Cellular Basis of Reduced Cortical Reelin Expression in Schizophrenia
Abstract
OBJECTIVE: The authors’ goals were to establish the cellular origin of the reduced cortical reelin expression that occurs in schizophrenia and to relate it to markers of synaptic pathology. METHOD: In situ hybridization was used to quantify reelin mRNA in the hippocampal formation and dorsolateral prefrontal cortex of brains from 13 subjects with schizophrenia and 12 subjects without schizophrenia. Results were correlated with the expression of three synaptic protein genes in the dentate gyrus. RESULTS: Reelin mRNA was expressed by layer I neurons, interneurons, and interstitial white matter neurons. In subjects with schizophrenia, less reelin mRNA was expressed by interstitial white matter neurons in the hippocampal formation and by all three cell types in the prefrontal cortex. Reelin and synaptic protein expression correlated positively. CONCLUSIONS: Interstitial white matter neurons, presumed remnants of the cortical subplate, contribute to the reduction in reelin mRNA in schizophrenia. Down-regulation of reelin expression may in turn contribute to the synaptic pathology of the disorder.
Reelin is a secretory protease with major roles in neurodevelopment and plasticity (1, 2). A reduction of reelin expression, shown initially by Impagnatiello et al. (3), is one of the most robust molecular findings in schizophrenia (2, 4). These observations, together with the known functions of reelin and the phenotype of reelin-haploinsufficient mice, encouraged the hypothesis that down-regulation of reelin is central to the pathogenesis of schizophrenia, with particular implications for involvement of γ-aminobutyric acid (GABA)-ergic neurons and synaptic plasticity (2, 5). However, the existing data do not identify clearly the cell types expressing less reelin in schizophrenia. The present study was designed to address this issue and to assess whether the reduction in reelin relates to the synaptic pathology apparent from molecular and other studies (6, 7).
Method
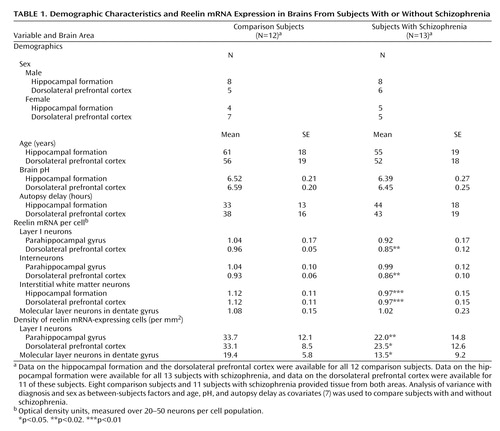
The subjects have been described elsewhere (7), and their demographic characteristics are summarized in Table 1. Sections from the hippocampal formation and dorsolateral prefrontal cortex (Brodmann’s area 9/46) were used to determine reelin mRNA by cellular in situ hybridization and emulsion autoradiography (7).
Results
In the dorsolateral prefrontal cortex, reelin mRNA was localized over layer I neurons, scattered interneurons, and some interstitial white matter neurons. In the hippocampal formation, reelin-expressing cells were prominent in the outer molecular layer of the dentate gyrus, close to the hippocampal fissure. Interstitial white matter neurons in the parahippocampal white matter as well as some interneurons and hilar neurons were also labeled. No other cell types, including pyramidal neurons or granule cells, were clearly labeled.
In the dorsolateral prefrontal cortex, reelin mRNA per cell was reduced in schizophrenia over all three neuron populations, including interstitial white matter neurons (Table 1). These cells also expressed less reelin mRNA in the hippocampal formation in schizophrenia, and the other neurons showed no significant differences. For the layer I and dentate gyrus molecular layer neurons, we also estimated the density of reelin-positive cells; we found a reduction of about 30% in schizophrenia (Table 1). Within the schizophrenia group, reelin expression did not relate to variables such as age at onset or negative symptoms.
In the dentate gyrus, the reelin-expressing neurons in the outer molecular layer make contact with granule cells and regulate connectivity (8). We examined whether there was a correlation between reelin expression and synaptic markers in this region. To do this, “reelin load” in the outer molecular layer was calculated by multiplying reelin mRNA signal per cell by the cell density; reelin load was then correlated with the levels of three synaptic protein mRNAs (synaptophysin, VGLUT1, and GAP-43), measured previously over the granule cells. There were significant positive correlations between reelin load and GAP-43 (r=0.56, N=18, p<0.03) and VGLUT1 (r=0.57, N=25, p=0.005) mRNAs and a nonsignificant correlation between reelin load and synaptophysin mRNA (r=0.44, N=19, p=0.08).
Discussion
Our data confirm that reelin expression is reduced in schizophrenia (2–5, 7) and show its cellular origin in the hippocampal formation and the dorsolateral prefrontal cortex. The distribution in the dorsolateral prefrontal cortex is similar to that in the superior temporal gyrus (7). The reduction in schizophrenia is apparent in terms of both reelin mRNA per cell and density of reelin-positive cells (Table 1). With regard to the latter finding, other studies show that there is no loss of the neurons themselves in schizophrenia (9, 10). Thus, the present results are indicative of a down-regulation of reelin expression.
In both areas studied, interstitial white matter neurons expressed less reelin mRNA, drawing renewed attention to this developmentally critical cell population—the presumed remnants of the cortical subplate—previously implicated in schizophrenia (see reference 7). In the dorsolateral prefrontal cortex, reelin mRNA was also reduced in layer I neurons, the survivors of Cajal-Retzius cells, essential for neuronal migration and lamination (1). Possibly, reduced reelin might be causal to a developmental anomaly affecting interstitial white matter neurons and Cajal-Retzius cells, but, equally, it might be consequential or unrelated to it. Regardless, since both neuron populations form synaptic contacts and persist in adulthood, and given that reelin has key roles in plasticity, the abnormality likely has ongoing effects on the functioning of neural circuits. Note that although reelin mRNA is not detected in pyramidal neurons, reelin is (11), perhaps because the neurons internalize the protein (12) after its secretion by the GABA-ergic cells with which they are synaptically connected. If so, reelin may have a role linking GABA-ergic with glutamatergic aspects of the disease pathophysiology, including its synaptic component (6). The correlations between reelin load and the presynaptic protein transcripts provide some empirical support for this proposal.
Although layer I neurons and most cortical interneurons are GABA-ergic, most interstitial white matter neurons are not, at least in rodents (13). Moreover, the various reelin-expressing cell populations differ in place and time of origin (14). Thus, our data suggest that the involvement of reelin in schizophrenia is not related directly to transmitter phenotype or embryological history of the neurons concerned. Instead, it may reflect an intrinsic property of the gene and its regulation. Possibilities include epigenetic factors (5) or an interaction with susceptibility genes, which themselves have effects on synaptic plasticity (15).
 |
Presented in part at the IXth International Congress on Schizophrenia Research, Colorado Springs, Colo., March 29–April 3, 2003, and the XIIth Winter Workshop on Schizophrenia, Davos, Switzerland, Feb. 7–13, 2004. Received May 9, 2005; revision received June 22, 2005; accepted July 15, 2005. From the Department of Psychiatry, University of Oxford. Address correspondence and reprint requests to Dr. Eastwood, University of Oxford, Department of Psychiatry, Neurosciences Bldg., Warneford Hospital, Oxford OX3 7JX, United Kingdom; [email protected] (e-mail).Supported by the Stanley Medical Research Institute and Medical Research Council. Dr. Eastwood held the British Medical Association Margaret Temple Fellowship.
1. Rice DS, Curran T: Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 2001; 24:1005–1039Crossref, Medline, Google Scholar
2. Fatemi SH: Reelin glycoprotein: structure, biology and roles in health and disease. Mol Psychiatry 2005; 10:251–257Crossref, Medline, Google Scholar
3. Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E: A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA 1998; 95:15718–15723Crossref, Medline, Google Scholar
4. Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB: Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 2005; 57:252–260Crossref, Medline, Google Scholar
5. Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C: Dendritic spine hypoplasticity and downregulation of reelin and GABAergic tone in schizophrenia vulnerability. Neurobiol Dis 2001; 8:723–742Crossref, Medline, Google Scholar
6. Harrison PJ: The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122:593–624Crossref, Medline, Google Scholar
7. Eastwood SL, Harrison PJ: Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry 2003; 8:821–831Crossref, Google Scholar
8. Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G: Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 2004; 41:71–84Crossref, Medline, Google Scholar
9. Benes FM, Barreta S: GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001; 25:1–27Crossref, Medline, Google Scholar
10. Lewis DA, Hashimoto T, Volk DW: Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324Crossref, Medline, Google Scholar
11. Roberts RC, Xu L, Roche JK, Kirkpatrick B: Ultrastructural localization of reelin in the cortex in post-mortem human brain. J Comp Neurol 2005; 482:294–308Crossref, Medline, Google Scholar
12. D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T: Reelin is a ligand for lipoprotein receptors. Neuron 1999; 24:471–479Crossref, Medline, Google Scholar
13. Arias MS, Baratta J, Yu J, Robertson RT: Absence of selectivity in the loss of neurons from the developing cortical subplate of the rat. Dev Brain Res 2004; 139:331–335Crossref, Google Scholar
14. Kriegstein AR, Noctor SC: Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 2004; 27:392–399Crossref, Medline, Google Scholar
15. Harrison PJ, Weinberger DR: Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10:40–68Crossref, Medline, Google Scholar



