Adjunctive Antidepressant Use and Symptomatic Recovery Among Bipolar Depressed Patients With Concomitant Manic Symptoms: Findings From the STEP-BD
Abstract
Objective: Practice guidelines have advised against treating patients with antidepressants during bipolar mixed states or dysphoric manias. However, few studies have examined the outcomes of patients with co-occurring manic and depressive symptoms who are treated with antidepressants plus mood stabilizing drugs. Method: The authors compared outcomes in patients with bipolar disorder who received a mood stabilizing agent with versus without an antidepressant for a bipolar depressive episode accompanied by ≥2 concurrent manic symptoms. The 335 participants were drawn from the first 2,000 enrollees in the National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Kaplan-Meier survival curves and Cox regression models were used to compare time to recovery. General linear models examined the relationship between antidepressant use or mania symptom load at the study entry and mania or depression symptom severity at the 3-month follow-up. Results: Adjunctive antidepressant use was associated with significantly higher mania symptom severity at the 3-month follow-up. The probability of recovery at 3 months was lower among patients with higher baseline depression severity. Antidepressant use neither hastened nor prolonged time to recovery once potential confounding factors were covaried in a Cox regression model. Conclusions: In bipolar depression accompanied by manic symptoms, antidepressants do not hasten time to recovery relative to treatment with mood stabilizers alone, and treatment with antidepressants may lead to greater manic symptom severity. These findings are consistent with those from the STEP-BD randomized trial for pure bipolar depression, in which adjunctive antidepressants did not yield higher recovery rates than did mood stabilizer monotherapy.
Appropriate treatments for bipolar depression with concurrent manic symptoms remain the subject of controversy. Clinically relevant depressive symptoms have been reported to co-occur during mania in 25%–40% of patients with bipolar disorder (1) . Conversely, mania symptoms during bipolar depression appear to be common, dimensional, and recurrent (2 – 7) phenomena, although subsyndromal mania with depression remains unrecognized as a formal entity in DSM-IV and ICD-10 (8) .
Nearly all practice guidelines advise against the use of antidepressants when depression and mania co-occur (9 – 12) , although data from clinical trials addressing this issue are scant. Mood stabilizing agents with demonstrated antidepressant properties, such as lamotrigine or quetiapine, are advocated by some guidelines for the treatment of bipolar depression, regardless of the presence of concomitant or subsyndromal mania symptoms (12) . Some studies indicate that recovery among manic patients with subsyndromal depressive symptoms is faster with divalproex than with lithium (13 , 14) , but there has been little empirical study of recovery from bipolar depression with subsyndromal mania. In pure bipolar depression, recent randomized controlled data show no advantage for antidepressants added to mood stabilizers over mood stabilizers alone (15) . Lacking from the empirical literature is a description of clinical or other factors to better identify when (rather than if) antidepressants are more likely to benefit or worsen treatment outcome for bipolar depression. The nature of a bipolar depressive episode—in particular, with respect to concomitant subsyndromal manic features—may represent one such prognostic variable and provided the impetus for the present study.
The present longitudinal, naturalistic study assessed time until recovery and subsequent relapse among patients with bipolar disorder treated for a bipolar depressive episode with concomitant manic symptoms. Participants were initially assessed upon entry to the National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). We sought to identify the prevalence and clinical correlates of antidepressant use in bipolar patients for whom depressive episodes were accompanied by mania symptoms and to compare times until recovery. Additionally, we hypothesized that short-term recovery would be less frequent and slower when antidepressants were added to mood stabilizing drugs for bipolar depression accompanied by syndromal or subsyndromal mania.
We chose to define the study group based on the presence of subsyndromal as well as syndromal mania during depression, rather than solely on the more rigorous DSM-IV criteria for mixed mania. We made this decision for two reasons. First, the construct of depression with subsyndromal mania (“mixed depression” [2] ) appears common, yet it is virtually unstudied (16 , 17) . Second, we reasoned that from the standpoint of relevance to ordinary clinical practice, the presence of a full depressive episode would likely prompt the average general practitioner to consider adding an antidepressant to mood stabilizing drugs. If, however, even low-grade mania symptoms were found to adversely influence outcome during antidepressant co-therapy for bipolar depression, the results would have robust generalizability for risk-benefit decisions about when to use or avoid antidepressants in bipolar depression.
Method
The study group was derived from the first 2,000 STEP-BD entrants. The nature and scope of the overall research program has previously been described (18) . STEP-BD is a multisite, nationwide clinical research program designed to study the treatment effectiveness and phenomenology, course, and outcome of individuals with bipolar disorder who are at least 15 years old. While STEP-BD included both naturalistic and randomized treatment, the present study concerns only individuals participating in naturalistic treatment. All subjects provided written informed consent to participate in the study protocol, which was approved by the institutional review boards at each of the STEP-BD sites. Subjects (N=335) were chosen based on the following criteria: 1) the presence of a full DSM-IV depressive episode, accompanied by ≥2 manic symptoms, and 2) a mood stabilizing agent (lithium, divalproex, carbamazepine, or lamotrigine) and/or an atypical antipsychotic prescribed at the first clinical assessment.
Subjects were then subdivided into those for whom an antidepressant at study entry was (N=145) or was not (N=190) prescribed. Antidepressants may have been started prior to enrollment in STEP-BD or may have been initiated at the study entry; the duration of antidepressant exposure during a current depressive episode was considered as a covariate in the outcome analyses. While the primary hypothesis concerned bipolar depression with syndromal or subsyndromal mania, we conducted separate analyses using a larger group of STEP-BD entrants (N=475) with full depression plus any number of manic symptoms at baseline (zero or one in addition to those with two or more) in order to examine the relationship between the symptom load and subsequent recovery.
Study Procedures and Measures
At study intake, subjects underwent a standardized diagnostic interview (Mini-International Neuropsychiatric Interview [19] ), and psychiatric history (Affective Disorders Evaluation [18] ). Ratings of DSM-IV symptoms of mania and depression and syndromic criteria for a full affective syndrome were recorded at baseline, and all subsequent visits were assessed using a standardized, validated semistructured interview (Clinical Monitoring Form [20] ). Clinical Monitoring Form ratings were conducted at baseline and on a quarterly basis thereafter. Additional treatment visits occurred between quarterly assessments based on clinical necessity, as determined by each treating physician in the study group. The Clinical Monitoring Form was also used to record treatment adherence in the week preceding each assessment. The severity of manic and depressive symptoms at follow-up was rated using the Young Mania Rating Scale (21) and the Montgomery-Åsberg Depression Rating Scale (22) , respectively.
Medication decisions were made according to “model practice procedures” from a “menu of reasonable choices,” to which the STEP-BD study physicians adhered (18) . Model practice procedures were developed based on first-tier recommendations of the 2000 Expert Consensus Guidelines for bipolar disorder (10) as well as other published treatment guidelines (9 , 11) . Clinicians were free to exercise autonomous judgment and decision making in choosing specific medications or drug classes consistent with model practice parameters. No specific guideline was mandated with respect to antidepressant use.
The STEP-BD has previously defined the construct of “recovery” based on the Clinical Monitoring Form-derived presence of ≤2 unequivocally present affective symptoms for at least 8 weeks (23) , which is consistent with DSM-IV criteria for partial or full remission as well as criteria used in the NIMH Collaborative Depression Study (24) . The clinical status of “recovering” designates this level of symptoms for at least 4 weeks and was chosen as an intermediate clinical status to identify significant improvement. The pooled endpoint status of either “recovered” or “recovering” has been used as a composite index of short-term outcome in previous studies of the STEP-BD cohort (25) and was used as the primary outcome point in the present study. Recovery status was examined at 3 months after study entry because prior investigations suggest that this timeframe captures the vast majority of events related to both immediate recovery (i.e., remission) and to adverse outcomes (i.e., acute mood destabilization) with antidepressants (26) .
Statistical Analyses
Initial univariate group comparisons were made by unpaired t tests for group mean differences and by chi square tests for analyses involving dichotomous variables. Achieving a clinical status of “recovered/recovering” was compared for subjects receiving antidepressants versus subjects not receiving antidepressants, using Kaplan-Meier survival analysis with an accompanying log rank statistic. A subsequent Cox regression model, with Wald chi square statistics and hazard ratios accompanied by 95% confidence intervals (CIs), was utilized to examine the likelihood for achieving a “recovered/recovering” status while controlling for the potential mediating effects of antidepressant use as well as other possible confounding factors. Finally, a general linear model was utilized to examine the potential interaction between antidepressant use at baseline and manic symptoms at baseline relative to mania symptom severity scores at the 3-month follow-up. To minimize heterogeneity within the study group, the Kaplan-Meier and Cox regression model analyses excluded subjects who began an antidepressant after study entry (N=26) and subjects with bipolar disorder, not otherwise specified (N=21). All statistical tests were two-tailed, with an alpha level of 0.05.
Results
Of the 2,000 subjects, 448 (22.4%) entered STEP-BD in a depressive episode with at least two manic symptoms at study entry. In the subgroup of 335 subjects who received lithium, an anticonvulsant, and/or an atypical antipsychotic at the time of the first clinical assessment, 145 (43.3%) were either already receiving an antidepressant or newly started on an antidepressant, while 190 (56.7%) were not. Twenty-one of the 448 subjects (4.7%) dropped out of the study protocol during the first 90 days after enrollment.
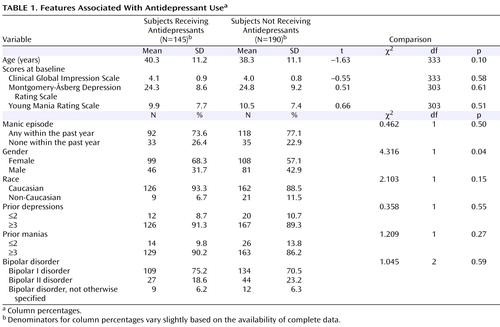
Table 1 summarizes the clinical and demographic features associated with the presence or absence of an antidepressant at study entry. Relative to those subjects who were not receiving an antidepressant, subjects who were receiving an antidepressant were older and significantly more often female. No significant group differences were evident in any other demographic or clinical variables between subjects receiving versus not receiving an antidepressant at study entry. Among the subjects who were either already receiving an antidepressant or newly started on an antidepressant (N=145), nonmutually exclusive antidepressants included bupropion (N=42 [29.0%]), citalopram (N=24 [16.6%]), fluoxetine (N=17 [11.7%]), paroxetine (N=19 [13.1%]), sertraline (N=8 [5.5%]), fluvoxamine (N=4 [2.8%]), venlafaxine (N=28 [19.3%]), mirtazapine (N=13 [19.0%]), nefazodone (N=8 [5.5%]), or nortriptyline (N=1 [0.7%]).
Pharmacotherapy Parameters: Mood Stabilizing Agents
Proportions of subjects who were receiving specific mood stabilizing agents were similar for those who did or did not receive an antidepressant. Lithium (mean dose=1019.7 [SD=366.9] mg/day) was taken by 52 subjects who were receiving antidepressants and 85 subjects who were not receiving an antidepressant (mean dose=1076.5 [SD=358.0] mg/day) (t=0.89, df=135, p=0.37). Sixty-four subjects who were given an antidepressant received divalproex (mean dose=1300.8 [SD=473.9] mg/day), and 85 subjects who were not given an antidepressant received divalproex (mean dose=1325.0 [SD=459.4] mg/day) (t=0.31, df=147, p=0.75). Twelve subjects who were given an antidepressant also received carbamazepine (mean dose=666.7 [SD=352.5] mg/day), and 10 subjects who were not given an antidepressant received carbamazepine (mean dose=720.0 [SD=139.8] mg/day) (t=0.48, df=14.9, p=0.64). Lamotrigine (mean dose=189.1 [SD=234.3] mg/day) was taken by 36 subjects receiving an antidepressant, and 64 subjects not receiving an antidepressant (mean dose=168.5 [SD=149.9] mg/day) (t=0.48, df=51.5, p=0.64). Finally, 61 subjects receiving an antidepressant also received an atypical antipsychotic, and 78 subjects not receiving an antidepressant received an atypical antipsychotic (χ 2 =0.04, df=1, p=0.85).
As shown in Figure 1 , Kaplan-Meier survival analyses of the time until achieving a status of “recovering/recovered” were comparable for subjects who did or did not receive an antidepressant (log rank statistic: p=0.651).
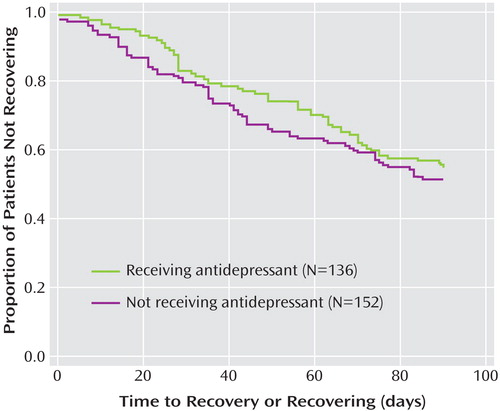
a Log rank test: χ 2 =0.205, df=1, p=0.651.
Figure 2 depicts the results from a Cox regression model with the likelihood for achieving a “recovering/recovered” status by 3 months, taken as the dependent variable. Independent variables for this analysis included parameters chosen from characteristics summarized in Table 1 as well as other variables hypothesized to be relevant to recovery and outcome with antidepressant therapy (e.g., time since last manic episode) (27) . Results indicated that a higher likelihood of recovery was associated only with lower baseline Montgomery-Åsberg Depression Rating Scale scores (Wald χ 2 =16.776, df=1, p<0.0001; hazard ratio=0.96 [95% CI=0.94–0.98]). The likelihood of recovery status was not significantly influenced by the presence or absence of antidepressants after controlling for potential covariates, including the duration of antidepressant treatment.
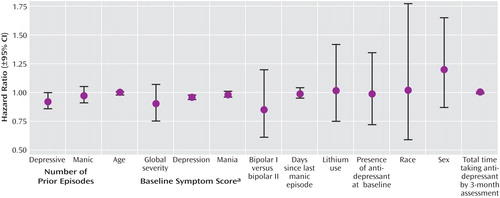
a Global Severity=Clinical Global Impression Scale; Depression=Montgomery-Åsberg Depression Rating Scale; Mania=Young Mania Rating Scale.
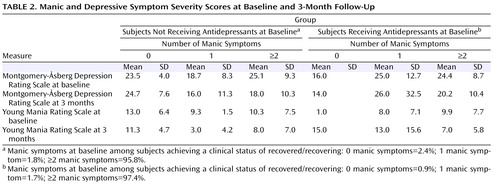
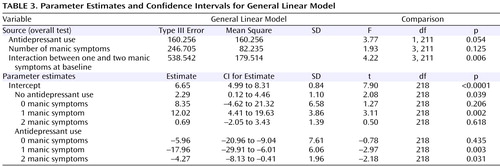
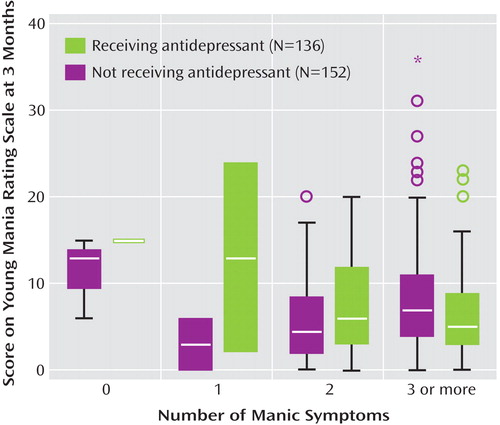
Table 2 presents the mean Montgomery-Åsberg Depression Rating Scale and Young Mania Rating Scale scores at baseline and at the 3-month follow-up, along with the percentage of subjects recovered, stratified by the presence of 0, 1, 2, or 3 or more mania symptoms at baseline in the larger group of 445 subjects with any manic symptoms at baseline. To evaluate changes in manic symptom severity over time in subjects who did or did not receive an antidepressant at baseline, a general linear model was constructed that assessed the following two independent variables: 1) the absence or presence of antidepressant use at intake and 2) the number of manic symptoms at study entry, relative to Young Mania Rating Scale scores at the 3-month assessment. This analysis, illustrated in Figure 2 and shown in parameter estimates in Table 3 , indicated a significant interaction between the number of manic symptoms at baseline and the use of antidepressants in predicting 3-month Young Mania Rating Scale scores (F=4.22, df=3, 211, p=0.006). The significant interaction indicates that in the context of manic symptoms at intake, adjunctive antidepressant use was subsequently associated with higher mania symptom severity levels at follow-up ( Figure 3 ).
Discussion
To our knowledge, this is the first study to empirically examine the association between adjunctive antidepressant use and short-term recovery from bipolar depression accompanied by manic symptoms. Time until symptomatic recovery was no faster with antidepressants added to mood stabilizers versus without antidepressants added to mood stabilizers. However, among depressed patients with manic symptoms at intake, those who received an antidepressant had more severe manic symptoms at the 3-month follow-up than those who did not receive an antidepressant. This observation strongly suggests that antidepressants do not provide benefit when used for bipolar depression in the presence of even subsyndromal mania, but may incur liability for exacerbating manic symptoms.
Prien et al. (28) found that the addition of imipramine to lithium provided neither an advantage nor disadvantage over lithium alone in preventing relapse after mixed manic episodes. In a nonrandomized, cross-sectional study, Goldberg et al. (29) reported that antidepressant use during mixed states was associated with a fourfold increased prevalence of suicidal ideation. Fagiolini et al. (30) observed that approximately two-thirds of depressed bipolar patients remit during mood stabilizer therapy without needing adjunctive antidepressants. These latter investigators observed that clinicians tended to prescribe antidepressants when the severity of depression was high and mania was low, but the point at which mania symptoms that accompany depression predict poorer antidepressant outcomes is unknown.
In the present study, the use of antidepressants for bipolar depression, despite co-occurring manic symptoms, did not appear to be driven by baseline levels of depression or global severity—in contrast to the prescribing patterns observed by Fagiolini et al. (30) . Prior work has shown that practitioners often fail to recognize manic symptoms during bipolar mixed states (31) . If clinicians underappreciate manic or hypomanic symptoms and overattend to concomitant depressive symptoms, they may assume a beneficial role for antidepressant pharmacotherapy above and beyond the effects of mood stabilizing drugs. Our findings suggest no advantage for such strategies and, in fact, raise the concern that baseline manic symptoms may simply worsen with such treatment. The conclusion that antidepressants plus mood stabilizers are no more advantageous than mood stabilizers alone is also consistent with prior randomized trials in pure bipolar depression (15 , 32 , 33) .
The nonrandomized, observational design of this study limits the extent to which causal inferences can be drawn regarding the consequences of antidepressant use in bipolar depression. Of particular concern, as in prior naturalistic studies, is the inability to determine why patients are or are not prescribed antidepressants. The nonrandomized design also did not permit the study of the clinicians’ rationale for treating mixed depression with mood stabilizers alone versus treating mixed depression with mood stabilizers plus an antidepressant. Although baseline Clinical Global Impression and Montgomery-Åsberg Depression Rating Scale scores did not differ between subjects who received or who did not receive antidepressants, it is possible that those who were receiving antidepressants prior to study entry had been more severely ill or treatment-resistant than those not receiving antidepressants, and therefore they were more liable to subsequent affective symptoms and slower recovery. These findings could also have been influenced by other unmeasured prognostic variables, such as axis II comorbidity. The STEP-BD database did not permit differentiation of antidepressant use prior to study entry from initiation upon entry. However, the likelihood of recovery was no different with or without antidepressant use when adjusting for the duration of antidepressant exposure. Groups receiving or not receiving an antidepressant were also comparable with regard to the use of concomitant mood stabilizers.
The present study focused on relatively short-term (3 months) outcomes in order to assess a plausible timeframe for resolution of an acute episode. The possible long-term benefits or adverse consequences of antidepressant use during continuation or maintenance phases of treatment, after an initial remission, should be a focus of future studies.
The present findings contrast with longer-term naturalistic data on relapse prevention during antidepressant use as reported elsewhere by Altshuler et al. (34) and by Joffe et al. (35) . Both of these observational studies reported relatively low relapse rates at 12 months after at least 6 months of exposure to antidepressants following acute remission. However, the nonrandomized designs and lack of statistical controls for potential confounding variables prevented the drawing of causal effects between treatment and outcome in these studies. While the former limitation also applies to our study, we were able to address the latter by applying a multivariate regression model that included a number of important clinical covariates. The studies by Altshuler et al. (34) and Joffe et al. (35) did not specify whether their bipolar depressed study groups included subjects with subsyndromal manic symptoms; thus, it is unclear whether the cohorts are directly comparable.
There remains uncertainty and debate concerning the clinical importance of subsyndromal manic symptoms that accompany bipolar depression (1 – 8) . Several research groups have argued in favor of a broader definition of “mixed” states than what is now described in DSM-IV (1 – 5 , 17) , on the grounds that even subsyndromal manic symptoms during depression are recurrent and elevate the longitudinal risk for suicidal behavior (36 , 37) . The present findings suggest that subsyndromal manic symptoms co-occurring with full depressive episodes hold at least short-term, negative prognostic implications for antidepressant response. It would be valuable for future controlled studies to examine the use of mood stabilizing agents with antidepressant properties as alternatives to traditional antidepressants for bipolar depression with concomitant manic symptoms.
1. McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI, Faedda GL, Swann AC: Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry 1992; 149:1633–1644Google Scholar
2. Benazzi F: Family history validation of a definition of mixed depression. Compr Psychiatry 2005; 46:159–166Google Scholar
3. Perugi G, Akiskal HS, Micheli C, Toni C, Madaro D: Clinical characterization of depressive mixed state in bipolar I patients: Pisa-San Diego Collaboration. J Affect Disord 2001; 67:105–114Google Scholar
4. Benazzi F: Mixed states in bipolar II disorder: should full hypomania always be required? Psychiatry Res 2004; 127:247–257Google Scholar
5. Sato T, Bottlender R, Sievers M, Moller HJ: Evidence of depressive mixed states. Am J Psychiatry 2005; 162:193–194Google Scholar
6. Cassidy F, Ahearn EP, Carroll BJ: Symptom profile consistency in recurrent manic episodes. Compr Psychiatry 2002; 43:179–181Google Scholar
7. Sato T, Bottlender R, Sievers M, Schroter A, Kleindienst N, Moller HJ: Evaluating the inter-episode stability of depressive mixed states. J Affect Disord 2004; 81:103–113Google Scholar
8. Sato T, Bottlender R, Kleindienst N, Tanabe A, Moller HJ: The boundary between mixed and manic episodes in the ICD-10 classification. Acta Psychiatr Scand 2002; 106:109–116Google Scholar
9. Bauer MS, Callahan AM, Jampala C, Petty F, Sajatovic M, Schaefer V, Wittlin B, Powell BJ: Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999; 60:9–21Google Scholar
10. Sachs G, Printz D, Kahn D, Carpenter D, Docherty JP: The Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder. Postgrad Med 2000; Spec No:1–104Google Scholar
11. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder (revision). Am J Psychiatry 2002; 159:1–50Google Scholar
12. Suppes T, Dennehy EB, Hirschfeld RM, Altshuler LL, Bowden CL, Calabrese JR, Crismon ML, Ketter TA, Sachs GS, Swann AC, Texas Consensus Conference Panel on Medication Treatment of Bipolar Disorder: The Texas Implementation of Medication Algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry 2005; 66:870–886Google Scholar
13. Swann AC, Bowden CL, Morris D, Calabrese JR, Petty F, Small J, Dilsaver SC, Davis JM: Depression during mania: treatment response to lithium or divalproex. Arch Gen Psychiatry 1997; 54:37–42Google Scholar
14. Clothier J, Swann AC, Freeman T: Dysphoric mania. J Clin Psychopharmacol 1992; 12(suppl 1):13S–16SGoogle Scholar
15. Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden CL, Fossey MD, Ostacher MJ, Ketter TA, Patel J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz MJ, Otto MW, Dennehy EB, Thase ME: Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–1722Google Scholar
16. Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum M: Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Biol Psychiatry 2003; 53:1028–1042Google Scholar
17. Bauer MS, Whybrow PC, Gyulai L, Gonnel J, Yeh HS: Testing definitions of dysphoric mania and hypomania. J Affect Disord 1994; 32:201–211Google Scholar
18. Bauer MS, Simon GE, Ludman E, Unutzer J: “Bipolarity” in bipolar disorder: distribution of manic and depressive symptoms in a treated population. Br J Psychiatry 2005; 187:87–88Google Scholar
19. Sheehan D, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59:22–33Google Scholar
20. Sachs GS, Guille C, McMurrich S: A clinical monitoring form for mood disorders. Bipolar Disord 2002; 4:323–327Google Scholar
21. Young RC, Biggs JT, Ziegler VT, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Google Scholar
22. Montgomery SA, Asberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Google Scholar
23. Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, Reilly-Harrington NA, Nierenberg AA, Sachs GS, Thase ME: Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry 2006; 163:217–224Google Scholar
24. Keller MB, Lavori PW, Coryell W, Endicott J, Mueller IT: Bipolar I: a five-year prospective follow-up. J Nerv Ment Dis 1993; 181:238–245Google Scholar
25. Weiss RD, Ostacher MJ, Otto MW, Calabrese JR, Fossey M, Wisniewski SR, Bowden CL, Nierenberg AA, Pollack MH, Salloum IM, Simon NM, Thase ME, Sachs GS; for STEP-BD Investigators: Does recovery from substance use disorder matter in patients with bipolar disorder? J Clin Psychiatry 2005; 66:730–735Google Scholar
26. Goldberg JF, Truman CJ: Antidepressant-induced mania: an overview of current controversies. Bipolar Disord 2003; 5:407–420Google Scholar
27. MacQueen GM, Young LT, Marriott M, Robb J, Begun H, Joffe RT: Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand 2002; 105:414–418Google Scholar
28. Prien RF, Himmelhoch JKM, Kupfer DJ: Treatment of mixed mania. J Affect Disord 1988; 15:9–15Google Scholar
29. Goldberg JF, Garno JL, Kocsis JH, Leon AC, Portera L, Whiteside JE: Correlates of suicidal ideation in dysphoric mania. J Affect Disord 1999; 56:75–81Google Scholar
30. Fagiolini A, Frank E, Cherry CR, Houck PR, Novick DM, Buysse DJ, Kupfer DJ: Clinical indicators for the use of antidepressants in the treatment of bipolar I depression. Bipolar Disord 2002; 4:277–282Google Scholar
31. Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L: Qualitative differences in mixed versus pure mania. Compr Psychatry 2000; 4:237–241Google Scholar
32. Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I: Double-blind comparison of adding a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000; 157:124–126Google Scholar
33. Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Google Scholar
34. Altshuler L, Suppes T, Black D, Nolen WA, Keck PE Jr, Frye MA, McElroy S, Kupka R, Grunze H, Walden J, Leverich G, Denicoff K, Luckenbaugh D, Post R: Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003; 160:1252–1262Google Scholar
35. Joffe TR, MacQueen GM, Marriott M, Young LT: One-year outcome with antidepressant–treatment of bipolar depression. Acta Psychiatr Scand 2005; 112:105–109Google Scholar
36. Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L: Association of recurrent suicidal ideation with nonremission from acute mixed mania. Am J Psychiatry 1998; 155:1753–1755Google Scholar
37. Sato T, Bottlender R, Tanabe A, Moller HJ: Cincinnati criteria for mixed mania and suicidality in patients with acute mania. Compr Psychiatry 2004; 45:62–69Google Scholar