Association of Schizophrenia and Autoimmune Diseases: Linkage of Danish National Registers
Abstract
OBJECTIVE: Individuals with schizophrenia and their relatives tend to have either higher or lower than expected prevalences of autoimmune disorders, especially rheumatoid arthritis, celiac disease, autoimmune thyroid diseases, and type 1 diabetes. The purpose of the study was to estimate the association of schizophrenia with these disorders as well as a range of other autoimmune diseases in a single large epidemiologic study. METHOD: The Danish Psychiatric Register, the National Patient Register, and a register with socioeconomic information were linked to form a data file that included all 7,704 persons in Denmark diagnosed with schizophrenia from 1981 to 1998 and their parents along with a sample of matched comparison subjects and their parents. The data linkage required that the autoimmune disease occur before the diagnosis of schizophrenia. RESULTS: A history of any autoimmune disease was associated with a 45% increase in risk for schizophrenia. Nine autoimmune disorders had higher prevalence rates among patients with schizophrenia than among comparison subjects (crude incidence rate ratios ranging from 1.9 to 12.5), and 12 autoimmune diseases had higher prevalence rates among parents of schizophrenia patients than among parents of comparison subjects (adjusted incidence rate ratios ranging from 1.3 to 3.8). Thyrotoxicosis, celiac disease, acquired hemolytic anemia, interstitial cystitis, and Sjögren’s syndrome had higher prevalence rates among patients with schizophrenia than among comparison subjects and also among family members of schizophrenia patients than among family members of comparison subjects. CONCLUSIONS: Schizophrenia is associated with a larger range of autoimmune diseases than heretofore suspected. Future research on comorbidity has the potential to advance understanding of pathogenesis of both psychiatric and autoimmune disorders.
Theories on autoimmune aspects of schizophrenia invoke the notion of early infection by microorganisms possessing antigens that are so similar to tissue in the CNS that resulting antibodies act against the brain (1–5). Comparisons of schizophrenia patients and healthy subjects have revealed differences in immunologic parameters (6), but there have been failures to replicate. There has been repeated evidence of a genetic locus for schizophrenia in the area of the human leukocyte antigens (HLA), also with failures to replicate (7–9). Obstetric complications have been implicated in schizophrenia, and some have speculated that infection in the mother produces antibodies that are transmitted to the fetus, producing autoantibodies that disrupt neural development and raise risk for schizophrenia (10, 11).
Schizophrenia patients or their relatives have been reported to have either higher or lower than expected prevalences of some autoimmune disorders, including rheumatoid arthritis (12), type 1 diabetes (13), thyroid disorders (13, 14), and celiac disease (15). This article presents a systematic comparison of the prevalence of 29 autoimmune disorders for all patients in the nation of Denmark diagnosed with schizophrenia between 1981 and 1998 and their parents along with a group of healthy subjects and their parents.
Method
The Registers
Data from Danish registers were linked using the unique personal identification number that has been allocated to all residents in Denmark since 1968 (16). Socioeconomic information was obtained from the Integrated Database for Longitudinal Labor Market Research, which was created by linking a number of employment and education databases (17).
The Danish Psychiatric Register, which records contacts with psychiatric facilities throughout Denmark, was the source of patients with schizophrenia (18, 19). There are no private psychiatric facilities in Denmark, and all treatment is free of charge. Information on autoimmune diseases originated from the National Patient Register, which has collected data on all admissions to Danish hospitals since 1977 (20). Since 1995 it has included all contacts in emergency rooms and outpatient clinics. Diagnoses in the psychiatric and patient registers were according to ICD-8 until the end of 1993 and according to ICD-10 from the beginning of 1994. The disease categories used here were designed to separate, as much as possible, syndromes with an autoimmune basis from similar syndromes with other causes.
The study procedures were approved by the Danish Data Protection Board and the Johns Hopkins Bloomberg School of Public Health Committee on Human Research.
Subjects
The 7,704 schizophrenia patients comprised all persons over age 15 admitted to a Danish psychiatric facility for the first time between 1981 and 1998 with a diagnosis of schizophrenia and known maternal identity. This group of patients, 66% of whom were male, has been described elsewhere (21). For each patient, 25 comparison subjects matched by year of birth and sex were selected randomly from a 5% sample of the Integrated Database for Longitudinal Labor Market Research. Comparison subjects were excluded if they had been admitted to a psychiatric facility before the first admission of the patient. In 92% of the patients and 96% of the comparison subjects, the father was known. Socioeconomic and disease information on the patients, comparison subjects, and their parents relates to the status just before the first contact with the patient. Wealth of the parents was organized into the highest quartile of the two parents for each individual, based on the distribution of wealth in the entire Integrated Database for Longitudinal Labor Market Research.
Statistical Analysis
Statistical methods included estimation of prevalence proportions in patients and comparison subjects, and conditional logistic regression analyses that yielded an incidence rate ratio. The strata for the logistic regression are formed by the matching variables of year of birth and sex. Multivariate models adjust for known risk factors for schizophrenia: urbanicity of birth, socioeconomic status, and family history of schizophrenia. An initial logistic regression model predicts the occurrence of schizophrenia from prior occurrence of any of 29 autoimmune diseases. Later models are developed that predict schizophrenia from the occurrence of each of the specific autoimmune diseases. Separate prediction models are developed from the autoimmune status of the patients and from the parents of the patients to suggest effects of genetics as distinct from environment.
Results
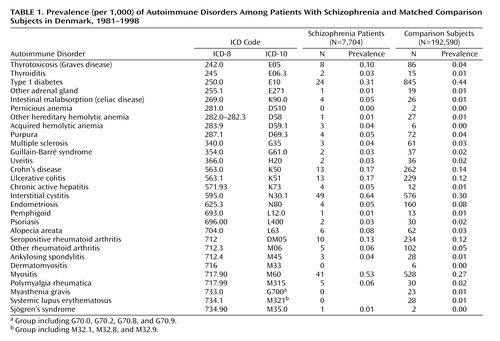
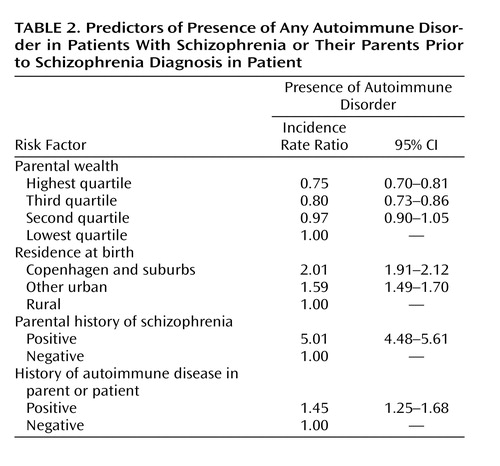
There were 29 autoimmune diseases with which either the patient or a parent was diagnosed before the patient had been diagnosed with schizophrenia. Table 1 shows prevalence data. There were 175, 16, and two patients with one, two, and three autoimmune diseases, respectively. Schizophrenia was associated with nearly 50% higher lifetime prevalence of one or more autoimmune disorders (Table 2). The analysis in Table 2 adjusts for known risk factors for schizophrenia (as well as controlling for sex and age in the matching process), revealing relationships that mirror the scientific literature (22).
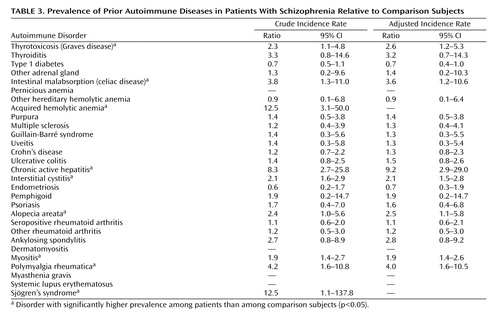
Nine autoimmune diseases had higher lifetime prevalence among schizophrenia patients than among comparison subjects at a 95% level of statistical significance: thyrotoxicosis, intestinal malabsorption, acquired hemolytic anemia, chronic active hepatitis, interstitial cystitis, alopecia areata, myositis, polymyalgia rheumatica, and Sjögren’s syndrome (Table 3). Two disorders had sizable incidence rate ratios but did not meet traditional levels of significance: thyroiditis (incidence rate ratio=3.3) and ankylosing spondylitis (incidence rate ratio=2.7). Even so-called significant findings were based on small numbers: a single case of Sjögren’s disorder produced the incidence rate ratio of 12.5; likewise three cases of acquired hemolytic anemia produced the large incidence rate ratio of 12.5.
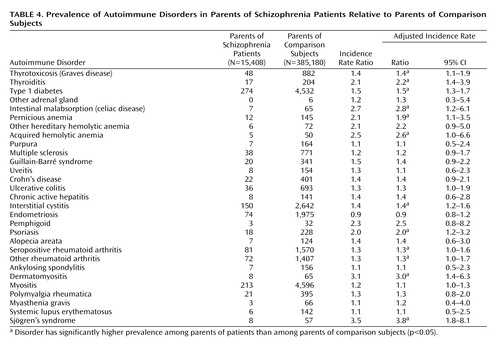
Twelve autoimmune diseases had higher prevalence among parents of schizophrenia patients than among parents of comparison subjects: thyrotoxicosis, thyroiditis, type 1 diabetes, intestinal malabsorption, pernicious anemia, acquired hemolytic anemia, interstitial cystitis, psoriasis, seropositive rheumatoid arthritis, other rheumatoid arthritis, dermatomyositis, and Sjögren’s syndrome (Table 4). Chronic active hepatitis, alopecia areata, myositis, and polymyalgia rheumatica were autoimmune disorders with higher prevalences than expected in the schizophrenia patients but not in their parents.
Discussion
Five autoimmune disorders appeared more frequently in patients with schizophrenia prior to schizophrenia onset as well as in the patients’ parents: thyrotoxicosis, intestinal malabsorption, acquired hemolytic anemia, interstitital cystitis, and Sjögren’s syndrome.
The relationship between intestinal malabsorption, or celiac disease, and schizophrenia was noticed as early as 1961 (23). Celiac disease is an immune-mediated reaction to gluten that presents with diarrhea, weight loss, and abdominal complaints as well as a range of less common signs and symptoms, including some psychiatric and neurological symptoms (24, 25). The psychological interpretation for the first case series was reinterpreted by Dohan (26) as an inherited defect in which the environmental trigger of gluten precipitated schizophrenia in some individuals. Dohan presented two series of ecological data supporting the idea: one temporal series from countries in Europe during World War II (26) and a number of comparisons in the western Pacific based on anthropological data (27). The literature includes case studies, biological explanations for the association, and clinical trials of gluten withdrawal (e.g., references 28–34). Data from the Oxford Record Linkage Study (35) revealed an odds ratio of about three for cross-sectional comorbidity of schizophrenia and celiac disease, and we have published a short report on celiac disease and schizophrenia that used a nearly identical dataset to this one (15).
Autoimmune thyroiditis is characterized by hypothyroidism with clinical manifestations of goiter and lymphocyte infiltration of the gland (36). Thyrotoxicosis (Graves disease) causes sustained hyperthyroidism with clinical complications in thyroid-associated ophthalmology and dermopathy (37). Kraepelin reported clinical observations of enlarged thyroid gland in dementia praecox (38). Subsequent clinical evidence has shown an excess of thyroid hormone dysfunction in schizophrenia, and some have attributed these observations to dysfunction of the hypothalamus-pituitary-thyroid axis and neuroleptic medications (39–42). Our findings on thyroid disorders confirm prior research (13, 14, 43, 44).
Acquired hemolytic anemia is the clinical manifestation of the production of antibodies against red blood cells (45, 46). Our findings on acquired hemolytic anemia are consistent with a prior study (13) that showed an excess of hemolytic anemia in (just two) relatives of schizophrenia patients compared with the single instance in healthy subjects (odds ratio=2.02, 95% CI=0.11–121.6).
Interstitial cystitis is a bladder condition characterized by increased urinary frequency or pelvic pain (47, 48). Although interstitial cystitis has been recently genetically linked to panic disorder (49), we are unaware of prior research linking it to schizophrenia. The causes and pathogenesis for interstitial cystitis are not known.
Sjögren’s syndrome is characterized by progressive destruction of the exocrine glands, manifesting in a decrease in the production of saliva and tears (50). Sjögren’s syndrome generally affects women, and the estimated prevalence varies from approximately 0.1%–6% (51–53). It sometimes occurs with other rheumatic disorders such as systemic lupus erythematosus, but we are unaware of any prior research linking it to schizophrenia.
This analysis does not support certain results in the epidemiologic literature on schizophrenia. For example, two studies have reported an excess of type I diabetes in the relatives of individuals with schizophrenia (13, 44), consistent with the data reported here in Table 4; other studies, however, have reported a negative association between the two disorders (54).
The most consistent finding in the area of schizophrenia and autoimmune diseases is the negative relationship with rheumatoid arthritis (12, 55, 56). The incidence rate ratio for the schizophrenia patients was very close to 1.0 in this analysis, whereas in most other studies rheumatoid arthritis is much less common in individuals with schizophrenia. Our analysis required that the rheumatoid arthritis appear before the patient was diagnosed with schizophrenia, which may have influenced this result, since many cases of rheumatoid arthritis have onset much later than the age at onset for schizophrenia. The incidence rate ratio for parents of schizophrenia patients was greater than 1.0, contrary to the expected direction. There are two small studies of rheumatoid arthritis in the mothers of schizophrenia patients (13, 57), which suggest an inverse relationship.
Parallel Genetic Studies of Schizophrenia and Autoimmune Diseases
One of the possible hypotheses for our observed results is that schizophrenia shares a genetic diathesis with the family of autoimmune diseases, yielding the nearly 50% increase in risk for other autoimmune disorders as shown in Table 2. For example, Becker and colleagues (58) hypothesized that common complex diseases may be a result of the collective effects of disease-specific loci, common nondisease-specific loci, and specific environmental triggers, the so-called common variants/multiple diseases hypothesis. This contrasts slightly with the notion that a single or limited number of genes specific to each autoimmune disorder might be associated with schizophrenia—straightforward pleiotropy (59). The data relevant to these hypotheses, for the most part, come from separate, parallel genetic research studies of specific disorders. For autoimmune diseases, a source of general vulnerability may be the HLA system. Association studies have highlighted the role of HLA genes for certain autoimmune diseases (60, 61). Case/control and family studies suggest that genes in HLA class II regions (e.g., HLA-DR3, DQA1, DRB1) are related to thyrotoxicosis and thyroiditis (60), but the evidence is limited (62). Several studies have suggested the HLA-related susceptibility for celiac disease lies in the DQ alleles (63, 64), but there is some evidence for linkage with other HLA regions (65, 66). There is a line of research on HLA class II (e.g., DQA, DRB1, and DQB1) in relation to the primary Sjögren’s syndrome (67–69). Little is known about associations linking HLA and interstitial cystitis. The epidemiologic association between all these disorders could be a result of 1) direct involvement of HLA antigens or 2) physical closeness between loci for the autoimmune disorders and schizophrenia loci in HLA regions.
Outside the HLA region, the search for variants for common autoimmune diseases has not, as yet, suggested many clusters related to schizophrenia (70). An exception is that linkage studies have suggested that schizophrenia and celiac disease may have genes that are close to each other or identical (71, 72). Another exception is that the HOPA (human opposite paired) gene on chromosome Xq13, which codes for a coactivator for the T4 receptor and in which mutations have been found associated with hypothyroidism (73), has recently been linked with the elevated risk of schizophrenia (74, 75). Because the HOPA gene is expressed throughout the CNS and other tissues, especially in the period of fetal development, the abnormality in the HOPA gene is hypothesized to raise risk for schizophrenia. Separate association studies have connected the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene to schizophrenia (76) and to rheumatoid arthritis (77), and a similar pattern exists for the IL1B gene (78, 79). The CTL-4 gene has been associated with schizophrenia in at least one study (80), and with type 1 diabetes, autoimmune thyroid diseases, and rheumatoid arthritis in numerous studies (58). There have also been suggestive association study findings for the IL10 gene (81) and type 1 diabetes, rheumatoid arthritis, and Sjögren’s syndrome (58) as well as for the TNF gene (82) and psoriasis, rheumatoid arthritis, and type 1 diabetes (58).
Limitations
These data suffer from important limitations. The rarity of the autoimmune disorders is problematic, even for this study that involved an entire nation. Ascertainment was based on diagnosis received in normal medical specialty settings. There is likely to be underascertainment, since individuals with many of these disorders will not always be in attendance at a specialty clinic or inpatient setting. The argument could be made that schizophrenia, and many autoimmune diseases, are so serious that they inevitably end up in specialty treatment and the register system. But some diseases, such as hypothyroid disorder and type 1 diabetes, may be treated by primary care practitioners and never enter the registers. However, for any given disease, these biases would exist equally for patients and comparison subjects, and for parents of both groups; as a consequence the net effect is to lower the degree of association, making the findings conservative.
Another explanation for the low prevalence of autoimmune diseases is that many of them have onset later than schizophrenia, so that the prevalence in patients is much lower than what might be expected. These findings—even findings on parents of patients—may be limited to a subset of autoimmune diseases that have early onset. This possibility cannot be addressed with these data. Another limitation of the present study is the fact that the analyses were carried out in subjects matched by gender, making it impossible to examine gender-related differences, even though the etiology of autoimmune diseases and of schizophrenia probably differ by sex (83, 84). Finally, we cannot be certain that treatment for autoimmune disease is not a risk factor for schizophrenia.
Conclusions from these analyses, especially when the focus is on individual disorders, must necessarily be circumspect because of the opportunistic nature of the statistical analysis. Results reviewed from genetic association and linkage studies likewise are suggestive at best. On the other hand, findings concerning celiac disease and autoimmune thyroid diseases are consistent with the scientific literature, and this analysis is a confirmation based on a stronger dataset than has existed before. In future clinical studies it may be interesting to search for a family history of autoimmune diseases, and specific autoantibodies, in patients with schizophrenia. Eventually, individual or family disease comorbidity may help to elucidate shared etiologic pathways.
 |
 |
 |
 |
Received Nov. 29, 2004; revision received Feb. 9, 2005; accepted March 10, 2005. From the Department of Mental Health, Bloomberg School of Public Health, The Johns Hopkins University; the National Centre for Register-Based Research, University of Aarhus, Aarhus, Denmark; and the Institute for Basic Psychiatric Research, Psychiatric Hospital, University Hospital, Aarhus, Denmark. Address correspondence and reprint requests to Dr. Eaton, Department of Mental Health, Bloomberg School of Public Health, The Johns Hopkins University, HH 852, 624 N. Broadway St., Baltimore, MD 21205; [email protected] (e-mail).Supported by the Danish National Research Foundation, the Stanley Medical Research Institute, and NIMH grant 53188.Dr. Ewald died in January 2004.The authors thank Noel Rose for advice provided for this study.
1. Rose NR: The role of infection in the pathogenesis of autoimmune disease. Semin Immunol 1998; 10:5–13Crossref, Medline, Google Scholar
2. DeLisi LE, Weber RJ, Pert CB: Are there antibodies against brain in sera from schizophrenic patients? review and prospectus. Biol Psychiatry 1985; 20:110–115Crossref, Medline, Google Scholar
3. Kirch DG: Infection and autoimmunity as etiologic factors in schizophrenia: a review and reappraisal. Schizophr Bull 1993; 19:355–370Crossref, Medline, Google Scholar
4. Wright P, Murray RM: Schizophrenia: prenatal influenza and autoimmunity. Ann Med 1993; 25:497–502Crossref, Medline, Google Scholar
5. Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Dow S, Zamkoff J, Dubbert BK, Lougee L: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998; 155:264–271; correction, 155:578Abstract, Google Scholar
6. Ganguli R, Brar JS, Chengappa KN, Yang ZW, Nimgaonkar VL, Rabin BS: Autoimmunity in schizophrenia: a review of recent findings. Ann Med 1993; 25:489–496Crossref, Medline, Google Scholar
7. Li T, Underhill J, Liu XH, Sham PC, Donaldson P, Murray RM, Wright P, Collier DA: Transmission disequilibrium analysis of HLA class II DRB1, DQA1, DQB1 and DPB1 polymorphisms in schizophrenia using family trios from a Han Chinese population. Schizophr Res 2001; 49:73–78Crossref, Medline, Google Scholar
8. Schwab SG, Knapp M, Freymann J, Albus M, Lerer B, Hallmayer J, Maier W, Wildenauer DB: The HLA DRB1 gene locus in schizophrenia: an association study in 55 families with linkage to chromosome 6p, in Ninth Biennial Winter Workshop on Schizophrenia Research. Davos, Switzerland, February 7-13, 1998. Schizophr Res 1998; 29(Jan suppl):1-216Google Scholar
9. Wright P, Donaldson PT, Underhill JA, Choudhuri K, Doherty DG, Murray RM: Genetic association of the HLA DRB1 gene locus on chromosome 6p21.3 with schizophrenia. Am J Psychiatry 1996; 153:1530–1533Link, Google Scholar
10. Chengappa KN, Nimgaonkar VL, Bachert C, Yang ZW, Rabin BS, Ganguli R: Obstetric complications and autoantibodies in schizophrenia. Acta Psychiatr Scand 1995; 92:270–273Crossref, Medline, Google Scholar
11. Wright P, Gill M, Murray RM: Schizophrenia: genetics and the maternal immune response to viral infection. Am J Med Genet 1993; 48:40–46Crossref, Medline, Google Scholar
12. Eaton WW, Hayward C, Ram R: Schizophrenia and rheumatoid arthritis: a review. Schizophr Res 1992; 6:181–192Crossref, Medline, Google Scholar
13. Gilvarry CM, Sham PC, Jones PB, Cannon M, Wright P, Lewis SW, Bebbington P, Toone BK, Murray RM: Family history of autoimmune diseases in psychosis. Schizophr Res 1996; 19:33–40Crossref, Medline, Google Scholar
14. DeLisi LE, Boccio AM, Riordan H, Hoff AL, Dorfman A, McClelland J, Kushner M, Van Eyl O, Oden N: Familial thyroid disease and delayed language development in first admission patients with schizophrenia. Psychiatry Res 1991; 38:39–50Crossref, Medline, Google Scholar
15. Eaton W, Mortensen PB, Agerbo E, Byrne M, Mors O, Ewald H: Coeliac disease and schizophrenia: population based case control study with linkage of Danish national registers. BMJ 2004; 328:438–439Crossref, Medline, Google Scholar
16. Malig C: The Civil Registration System in Denmark: IIVRS Technical Paper 66. Bethesda, Md, International Institute for Vital Registration and Statistics, 1996Google Scholar
17. Danmarks Statistiks: IDA: En Integreret Database for Arbejdsmarkedsforskning. Copenhagen, Danmarks Statistiks Trykkeri, 1991Google Scholar
18. Munk-Jorgensen P, Kastrup M, Mortensen PB: The Danish Psychiatric Register as a tool in epidemiology. Acta Psychiatr Scand Suppl 1993; 370:27–32Crossref, Medline, Google Scholar
19. Mortensen PB, Cantor-Graae E, McNeil TF: Increased rates of schizophrenia among immigrants: some methodological concerns raised by Danish findings. Psychol Med 1997; 27:813–820Crossref, Medline, Google Scholar
20. Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH: The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull 1999; 46:263–268Medline, Google Scholar
21. Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB: Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry 2003; 60:673–678Crossref, Medline, Google Scholar
22. Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, Andersen PK, Melbye M: Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med 1999; 340:603–608Crossref, Medline, Google Scholar
23. Graff H, Handford A: Celiac syndrome in the case histories of five schizophrenics. Psychiatr Q 1961; 35:306–313Crossref, Medline, Google Scholar
24. Farrell RJ, Kelly CP: Celiac sprue. N Engl J Med 2002; 346:180–188Crossref, Medline, Google Scholar
25. Hadjivassiliou M, Grunewald RA, Davies-Jones GA: Gluten sensitivity as a neurological illness. J Neurol Neurosurg Psychiatry 2002; 72:560–563Crossref, Medline, Google Scholar
26. Dohan FC: Hypothesis: genes and neuroactive peptides from food as cause of schizophrenia. Adv Biochem Psychopharmacol 1980; 22:535–548Medline, Google Scholar
27. Dohan FC, Harper EH, Clark MH, Rodrigue RB, Zigas V: Is schizophrenia rare if grain is rare? Biol Psychiatry 1984; 19:385–399Medline, Google Scholar
28. Vlissides DN, Venulet A, Jenner FA: A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry 1986; 148:447–452Crossref, Medline, Google Scholar
29. De Santis A, Addolorato G, Romito A, Caputo S, Giordano A, Gambassi G, Taranto C, Manna R, Gasbarrini G: Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J Intern Med 1997; 242:421–423Crossref, Medline, Google Scholar
30. Dseplat-Jego S, Bernard D, Bagneres D, Frances Y: Neuropsychiatric symptoms in the elderly: let us not forget celiac disease. J Am Geriatr Soc 2003; 51:884–885Crossref, Medline, Google Scholar
31. Jansson B, Kristjansson E, Nilsson L: [Schizophrenic psychosis disappearing after patient is given gluten-free diet]. Lakartidningen 1984; 81:448–449 (Swedish)Medline, Google Scholar
32. Dohan FC: Genetic hypothesis of idiopathic schizophrenia: its exorphin connection. Schizophr Bull 1988; 14:489–494Crossref, Medline, Google Scholar
33. Paroli E: Opioid peptides from food (the exorphins). World Rev Nutr Diet 1988; 55:58–97Crossref, Medline, Google Scholar
34. Smith RS: The GI T-lymphocyte theory of schizophrenia: some new observations. Med Hypotheses 1992; 37:27–30Crossref, Medline, Google Scholar
35. Baldwin JA: Schizophrenia and physical disease: a preliminary analysis of the data from the Oxford Record Linkage Study, in Biochemistry of Schizophrenia and Addiction. Edited by Hemmings G. Baltimore, University Park Press, 1980, pp 297-318Google Scholar
36. Barbesino G, Chiovato L: The genetics of Hashimoto’s disease. Endocrinol Metab Clin North Am 2000; 29:357–374Crossref, Medline, Google Scholar
37. Weetman AP: Graves’ disease. N Engl J Med 2000; 343:1236–1248Crossref, Medline, Google Scholar
38. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
39. Spratt DI, Pont A, Miller MB, McDougall IR, Bayer MF, McLaughlin WT: Hyperthyroxinemia in patients with acute psychiatric disorders. Am J Med 1982; 73:41–48Crossref, Medline, Google Scholar
40. Othman SS, Abdul Kadir K, Hassan J, Hong GK, Singh BB, Raman N: High prevalence of thyroid function test abnormalities in chronic schizophrenia. Aust NZ J Psychiatry 1994; 28:620–624Crossref, Medline, Google Scholar
41. Baumgartner A, Pietzcker A, Gaebel W: The hypothalamic-pituitary-thyroid axis in patients with schizophrenia. Schizophr Res 2000; 44:233–243Crossref, Medline, Google Scholar
42. Degner D, Meller J, Bleich S, Schlautmann V, Ruther E: Affective disorders associated with autoimmune thyroiditis. J Neuropsychiatry Clin Neurosci 2001; 13:532–533Crossref, Medline, Google Scholar
43. MacSweeney D, Timms P, Johnson A: Thryo-endocrine pathology, obstetric morbidity and schizophrenia: survey of a hundred families with a schizophrenic proband. Psychol Med 1978; 8:151–155Crossref, Medline, Google Scholar
44. Wright P, Sham PC, Gilvarry CM, Jones PB, Cannon M, Sharma T, Murray RM: Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophr Res 1996; 20:261–267Crossref, Medline, Google Scholar
45. Gehrs BC, Friedberg RC: Autoimmune hemolytic anemia. Am J Hematol 2002; 69:258–271Crossref, Medline, Google Scholar
46. Kelton JG, Chan H, Heddle N, Whittaker S: Acquired hemolytic anemia, in Blood and Bone Marrow Pathology. Edited by Wickramasinghe SN, McCullough J. New York, Churchill Livingstone, 2002, pp 185-202Google Scholar
47. Parsons CL: Interstitial cystitis: epidemiology and clinical presentation. Clin Obstet Gynecol 2002; 45:242–249Crossref, Medline, Google Scholar
48. Kusek JW, Nyberg LM: The epidemiology of interstitial cystitis: is it time to expand our definition? Urology 2001; 57:95–99Crossref, Medline, Google Scholar
49. Weissman MM, Gross R, Fyer A, Heiman GA, Gameroff MJ, Hodge SE, Kaufman D, Kaplan SA, Wickramaratne PJ: Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatry 2004; 61:273–279Crossref, Medline, Google Scholar
50. Manthorpe R, Asmussen K, Oxholm P: Primary Sjogren’s syndrome: diagnostic criteria, clinical features, and disease activity. J Rheumatol Suppl 1997; 50:8–11Medline, Google Scholar
51. Thomas E, Hay EM, Hajeer A, Silman AJ: Sjogren’s syndrome: a community-based study of prevalence and impact. Br J Rheumatol 1998; 37:1069–1076Crossref, Medline, Google Scholar
52. Tomsic M, Logar D, Grmek M, Perkovic T, Kveder T: Prevalence of Sjogren’s syndrome in Slovenia. Rheumatology (Oxford) 1999; 38:164–170Crossref, Medline, Google Scholar
53. Bowman SJ, Ibrahim GH, Holmes G, Hamburger J, Ainsworth JR: Estimating the prevalence among Caucasian women of primary Sjogren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol 2004; 33:39–43Crossref, Medline, Google Scholar
54. Finney GO: Juvenile onset diabetes and schizophrenia? (letter). Lancet 1989; 2:1214–1215Crossref, Medline, Google Scholar
55. Mors O, Mortensen PB, Ewald H: A population-based register study of the association between schizophrenia and rheumatoid arthritis. Schizophr Res 1999; 40:67–74Crossref, Medline, Google Scholar
56. Oken RJ, Schulzer M: At issue: schizophrenia and rheumatoid arthritis: the negative association revisited. Schizophr Bull 1999; 25:625–638Crossref, Medline, Google Scholar
57. McLaughlin DG: Racial and sex differences in length of hospitalization of schizophrenics. Honolulu, VI World Congress of Psychiatry, 1977Google Scholar
58. Becker KG, Barnes KC, Bright TJ, Wang SA: The Genetic Association Database. Nat Genet 2004; 36:431–432Crossref, Medline, Google Scholar
59. Hodgkin J: Seven types of pleiotropy. Int J Dev Biol 1998; 42:501–505Medline, Google Scholar
60. Gough SC: The genetics of Graves’ disease. Endocrinol Metab Clin North Am 2000; 29:255–266Crossref, Medline, Google Scholar
61. Simmonds MJ, Gough SC: Unravelling the genetic complexity of autoimmune thyroid disease: HLA, CTLA-4 and beyond. Clin Exp Immunol 2004; 136:1–10Crossref, Medline, Google Scholar
62. Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, Large DM, Toft AD, McCarthy MI, Kendall-Taylor P, Pearce SH: The cytotoxic T lymphocyte antigen-4 is a major Graves’ disease locus. Hum Mol Genet 1999; 8:1195–119Crossref, Medline, Google Scholar
63. King AL, Ciclitira PJ: Celiac disease: strongly heritable, oligogenic, but genetically complex. Mol Genet Metab 2000; 71:70–75Crossref, Medline, Google Scholar
64. Clot F, Babron MC: Genetics of celiac disease. Mol Genet Metab 2000; 71:76–80Crossref, Medline, Google Scholar
65. Greco L, Corazza G, Babron MC, Clot F, Fulchignoni-Lataud MC, Percopo S, Zavattari P, Bouguerra F, Dib C, Tosi R, Troncone R, Ventura A, Mantavoni W, Magazzu G, Gatti R, Lazzari R, Giunta A, Perri F, Iacono G, Cardi E, de Virgiliis S, Cataldo F, De Angelis G, Musumeci S, Clerget-Darpoux F, et al: Genome search in celiac disease. Am J Hum Genet 1998; 62:669–675Crossref, Medline, Google Scholar
66. Popat S, Bevan S, Braegger CP, Busch A, O’Donoghue D, Falth-Magnusson K, Godkin A, Hogberg L, Holmes G, Hosie KB, Howdle PD, Jenkins H, Jewell D, Johnston S, Kennedy NP, Kumar P, Logan RF, Love AH, Marsh MN, Mulder CJ, Sjoberg K, Stenhammar L, Walker-Smith J, Houlston RS: Genome screening of coeliac disease. J Med Genet 2002; 39:328–331Crossref, Medline, Google Scholar
67. Feighery C: Fortnightly review: coeliac disease. BMJ 1999; 319:236–239Crossref, Medline, Google Scholar
68. Gottenberg JE, Busson M, Loiseau P, Cohen-Solal J, Lepage V, Charron D, Sibilia J, Mariette X: In primary Sjogren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum 2003; 48:2240–2245Crossref, Medline, Google Scholar
69. Chused TM, Kassan SS, Opelz G, Moutsopoulos HM, Terasaki PI: Sjogren’s syndrome association with HLA-Dw3. N Engl J Med 1977; 296:895–897Crossref, Medline, Google Scholar
70. The Genetic Association Database. Bethesda, Md, National Institutes of Health, 2004Google Scholar
71. Zhong F, McCombs CC, Olson JM, Elston RC, Stevens FM, McCarthy CF, Michalski JP: An autosomal screen for genes that predispose to celiac disease in the western counties of Ireland. Nat Genet 1996; 14:329–333Crossref, Medline, Google Scholar
72. Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Webb BT, Zhang J, Walsh D: A potential vulnerability locus for schizophrenia on chromosome 6p24-22: evidence for genetic heterogeneity. Nat Genet 1995; 11:287–293Crossref, Medline, Google Scholar
73. Naar AM, Beaurang PA, Zhou S, Abraham S, Solomon W, Tjian R: Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature 1999; 398:828–832Crossref, Medline, Google Scholar
74. DeLisi LE, Smith AB, Razi K, Stewart J, Wang Z, Sandhu HK, Philibert RA: Investigation of a candidate gene for schizophrenia on Xq13 previously associated with mental retardation and hypothyroidism. Am J Med Genet 2000; 96:398–403Crossref, Medline, Google Scholar
75. Philibert RA, Sandhu HK, Hutton AM, Wang Z, Arndt S, Andreasen NC, Crowe R, Wassink TH: Population-based association analyses of the HOPA12bp polymorphism for schizophrenia and hypothyroidism. Am J Med Genet 2001; 105:130–134Crossref, Medline, Google Scholar
76. Joober R, Benkelfat C, Lal S, Bloom D, Labelle A, Lalonde P, Turecki G, Rozen R, Rouleau GA: Association between the methylenetetrahydrofolate reductase 677C—>T missense mutation and schizophrenia. Mol Psychiatry 2000; 5:323–326Crossref, Medline, Google Scholar
77. Urano W, Taniguchi A, Yamanaka H, Tanaka E, Nakajima H, Matsuda Y, Akama H, Kitamura Y, Kamatani N: Polymorphisms in the methylenetetrahydrofolate reductase gene were associated with both the efficacy and the toxicity of methotrexate used for the treatment of rheumatoid arthritis, as evidenced by single locus and haplotype analyses. Pharmacogenetics 2002; 12:183–190Crossref, Medline, Google Scholar
78. Meisenzahl EM, Rujescu D, Kirner A, Giegling I, Kathmann N, Leinsinger G, Maag K, Hegerl U, Hahn K, Möller H-J: Association of an interleukin-1β genetic polymorphism with altered brain structure in patients with schizophrenia. Am J Psychiatry 2001; 158:1316–1319Link, Google Scholar
79. Genevay S, Di Giovine FS, Perneger TV, Silvestri T, Stingelin S, Duff G, Guerne PA: Association of interleukin-4 and interleukin-1β gene variants with Larsen score progression in rheumatoid arthritis. Arthritis Rheum 2002; 47:303–309Crossref, Medline, Google Scholar
80. Jun TY, Pae CU, Chae JH, Bahk WM, Kim KS, Han H: Polymorphism of CTLA-4 gene at position 49 of exon 1 may be associated with schizophrenia in the Korean population. Psychiatry Res 2002; 110:19–25Crossref, Medline, Google Scholar
81. Chiavetto LB, Boin F, Zanardini R, Popoli M, Michelato A, Bignotti S, Tura GB, Gennarelli M: Association between promoter polymorphic haplotypes of interleukin-10 gene and schizophrenia. Biol Psychiatry 2002; 51:480–484Crossref, Medline, Google Scholar
82. Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M: Association between -G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry 2001; 6:79–82Crossref, Medline, Google Scholar
83. Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E: Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect 1999; 107(suppl 5):681-686Google Scholar
84. Hafner H, an der Heiden W, Behrens S, Gattaz WF, Hambrecht M, Loffler W, Maurer K, Munk-Jorgensen P, Nowotny B, Riecher-Rossler A, Stein A: Causes and consequences of the gender difference in age at onset of schizophrenia. Schizophr Bull 1998; 24:99–113Crossref, Medline, Google Scholar



