropout among control conditions was 44.6%. Across all substance use groups, cocaine and opiate patients tended to have higher mean dropout rates (42.0% and 37.0%, respectively) than patients treated for cannabis and polysubstance use (27.8% and 31.3%, respectively). Contingency management demonstrated the lowest dropout rates (29.4%), followed by general cognitive behavior therapy (35.3%) and cognitive behavior therapy plus contingency management (44.5%). Only two studies provided relapse prevention dropout rates (57.0%), and these studies were specific to cocaine treatment.
Aggregate Effect Sizes
Table 1 presents aggregate effect sizes across all studies. The aggregate effect size across all conditions and all substances was in the moderate range (d=0.45), with a 95% confidence interval (CI) of 0.27 to 0.63. Although there was an apparent difference in effect sizes depending on the outcome measure used, with self-report yielding a high-moderate effect size (d=0.61, 95% CI=0.35 to 1.20) and toxicology screen outcomes yielding a low-moderate effect size (d=0.33, 95% CI=0.17 to 0.49), this difference did not reach significance according to a within-sample t test, which included only the 12 studies that provided both measures (t=1.16, df=11, p<0.28). Urine analysis detection time differs across drugs of abuse. Whereas cocaine and opiates have an approximate window of detection of 1–3 days, the window of detection time for marijuana (cannabis) can extend to weeks to months for individuals who are chronic heavy users. In the current meta-analysis, only one study of marijuana used urine analysis as an outcome variable ( Table 1 ).
Effect Sizes Across Substance Type
Figure 1 represents overall effect sizes for psychosocial treatments (collapsed across treatment type) in terms of the substance use being targeted. Independent-sample t tests revealed that psychosocial treatments had their lowest efficacy for polysubstance use, with a significant difference between outcomes for polysubstance use (d=0.24, 95% CI=0.03 to 0.44) and cannabis use (d=0.81, 95% CI=0.25 to 1.36) disorders (t=2.42, df=17, p<0.03). Treatments targeting cocaine use yielded medium to large effect sizes (d=0.62, 95% CI=0.16 to 1.08), and treatments targeting opiate use yielded small to medium effect sizes (d=0.39, 95% CI=0.18 to 0.60). There were no other significant differences between the substance use disorders treated.
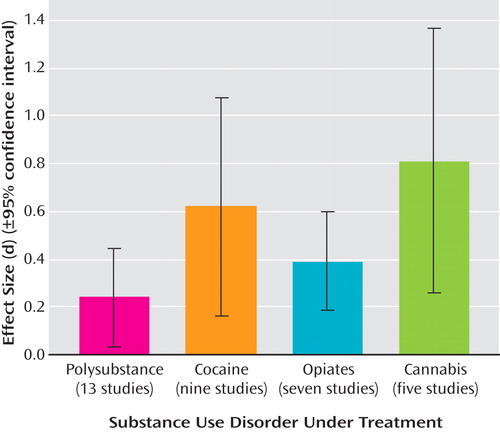
Effect Sizes Across Treatment Type
Figure 2 presents effect sizes for treatment outcome in terms of treatment type across all substances. The results indicate that treatments incorporating both cognitive behavior therapy and contingency management had the highest effect sizes (d=1.02); however, this result must be interpreted cautiously as there were few studies in this category (N=2; 95% CI=–0.05 to 2.09). Treatments using contingency management alone produced moderate-high effect sizes (d=0.58, 95% CI=0.25 to 0.90). Cognitive behavior therapy alone and relapse prevention evidenced low moderate effect sizes: d=0.28 (95% CI=0.06 to 0.51) and d=0.32 (95% CI=0.06 to 0.56), respectively.
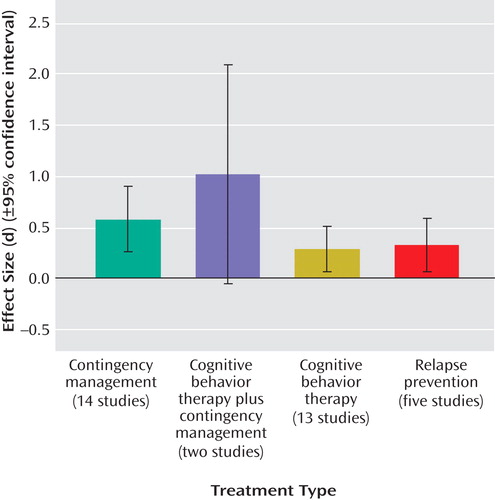
Abstinence Rates
Across all active treatment conditions, almost one-third of the participants (31%) achieved posttreatment and/or clinically significant abstinence. Alternately, only 13% of all participants in control conditions achieved abstinence. Across drug use groups, rates were similar, with 36.2% of opiate users, 31.7% of cocaine users, and 26.0% of cannabis users achieving abstinence during the study period.
Although the combination of cognitive behavior therapy and contingency management evidenced the largest effect size, this advantage was not evident for the percent of abstinence posttreatment (26.5%). Treatment involving relapse prevention evidenced the largest posttreatment abstinence rates, with 39.0% abstinent. Posttreatment percent abstinent for general cognitive behavior therapy alone was 27.1%, and for contingency management alone, it was 31.0%.
Moderators
In an attempt to better understand our outcome findings, we examined variables that may potentially moderate the association between aggregate effect size estimates and treatment dropout rates. In terms of sample demographics, we used Pearson’s partial correlations to assess the relationship between age, sex (percent male in each study), ethnicity (percent white), marital status (percent single/unmarried), employment status (percent employed), and average years of substance use in relation to outcome variables (e.g., treatment retention, overall effect size).
A significant negative correlation was found between age and effect size (r=–0.37, p<0.05), suggesting that younger samples were more likely to have larger effect sizes. A significant negative correlation was also found between average years of substance use and treatment dropout rate (r=–0.68, p<0.05), indicating that participants with longer histories of substance use were less likely to drop out of treatment than those with shorter histories of use. No other demographic variables were significantly associated with treatment outcome.
In addition, we also examined a number of treatment variables in relation to key outcome variables. Treatment variables included the number of weeks treatment was administered, the number of treatment sessions per week, and whether or not medication maintenance was employed. The results indicated a significant negative correlation between the number of treatment weeks and effect size (r=–0.34, p<0.05). Number of treatment sessions per week, however, was not significantly related to treatment outcome or treatment retention. Receipt of medication maintenance was negatively associated with dropout rates (r=–0.51, p<0.05); patients in studies in which medication maintenance provided a backdrop to the psychosocial conditions under study were less likely to drop out of treatment than patients in treatment that did not use medication.
Agonist Versus Other/No Drug Therapies
An additional issue that may influence the size of treatment effects is the background drug treatment condition. Agonist therapies, stimulating the relevant drug receptor, attenuate withdrawal and drug craving and may provide either a better or worse backdrop for other treatments. In a controlled effect size analysis, this drug condition affects changes in both the experimental and control treatment conditions, and hence, for there to be differential effects evident for comparisons between the psychosocial treatment conditions, the drug must lead to interaction effects. For example, if cravings are attenuated, a subsequent psychosocial treatment may have a differential “foothold” for changing drug use behaviors. Alternatively, the drug benefits applied to both the experimental treatment group and the control condition may make it more difficult to show additive effects by providing all patients in the trial with initial changes in drug use that may approach ceiling effects for short-term treatment.
To assess the potential influence of agonist therapies, we compared studies using this approach (N=13, including both methadone and buprenorphine treatment) to those that offered no drug treatment (N=18) or antagonist therapy (N=3, naltrexone treatment). Agonist treatment was used in seven of 13 polysubstance use studies, three of nine cocaine studies, and three of seven opiate studies. No agonist treatment was offered in studies of cannabis treatment; hence, these studies (N=5) were not considered in the following analysis. Overall, we found no significant difference or tendency in effect sizes for the combined drug groups, excluding marijuana (agonist overall: mean d=0.38, not agonists: d=0.40; t=0.1, df=27, p<0.90). Variability in effects was high, with agonist therapies showing both the highest and lowest effect sizes in the entire sample of studies included in this meta-analysis. No differences between agonist use and the remainder of the sample were significant when they were examined separately in the polysubstance, cocaine, and opiate use groups (all p values >0.14).
Publication Bias and Associations With Sample Size and Publication Year
Investigators have recognized the potential discrepancy between the number of trials completed and the number of trials published. If studies are not published because the findings are not significant, a meta-analysis of published studies may overestimate effect sizes. This problem has been labeled “the file drawer problem” (62) .
Using a conservative method of addressing this problem, one must assume that the effect sizes of all current or future unpublished studies are equal to 0 and compute the number of studies it would require to reduce the overall effect size to a minimally informative level, in this case, a small effect size (d=0.2). With the guidelines of Orwin (63) , more than 42 “file drawer studies” with null results would be required to reduce the overall effect size to a small level, according to Cohen’s standards (27) , a result indicating that the findings to date (based on 34 studies) are fairly robust. Moreover, we found no significant relationship between sample size and effect size (r=0.02, p>0.90), and there was no significant association between publication year and effect size (r=–0.09, p>0.50).
Discussion
Overall, our meta-analytic review of cognitive behavioral treatment trials published through March of 2005 provided consistent evidence for benefit from a variety of psychosocial interventions. Effect sizes were in the low-moderate to high-moderate range, depending on the substance disorder and treatment type. Given the long-term social, emotional, and cognitive difficulties associated with substance abuse and dependence, these effect sizes and abstinence rates are noteworthy and comparable to those of interventions for other psychiatric disorders. Specifically, they are in the same range as those obtained in meta-analytic reviews using similar methods to examine the benefits of pharmacotherapy for anxiety disorders (64 , 65) .
Among the disorders under treatment, interventions for cannabis and cocaine yielded the largest effect sizes. However, cocaine treatments also yielded the largest dropout rates, and this dichotomous finding may suggest that rather than pursuing stepwise gains, many patients make an early decision between targeting abstinence or dropping out of treatment. Not surprisingly, treatment targeting polysubstance use yielded the lowest effect size and the lowest percent posttreatment abstinence. Polysubstance users, as a group, tend to have the highest rates of comorbid psychiatric and medical conditions, which may enhance dysfunctional coping motives while also interfering with treatment participation (1) .
Overall, the highest effect size estimates were obtained for contingency management approaches, followed by relapse prevention and other cognitive behavioral therapy approaches. The combination of cognitive behavioral therapy and contingency management had particularly high effect sizes, but confidence in these estimates was limited by having only two studies for evaluation. Contingency management therapies often involve monetary incentives and frequent drug testing, which may challenge some treatment networks, although there has been some promise with the development of more cost-effective incentives (66) .
Our meta-analysis was limited by the small number of studies for the combination of contingency management and cognitive behavioral therapy as well as for studies of relapse prevention. Also, fewer studies were completed for cannabis and opiate use disorders; hence, confidence in our effect size estimates is most limited for these disorders. Also, it is noteworthy that none of the relapse prevention studies analyzed included polysubstance users, the group with the lowest effect size estimates.
Although the backdrop condition of medication use (e.g., the use of agonist therapies) may have an effect on the overall responsivity of the treatment samples, this treatment effect should be applied equally to the experimental treatment and the comparison treatment conditions unless there is an interaction effect. For example, agonist therapy may make it easier for patients to respond to other treatment resources if craving is reduced; however, it is not clear whether this will differentially advantage the experimental versus the comparison treatment. In our meta-analysis, we found no reliable evidence of differential benefit in terms of the controlled effect sizes showing the effects of cognitive behavioral treatment over the control condition.
Overall, meta-analytic review of the psychosocial treatment literature for illicit drug use revealed promising findings. Given the aggregate effect size for active treatment, the current evidence suggests that the average patient undergoing psychosocial interventions achieves acute outcomes better than approximately 67% of the patients in control conditions. Directions for future research include studies aimed at improving retention rates for all substance use groups, as well as at improving treatment efficacy for polysubstance users.
1. Carroll KM, Onken LS: Behavioral therapies for drug abuse. Am J Psychiatry 2005; 162:1452–1460Google Scholar
2. Kidorf M, Stitzer ML: Contingency use of take-homes and split-dosing to reduce illicit drug use of methadone patients. Behav Ther 1996; 27:41–51Google Scholar
3. Budney ST, Higgens ST, Mercer DE, Carpenter G: A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction, NIH Publication 98–4309. Rockville, Md, National Institute on Drug Abuse, 1998Google Scholar
4. Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL: Contingent reinforcement increases cocaine abstinence during out-patient treatment and 1 year of follow-up. J Consult Clin Psychol 2000; 68:64–72Google Scholar
5. Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S: Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry 2003; 60:1043–1052Google Scholar
6. Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W: Smoking cessation in methadone maintenance. Addiction 2002; 97:1317–1328Google Scholar
7. Miller WR, Willbourne PL: Mesa grande: a methodological analysis of clinical trials of treatments for alcohol disorders. Addiction 2002; 97:265–277Google Scholar
8. Carroll KM, Rounsaville BJ, Nich C, Gordon LT: One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: delayed emergence of psychotherapy effects. Arch Gen Psychiatry 1994; 51:989–997Google Scholar
9. Stephens RS, Roffman RA, Curtin L: Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol 2000; 68:898–908Google Scholar
10. Azrin NH, McMahon PT, Donohue B, Beselei VA: Behavior therapy for drug abuse: a controlled treatment outcome study. Behav Res Ther 1994; 32:857–866Google Scholar
11. Barnett PG, Rodgers JH, Bloch DA: A meta-analysis comparing buprenorphine to methadone for treatment of opiate dependence. Addiction 2001; 96:683–690Google Scholar
12. Levin FR, McDowell D, Evans SM, Nunes E, Akerele E: Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict 2004; 13:21–32Google Scholar
13. Marsch LA: The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction 1998; 93:515–532Google Scholar
14. West SL, O’Neal KK, Graham CW: A meta-analysis comparing the effectiveness of buprenorphine and methadone. J Subst Abuse 2000; 12:405–414Google Scholar
15. Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD: A contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend 2000; 58:55–66Google Scholar
16. Agosti V: The efficacy of treatments in reducing alcohol consumption: a meta-analysis. Int J Addict 1995; 30:1067–1077Google Scholar
17. Apodaca TR, Miller WR: A meta-analysis of the effectiveness of bibliotherapy for alcohol problems. J Clin Psychol 2003; 59:289–304Google Scholar
18. Barth J, Critchley J, Bengel J: Efficacy of psychosocial interventions for smoking cessation in patients with coronary heart disease: a systematic review and meta-analysis. Ann Behav Med 2006; 32:10–20Google Scholar
19. Prochaska JJ, Delucchi K, Hall SM: A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol 2004; 72:1144–1156Google Scholar
20. Sussman S, Sun P, Dent CW: A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006; 25:549–557Google Scholar
21. Rubak S, Sandbaek A, Lauritzen T, Christensen B: Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005; 55:305–312Google Scholar
22. Burke BL, Arkowitz H, Menchola M: The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003; 71:843–861Google Scholar
23. Vasilaki EI, Hosier SG, Cox WM: The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol 2006; 41:328–335Google Scholar
24. Covi L, Hess JM, Schroeder JR: A dose response study of cognitive behavioral therapy in cocaine abusers. J Subst Abuse Treat 2002; 23:191–197Google Scholar
25. McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M: The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 1992; 9:199–213Google Scholar
26. Sobell LC, Sobell MB: Timeline Follow-Back: a technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods. Edited by Allen J and Litten RZ. Totowa, NJ, Humana Press, 1992, pp 41–72Google Scholar
27. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Erlbaum, 1988Google Scholar
28. Stitzer ML, Iguchi MY, Felch LJ: Contingent take-home incentive: effects on drug use of methadone maintenance patients. J Consult Clin Psychol 1992; 60:927–934Google Scholar
29. Iguchi MY, Belding MA, Morral AR, Lamb RI, Husband SD: Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. J Consult Clin Psychol 1997; 65:421–428Google Scholar
30. Downey KK, Helmus TC, Schuster CR: Treatment of heroin-dependent poly drug abusers with contingency management and buprenorphine maintenance. Exper Clin Psychopharmacol 2000; 8:176–184Google Scholar
31. Chutuape MA, Silverman K, Stitzer ML: Effects of urine testing frequency on outcome in a methadone take-home contingency program. Drug Alcohol Depend 2001; 62:69–76Google Scholar
32. Katz EC, Chutuape MA, Jones HE, Stitzer ML: Voucher reinforcement for heroin and cocaine abstinence in an outpatient drug-free program. Exper Clin Psychopharmacol 2002; 10:136–143Google Scholar
33. Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K: Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend 2003; 70:315–325Google Scholar
34. Petry NM, Martin B: Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol 2004; 70:398–405Google Scholar
35. Linehan MM, Schmidt H, Dimeff LA, Craft CJ, Kanter J, Comtois KA: Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addict 1999; 8:279–292Google Scholar
36. Schmitz JM, Averill P, Sayre S, McCleary P, Moeller F, Swann A: Cognitive-behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addict Disord Treat 2002; 1:17–24Google Scholar
37. Pollack MH, Penava SA, Bolton E, Worthington JJ III, Allen GL, Farach FJ Jr, Otto MW: A novel cognitive-behavioral approach for treatment-resistant drug dependence. J Subst Abuse Treat 2002; 23:335–342Google Scholar
38. Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C: Promising treatments for women with comorbid PTSD and substance use disorders. Am J Psychiatry 2004; 161:1426–1432Google Scholar
39. Azrin NH, McMahon PT, Donohue B, Besalel VA, Lapinski KJ, Kogan ES, Acierno RE, Galloway E: Behavior therapy for drug abuse: a controlled treatment outcome study. Behav Res Ther 1994; 32:857–866Google Scholar
40. Gottheil E, Thornton C, Weinstein S: Effectiveness of high versus low structure individual counseling for substance abuse. Am J Addict 2002; 11:279–290Google Scholar
41. Silverman K, Higgins ST, Brooner RK: Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry 1996; 53:409–415Google Scholar
42. Jones HE, Johnson RE, Bigelow GE: Safety and efficacy of L-tryptophan and behavioral incentives for treatment of cocaine dependence: a randomized clinical trial. Am J Addict 2004; 13:421–437Google Scholar
43. Sigmon SC, Correia CJ, Stitzer ML: Cocaine abstinence during methadone maintenance: effects of repeated brief exposure to voucher-based reinforcement. Exper Clin Psychopharmacol 2004; 12:269–275Google Scholar
44. Silverman K, Robles E, Mudric T: A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J Consult Clin Psychol 2004; 72:839–854Google Scholar
45. Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G: Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry 1993; 150:763–769Google Scholar
46. Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ: Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 1998; 93:713–727Google Scholar
47. Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT: Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry 1999; 56:493–502Google Scholar
48. Carroll KM, Rounsaville BJ, Gordon LT: Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch Gen Psychiatry 1994; 51:177–187Google Scholar
49. Schmitz JM, Averill P, Sayre S, McCleary P, Moeller F, Swann A: Cognitive-behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addict Disord Treat 2002; 1:17–24Google Scholar
50. Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ: Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch Gen Psychiatry 2001; 58:755–761Google Scholar
51. Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ: Contingency management to enhance naltrexone treatment of opioid dependence: a randomized clinical trial of reinforcement. Exper Clin Psychopharmacol 2002; 10:54–63Google Scholar
52. Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA: Effects of adding behavioral treatment to opioid detoxification with buprenorphine. J Consult Clin Psychol 1997; 65:803–810Google Scholar
53. Rawson RA, McCann MJ, Shoptaw SJ, Miotto KA, Frosch DL, Obert JL, Ling W: Naltrexone for opioid dependence: evaluation of a manualized psychosocial protocol to enhance treatment response. Drug Alcohol Rev 2001; 20:67–78Google Scholar
54. Linehan MM, Dimeff LA, Reynolds SK, Comtois KA, Welch SS, Heagerty P, Kivlahan DR: Dialectal behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002; 67:13–26Google Scholar
55. Abbott PJ, Weller SB, Delaney HD, Moore BA: Community reinforcement approach in the treatment of opiate addicts. Am J Drug Alcohol Abuse 1998; 24:17–30Google Scholar
56. McAuliffe WE: A randomized controlled trial of recovery training and self-help for opioid addicts in New England and Hong Kong. J Psychoactive Drugs 1990; 22:197–209Google Scholar
57. Sinha R, Easton C, Renee-Aubin L, Carroll KM: Engaging young probation-referred marijuana-abusing individuals in treatment: a pilot trial. Am J Addict 2003; 12:314–323Google Scholar
58. Copeland J, Swift W, Roffman R, Stephans R: A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat 2001; 21:55–64Google Scholar
59. Budney AJ, Higgins ST, Radonovich KJ, Novy PL: Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol 2000; 68:1051–1061Google Scholar
60. Roffman RA, Stephens RS, Simpson EE, Whitaker DL: Treatment of marijuana dependence: preliminary results. J Psychoactive Drugs 1988; 20:129–137Google Scholar
61. Stephens RS, Roffman RA, Curtin L: Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol 2000; 68:898–908Google Scholar
62. Rosenthal R: The “file drawer problem” and tolerance for null results. Psychol Bull 1991; 86:638–641Google Scholar
63. Orwin RG: A fail-safe N for effect size in meta-analysis. J Educ Stat 1983; 8:157–159Google Scholar
64. Gould RA, Otto MW, Pollack MH, Yap L: Cognitive-behavioral and pharmacological treatment of generalized anxiety disorder: a preliminary meta-analysis. Behav Ther 1997; 28:285–305Google Scholar
65. Otto MW, Tuby KS, Gould RA, McLean RY, Pollack MH: An effect-size analysis of the relative efficacy and tolerability of serotonin selective reuptake inhibitors for panic disorder. Am J Psychiatry 2001; 158:1989–1992Google Scholar
66. Petry NM, Martin B: Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol 2004; 70:398–405Google Scholar