Anticraving Medications for Relapse Prevention: A Possible New Class of Psychoactive Medications
Abstract
Psychiatrists have gradually developed a list of medications that are effective in the treatment of addictive disorders. Although alcoholism has received the most attention, nicotine, heroin, and cocaine have all been shown to be influenced by heredity. Of course, the immediate goal is the reduction of drug craving and the prevention of relapse to compulsive drug taking. A medication that can aid in the maintenance of the opiate-free state is naltrexone, a specific opiate antagonist. Naltrexone is also a good example of an anticraving medication used in the treatment of alcoholism. Clinicians currently have two types of medication to aid in the treatment of tobacco use disorder, arguably the most important addiction. Bupropion and nicotine replacement can be given in a coordinated fashion to provide the best available results. At present, no medication is approved by the Food and Drug Administration for the indication of cocaine addiction. Recently, however, five different medications, already approved for other purposes, have been found to be effective among cocaine addicts. Despite clinical trials that show benefit, anticraving medications are not well known and are underused by clinicians. Addiction is a heterogeneous condition, with variability in reactivity to the drug of abuse and to the medications available to treat it. Recent developments in pharmacogenetics may result in improved selection of medications based on genotype.
All psychiatrists are familiar with antipsychotic, antianxiety, and antidepressant medications, whose origins date to the 1950s. We who have been in the field since the early days of psychoactive medications will recall that there were real questions in the 1960s about whether these medications were specific treatments or just sedating agents. More recently, we have come to rely on medications that are effective against bipolar disorder, phobias, posttraumatic stress disorder, and obsessive-compulsive disorder. Although most psychiatrists would now agree on the specificity of these medications, it took time and multiple randomized, controlled trials for clinicians to become convinced.
Over the past 25 years, we have gradually developed a list of medications that have been found in randomized, controlled trials to be effective in the treatment of addictive disorders. The immediate goal is the reduction of drug craving and the prevention of relapse to compulsive drug taking. The number of such medications is increasing, with an additional one (acamprosate) approved by the Food and Drug Administration (FDA) in 2004 joining buprenorphine, which became available to clinicians in 2002. Still, many psychiatrists are not aware that medications are effective in the treatment of addictive disorders. Some still subscribe to the adage, “You can’t treat a drug problem with a drug.”
Detoxification of drug-dependent patients is noncontroversial, time-limited, and easy to understand. The patient is simply given a medication in the same category as the drug of dependence, and withdrawal symptoms are blocked. Then the medication can be gradually reduced at a rate determined by the half-life of the drug of dependence. Evidence has not been presented to show that long-term outcome after very rapid detoxification under general anesthesia is more effective than detoxification over days (1). Thus, opiate withdrawal symptoms can be treated by methadone or buprenorphine, and nicotine withdrawal can be treated with a nicotine patch. Another approach to opiate withdrawal is to block brain pathways involved in producing the symptoms with a medication such as clonidine or lofexidine (2). Lofexidine is not yet available in the United States, but it is widely used in the United Kingdom as a nonopiate medication for opiate withdrawal.
Addiction, however, is a chronic condition, and the changes in brain pathways created by years of drug use do not revert to normal when the body is cleansed of the drug. Thus, long-term treatment beyond detoxification is usually necessary. Because of the chronic nature of addiction, with relapses and remissions and a strong genetic component, we have compared it to other chronic diseases, such as diabetes and hypertension (3).
Relapse-Prevention Psychotherapies
Although the focus of this section is medications, I must state that medications are most effective in the context of psychotherapy or counseling. A variety of psychotherapeutic and behavioral techniques have been developed for the prevention of relapse (4, 5). Still, even the best behavioral programs, aided by self-help movements such as Alcoholics Anonymous, have a high rate of relapse. This article will not review the extensive literature on manualized relapse-prevention psychotherapy approaches, including supportive expressive, cognitive behavior, motivation enhancement, and others (e.g., references 6, 7). It is important to note, however, that none of these psychotherapeutic approaches conflicts in any way with the use of medication to further reduce the probability of relapse. Indeed, the course of addiction can be so devastating that the therapist should take advantage of all available means to improve the likelihood of a successful outcome for the patient. Unfortunately, medications are sometimes disparaged as a “crutch,” and the patient is encouraged to remain totally “drug free.”
Medications to Prevent Relapse
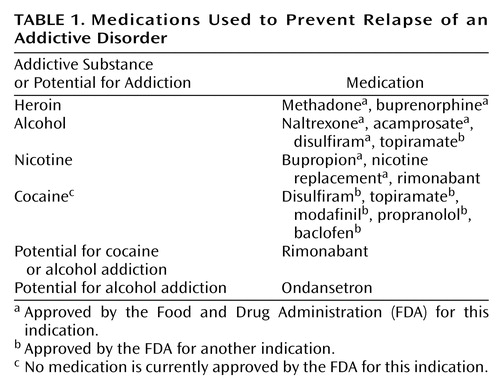
There is now a range of medications available for each of the major classes of addictive drugs: alcohol, opiates, stimulants, and nicotine. In each case, there is evidence that, in combination with psychotherapy or counseling, they can reduce the likelihood of relapse. A list of such medications is shown in Table 1.
Opiate Addiction
Since relapse after a period of abstinence is so common, one approach to treatment has been to simply maintain the patient with a medication in the same pharmacological category as the prior drug of dependence. This strategy has been found to be very effective for opiate addiction, where maintenance with methadone or buprenorphine can enable the former addict to function normally with little or no drug craving. One can argue that the opiate addict has a deficiency in the endogenous opioid system such that he or she feels uncomfortable without the help of prescribed opioids. In this model, methadone is analogous to prednisone for adrenal insufficiency or thyroxine for thyroid insufficiency. Although it is not yet possible to measure the integrity of the endogenous opioid system as we can for the adrenal or thyroid systems, there are clinical findings that support this scenario. Perhaps the best data are the numbers of former heroin addicts who are able to lead successful lives while being maintained with methadone for years, yet they cannot function when their medication is discontinued.
Of course, there are former opiate addicts who can maintain a stable, opiate-free existence. A medication that can aid in the maintenance of the opiate-free state is naltrexone, a specific opiate antagonist. Although it seems paradoxical in view of the analogy with endocrine disorders, there are patients who can live comfortably for years taking a medication that blocks opiate receptors. If a former addict “slips” and takes a dose of heroin or other opiates while taking maintenance naltrexone, the effects will be neutralized. Many report no craving while taking naltrexone, possibly because they learn that opiates are simply unavailable because of the receptor blockade (8). There is no aversive consequence to taking heroin, simply a lack of pleasurable effect. Of course, oral naltrexone has been poorly accepted by typical heroin addicts, although it has been very useful in the treatment of physicians and other medical personnel with opiate addiction. There is growing acceptance of naltrexone for the prevention of relapse after release from prison (9), and the recent development of depot naltrexone preparations (10, 11) suggests that this medication may yet have a larger impact on the treatment of opiate addiction.
Other Addictions
Although maintenance on an agonist works well for opiate dependence, the analogy has not been found useful for dependence on nicotine, alcohol, or stimulants. The first step in the treatment of these addictions is detoxification, which can be facilitated by appropriate medications. Thus, a nicotine patch, nicotine nasal spray, or nicotine gum reduces withdrawal symptoms in smokers, benzodiazepines reliably block alcohol withdrawal symptoms, and stimulant withdrawal often needs no medication. The major problem in treatment of all of these conditions is relapse, and this risk of relapse continues for months and years after detoxification. Even patients who perform extremely well during inpatient treatment, exhibiting insight into their drug problems during therapy sessions and a firm intention to avoid all persons, places, and things associated with alcohol or other drugs, usually relapse soon after leaving the program. Thus, aftercare is critical and should continue for at least a year and preferably longer.
The list in Table 1 includes medications not yet approved by the FDA and some that have been approved for other indications but also have had positive results in randomized, clinical trials for addictive disorders. One of the effects often attributed to these drugs is the reduction of the strong urge or desire to take the drug. The word “craving” is usually assigned to this feeling. So far, no medication has been approved for the specific indication of “anticraving,” and indeed, even the concept of craving is controversial. In clinical trials, however, reductions in craving usually predict reduced drug use or maintenance of abstinence. Craving has been measured in a variety of ways, from a 100-mm Likert scale (12) to a more complex questionnaire (13). Some have argued that “craving” is not a useful concept (14), and certainly, no one would claim that drug use never occurs without a conscious craving or that craving always leads to drug use. Some compulsive drug use has an automatic quality without a conscious buildup of desire. Yet most former addicts recognize a state of intense desire for drugs that may be triggered by environmental cues or that appear spontaneously. Despite the controversy, craving has been measured in many clinical trials, and there is at least some evidence to support an anticraving effect for the medications listed in Table 1.
Naltrexone as a Model Anticraving Medication
A good example of an anticraving medication is naltrexone for the treatment of alcoholism. Naltrexone was developed for the treatment of opiate addiction and was only applied to alcoholics because of animal studies showing that alcohol activated the endogenous opioid system (15). It was tested in a series of open trials in the 1980s and subsequently in a randomized, controlled trial in the alcohol program of the Philadelphia Veterans Administration Medical Center (12, 16). After replication at another site (17), the FDA added alcoholism to the indications for naltrexone. It is interesting to note that both of these first two studies measured craving for alcohol, and it was lower in the group randomly assigned to naltrexone (who also drank less alcohol).
In recent years, animal models of addiction relapse have been developed. The animal is allowed to establish consistent drug-taking behavior and then is withdrawn from the drug. After a period of abstinence, it is tested for reinstatement (relapse) of drug taking. Relapse test procedures fall into three categories: stress, conditioned cues, and drug priming. Stress is modeled by small amounts of foot shock that provoke reinstatement of drug taking. Conditioned cues are stimuli (sound, light, and odor) that previously have been paired with drug availability, and drug priming involves giving a small dose of the drug the animal was taking. With these procedures, the animal quickly reinstates the prior level of response to the drug. Medications can be tested in these animal models, and so far, they are proving to be fairly predictive of the response in patients.
Naltrexone is an example of a medication that consistently reduces alcohol consumption in animals. It appears to do so by blocking opiate receptors that modulate the release of dopamine in the reward system, specifically, the nucleus accumbens (18). Alcohol alone increases extracellular dopamine in the nucleus accumbens, but in animals pretreated with naltrexone, the alcohol-induced dopamine increase is blocked. Further evidence of the role of the endogenous opioid system in alcohol reward is that mu receptor knockout mice do not self-administer alcohol (19).
Dopamine has not been measured directly in humans given alcohol, but there are studies that are consistent with the hypothesis that alcohol releases endogenous opioids that are involved in the activation of neurotransmitters, such as dopamine, resulting in the rewarding effects of alcohol. Gianoulakis et al. (20) reported that test doses of alcohol produce significantly larger increases in plasma beta endorphin in nonalcoholic volunteers with a family history of alcoholism than in volunteers with no alcoholism in their family backgrounds. Although this is a pituitary response and is not necessarily reflective of central endogenous opioids, a parallel CNS effect is suggested by reports of naltrexone blockade of the stimulation or a “high” produced by alcohol in volunteers with a family history of alcoholism. Volunteers with no family history of alcoholism did not report stimulation from alcohol (21). Furthermore, during randomized, controlled trials, patients randomly assigned to naltrexone reported that when they slipped and drank alcohol, the expected high was blocked (22, 23). Thus, both animal data and clinical studies support the hypothesis that the endogenous opioid system is involved in the subjective effects of alcohol.
The animal models of relapse indicate that naltrexone blocks cue-induced relapse but is less effective against stress-induced relapse (24). In human alcoholics, most studies have examined the effects of naltrexone or placebo in combination with some form of counseling or psychotherapy in the prevention of relapse to clinically significant drinking. In these studies, relapse could be related to stress, cues, the priming effect of a small dose of alcohol, or other nonspecified factors. A large majority of randomized, controlled trials found that patients who were randomly assigned to naltrexone had significantly fewer relapses than the placebo groups, but the reasons for relapses are usually not known. One study, however, examined the effects of naltrexone on cues for alcohol drinking and found a reduction in the urge to drink in response to alcohol cues (25) and improved outcome at 1 year (26).
Craving is based on subjective reports, and no animal model has yet been developed to study this human phenomenon. There is evidence, however, that the dopamine system can be conditioned so that cues previously associated with drug availability can activate the reward system. This has been reported in humans by using brain imaging (27, 28) and in animals by using microdialysis. An example of the latter is the work of Gonzales and Weiss (18), who demonstrated an increase in dopamine levels in the nucleus accumbens as soon as an animal was placed in a chamber where it expected to have access to alcohol. Naltrexone did not block this conditioned dopamine increase, but it did block the pharmacological increase in dopamine produced by alcohol. In humans during randomized, controlled trials, naltrexone was reported to suppress craving compared to alcoholics given placebo in several studies where craving was measured (e.g., references 12, 17, 29).
One of the most elegant models for studying the effects of a priming dose of alcohol on craving in humans was developed by O’Malley and colleagues (30). Nontreatment-seeking alcoholics who were pretreated with naltrexone or placebo were given a single priming drink of alcohol in the laboratory. Those receiving naltrexone had lower craving levels at baseline, and craving did not significantly change after alcohol priming. The placebo-treated patients reported higher craving at baseline, and craving was significantly increased by the priming drink. The reports of craving correlated well with drinking behavior because alcohol was made available to the participants as part of the experiment. Participants had the choice of more alcoholic drinks or money instead of each drink after the priming drink. The placebo group chose to drink significantly more than the naltrexone group, resulting in a progressively increasing blood alcohol level, whereas the naltrexone group reported less craving during alcohol availability, consumed fewer drinks, and drank them more slowly when they did drink. This human laboratory study was remarkable in that it produced results consistent with both animal models and clinical trials. A human laboratory model for stress-induced relapse has also been reported but has not yet been used to test medications (31).
A major problem with all outpatient treatments of long duration, whatever the diagnosis, is variability of adherence to a medication regimen. Obviously, a medication cannot help if it is not taken regularly. Naltrexone, despite a plasma half-life of only 10–12 hours (including its active metabolite), has activity at mu opiate receptors in the human brain for over 48 hours (32; unpublished 2004 paper by M.E. McCaul et al.). However, activity at delta receptors is less and more variable, and the duration of activity at kappa opiate receptors is unknown. Activity at all three types of receptors may be essential for full clinical benefit (33), and thus, a daily dose of naltrexone is recommended. Several studies have shown efficacy in preventing relapse only if patients not taking naltrexone regularly are excluded (34, 35). Despite heterogeneity among alcoholics and problems of variable medication adherence, the number of randomized, controlled trials finding naltrexone effective in alcoholics greatly outnumbers the negative trials. To address the problem of adherence, a major advance in the clinical usefulness of naltrexone is currently underway with the introduction of depot formulations. These new formulations provide active blood levels for 30–40 days after a single injection (10, 11). FDA approval of one of these formulations is expected within the next 2 years and will greatly facilitate the treatment of both alcoholism and heroin addiction.
Heredity has been found to play a major role in all forms of drug addictions. Although alcoholism has received the most attention, nicotine, heroin, and cocaine all have been shown to be influenced by heredity (36). Genes clearly influence the pharmacological effects of alcohol. The well-known alcohol-flushing reaction has been shown to be mediated by the genes for both alcohol dehydrogenase and aldehyde dehydrogenase (37), and the risk of developing alcoholism varies as expected, according to the severity of the genetically determined flushing after alcohol ingestion. Heredity also influences the behavioral sensitivity to alcohol. A low response to alcohol (tolerance) was found to be more common in the sons of alcoholics, and this tolerance predicted a fourfold increase in the risk of developing alcoholism within 10 years (38). As described, volunteers with a family history of alcoholism also showed a greater beta endorphin response to alcohol (20) and a greater stimulation effect from alcohol (21). In the laboratory study by King et al. (21), naltrexone was found to block stimulation in the volunteers with a family history of alcoholism. These human laboratory findings are consistent with animal laboratory data and data from clinical trials.
Heterogeneity of Response
If we consider the evidence for a genetic component to addictive disorders, it is not surprising that there are a wide variety of responses to anticraving medications. Among alcoholics, there appears to be a subgroup that responds to alcohol with an increase in plasma beta endorphin and probably a similar increase in CNS endogenous opioids that can be neutralized by an opiate receptor antagonist. Although there is no practical way to identify this subcategory at present, analyses of randomized, controlled trials have provided some help to clinicians. Thus, a strongly positive family history of alcoholism points to a poor prognosis for a placebo group but a good response to treatment with naltrexone (39, 40). Similarly, the subgroup of alcoholics that reported a high level of alcohol craving did poorly when they were randomly assigned to placebo but achieved a good response to naltrexone therapy (40). Previous typologies of alcoholism focused on age at onset and clinical characteristics (41–43) have not been studied with respect to naltrexone response.
Obviously, greater precision is desirable in the selection of patients for naltrexone treatment. One potential advance would be to identify alcoholics likely to respond to treatment involving the blockade of opiate receptors. The activation of the endogenous opioid system by alcohol could vary according to the opioid peptides released (44) and according to the sensitivity of the opiate receptors. A number of polymorphisms have been identified for the gene encoding the mu opiate receptor, and one of them, the Asp40 variant, has been found to have high affinity for beta endorphin in vitro (45) and altered function during in vivo studies (46, 47). Alcoholics with this variant who were enrolled in randomized, controlled trials had a poor outcome when they were randomly assigned to placebo but a good outcome when they received naltrexone (48). Of course, there is evidence from animal studies that the blockade of delta and kappa opiate receptors, in addition to mu receptors, is involved in the effects of naltrexone on alcohol drinking (33). Nevertheless, the finding of a genotype that predicts success with naltrexone, if replicated, will provide a pharmacogenetic tool to enhance the matching of patients to treatment and encourage the search for additional functional polymorphisms.
Other Anticraving Medications
Naltrexone is a particularly interesting medication in that its usefulness was discovered in animal models, and there is a remarkable consistency among studies in the preclinical laboratory, the human laboratory, and the clinic. The other medications listed in Table 1 have been the subject of fewer studies but show great promise. They will be described briefly to provide an overview of the rest of the members of the “anticraving” class.
Alcoholism
In addition to naltrexone, the clinician has three other medication options to aid in the treatment of alcoholism. Acamprosate was approved by the FDA for the treatment of alcoholism in 2004. This medication has multiple actions, including a decrease in glutamate release, a decrease in calcium ion influx through voltage-dependent calcium channels, a decrease in N-methyl-d-aspartic acid (NMDA) receptor excitability, and an increased level of taurine, an inhibitory neurotransmitter (49). Because acamprosate’s effects on craving do not directly involve the endogenous opioid system, the addition of naltrexone should provide additive benefit. The combination showed increased efficacy in one alcoholism study in Europe (50), and the combination is currently the subject of a large trial in the United States.
A third option is disulfiram, the first drug that was approved for the prevention of relapse in alcoholism. This is a medication that does not specifically block craving, although, if taken regularly, it may simplify the daily struggle against urges toward alcohol. Disulfiram blocks the enzyme alcohol dehydrogenase so that if the patient takes a drink, acetaldehyde is produced, causing a very unpleasant flushing reaction. In large randomized, controlled trials, disulfiram has not been effective because of the lack of medication adherence (51), but in situations where it can be prescribed with daily monitoring of ingestion, it can be useful.
A fourth option, although only approved by the FDA for seizure disorders, is topiramate. This γ-aminobutyric acid (GABA)-ergic medication has been found in a single randomized, controlled trial to significantly reduce drinking in alcoholics (52). Additional studies are in progress.
One other promising medication, the serotonin 5-HT3 antagonist ondansetron, is not available for prescription as an oral medication. It has FDA approval for the treatment of nausea by the parenteral route, but a randomized, controlled trial with a special formulation for research in alcoholics found a significant reduction in drinking for early-onset alcoholics (53). This early-onset subtype is postulated to have greater involvement of the serotonergic system, with a higher probability of antisocial behavior. Additional clinical trials are indicated.
Nicotine Addiction
Clinicians currently have two types of medication to aid in the treatment of tobacco use disorder, arguably the most important addiction. Bupropion and nicotine replacement can be given in a coordinated fashion to provide the best available results. Bupropion is well known as an antidepressant, but an astute clinician noted that it had a specific effect on nicotine craving (54). It has subsequently been found in randomized, controlled trials to be the single most effective treatment for cigarette smoking. In combination with the nicotine patch, the success rate for smoking cessation has improved. After a week of taking bupropion, a smoker can set a quit date and use nicotine replacement in the form of a patch, gum, or nasal spray to ease withdrawal symptoms.
Additional medications that aid in the treatment of nicotine addiction are in clinical trials. One that is advanced in the FDA approval process is rimonabant. This medication is similar to naltrexone in that it acts specifically by interfering with the function of a physiological system that has widespread but poorly understood normal functions: the endogenous cannabinoid system. Cannabinoid (CB-1) receptors are widely distributed throughout multiple brain systems and, based on animal studies, they play a critical role in memory, motor control, pain perception, and many other functions. Rimonabant is a specific antagonist to CB-1 receptors and has been tested in a variety of animal models. Besides blocking the psychoactive effects of marijuana, this medication has effects in the absence of external marijuana because it blocks endogenous cannabinoids. Under certain conditions, this medication can reduce food intake, alcohol intake, and block reinstatement of cocaine and heroin self-administration (55–57). Randomized, controlled trials have now been completed in smokers and in obese persons. Although the results have not yet been published, they have been presented at meetings and indicate that rimonabant is effective for patients wishing to lose weight and for patients wishing to stop smoking. This drug is advancing through the FDA approval process, and although it is too early to call it an anticraving medication, animal data and early clinical trials point to that classification. Moreover, there is an indication that it will have a general usefulness in reducing appetite for food as well as the craving for a range of drugs, including nicotine, alcohol, cocaine, and heroin.
Cocaine Addiction
There is a very active drug discovery program supported by the National Institute on Drug Abuse to develop medications for cocaine addiction, but there are still no FDA-approved medications for this indication. Animal models have generally not replicated the bingeing pattern displayed by human addicts. Low, intermittent doses in rodents have demonstrated behavioral sensitization. This means that the same dose produces progressively increasing motor activity instead of decreased activity or tolerance. This sensitization is hypothesized to play a major role in compulsive drug seeking in humans (58). In human addicts, there is subjective evidence of tolerance and no clear signs of sensitization, although some have speculated that stimulant psychosis represents a form of sensitization. Since only 16% of those who try cocaine develop addiction (59), perhaps the majority of users experience sensitization as unpleasant and stop using cocaine.
Recently, however, five different medications already approved for other purposes have been found in randomized, controlled trials to be effective among cocaine addicts.
Disulfiram was initially tested by Carroll and colleagues (60) in patients suffering from alcohol abuse and dependence coexisting with cocaine dependence. They discovered, however, that the medication was also helpful to those who met criteria for cocaine addiction alone, with no significant accompanying alcohol problem. The mechanism whereby disulfiram reduces the frequency of cocaine use is unknown, but it does not appear to be related to the prevention of alcohol use. Rather, abusers of cocaine—but not alcohol—showed more benefits from disulfiram than those with a history of combined alcohol/cocaine abuse or dependence. The mechanism for this effect is unknown, but disulfiram is known to increase plasma concentrations of cocaine three- to sixfold (61) and to inhibit dopamine beta hydroxylase (62), thus increasing the effects of cocaine and possibly making cocaine use aversive.
Modafinil, approved for the treatment of narcolepsy, has been found by two groups to reduce the high from cocaine and to reduce cocaine craving (63). After positive results from an open trial, the results from a randomized, controlled trial were also positive (64), and a multiclinic trial is in progress. The mechanism of action of modafinil is unknown, but it does increase glutamate levels in the brain, thus reversing the effect of chronic cocaine use, as measured in animal models (65). Modafinil is very well accepted by patients, who report a reduction of cocaine withdrawal symptoms and a blunting of the effects of cocaine if they begin to relapse while taking the medication. The medication has not been reported to produce euphoria, and there has been no indication of excessive use or abuse in clinical trials (64).
Propranolol, a beta-blocker, was found in a randomized, controlled trial to significantly reduce relapse in cocaine addicts with high cocaine withdrawal scores (66), and confirmatory work is in progress. It was selected for a randomized, controlled trial because of its demonstrated effect on stress-related memories and the hypothesis that this might reduce the strength of cocaine-conditioned relapse cues.
A final category of anticraving relapse-prevention medications to consider is that of GABA-enhancing medications. GABA is the principal inhibitory neurotransmitter in the brain, and these drugs were originally developed for the treatment of seizure disorders. Subsequently, they were shown to be beneficial as mood stabilizers. More recently, they have been found to have benefit in the treatment of addictive disorders. Topiramate (already mentioned) was reported to reduce relapse in alcoholics (52) and in cocaine addicts (67). A limiting side effect of this medication is the production of reversible cognitive impairment, necessitating very slow titration of the drug to therapeutic levels. At present, topiramate is the subject of additional clinical trials, and it is prescribed cautiously by some clinicians for selected cases of cocaine or alcohol dependence.
An even more potent medication for augmenting the GABA system is vigabatrin, an inhibitor of GABA transaminase. This results in very high levels of GABA that makes it effective for control of infantile spasms but produces visual scotomata in up to 30% of the patients receiving the medication. In animal models, it was very effective as an anticraving relapse-prevention medication (68) and in a preliminary study in human cocaine addicts (69). However, vigabatrin trials have been suspended in the United States because of concerns over problems with the visual field. This category of medications is very promising, however, and research is underway to find nontoxic medications that increase GABA and reduce drug craving. The GABA B agonist baclofen has also been reported to reduce cocaine use (70), and it is now the subject of a multiclinic trial.
Summary
The major problem facing clinicians who treat patients with addictive disorders is relapse. Addiction is a chronic disorder that requires long-term treatment, and in recent years, medications have been developed that add to the benefits of psychosocial interventions for the prevention of relapse. These medications generally reduce drug craving and reduce the likelihood of relapse to compulsive drug use. Animal models have been useful in the development of these medications, and there is consistency among the results of animal models, human laboratory tests, and clinical trials. Naltrexone is an example of a medication that blocks opiate receptors and was developed as a treatment for heroin addiction. Through animal models, it was discovered to have a role in the treatment of one subcategory of alcoholism. Another line of research has discovered a role for medications that affect the endogenous cannabinoid system. Other examples of medications both currently available and still in development are discussed in this review.
Despite clinical trials that show benefit, anticraving medications are not well known and are underused by clinicians. Addiction is a heterogeneous condition with variability in reactivity to the drug of abuse and to the medications available to treat it. Recent developments in pharmacogenetics may result in improved selection of medications based on genotype.
 |
Presented in part at the 157th annual meeting of the American Psychiatric Association, New York, May 1–6, 2004. Received Sept. 8, 2004; revision received Nov. 10, 2004; accepted Dec. 3, 2004. From the University of Pennsylvania/Philadelphia VA Medical Center. Address correspondence and reprint requests to Dr. O’Brien, University of Pennsylvania, Treatment Research Center, 3900 Chestnut St., Rm. 6178, Philadelphia, PA 19104-6178; [email protected] (e-mail). Supported by NIH grants DA-P60-05186 and DA-P50-12756 from the National Institute on Drug Abuse. Dr. O’Brien is a consultant to Alkermes Inc., Forest Laboratory, and Drug Abuse Sciences, Ltd.
1. Lawental E: Ultra rapid opiate detoxification as compared to a 30-day inpatient detoxification program—a retrospective follow-up study. J Subst Abuse 2000; 11:173–181Crossref, Medline, Google Scholar
2. Gerra G, Zaimovic A, Giusti F, Di Gennaro C, Zambelli U, Gardini S, Delsignore R: Lofexidine versus clonidine in rapid opiate detoxification. J Subst Abuse Treat 2001; 21:11–17Crossref, Medline, Google Scholar
3. McLellan AT, Lewis DC, O’Brien CP, Kleber HD: Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000; 284:1689–1695Crossref, Medline, Google Scholar
4. Carroll KM, Rounsaville BJ, Gawin FH: A comparative trial of psychotherapies for ambulatory cocaine abusers: relapse prevention and interpersonal psychotherapy. Am J Drug Alcohol Abuse 1991; 17:229–247Crossref, Medline, Google Scholar
5. Volpicelli JR, Pettinati HM, McLellan AT, O’Brien CP (eds): Combining Medication and Psychosocial Treatments for Addiction—The Brenda Approach. New York, Guilford, 2001Google Scholar
6. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol 1997; 58:7–29Crossref, Medline, Google Scholar
7. Crits-Christoph PL, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody G, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck S: Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry 1999; 56:493–502Crossref, Medline, Google Scholar
8. Meyer R, Mirin S: The Heroin Stimulus. New York, Plenum, 1979Google Scholar
9. Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, O’Brien CP: Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat 1997; 14:529–534Crossref, Medline, Google Scholar
10. Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW (Vivitrex Study Group): Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 2005; 293:1617–1625Crossref, Medline, Google Scholar
11. Kranzler HR, Wesson DR, Billot L (Drug Abuse Sciences Naltrexone Depot Study Group): Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res 2004; 28:1051–1059Crossref, Medline, Google Scholar
12. Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP: Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 1992; 49:876–880Crossref, Medline, Google Scholar
13. Anton RF, Moak DH, Latham PK: The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry 1996; 53:225–231Crossref, Medline, Google Scholar
14. Tiffany ST: Cognitive concepts of craving. Alcohol Res Health 1999; 23:215–224Medline, Google Scholar
15. Altshuler HL, Phillips PA, Feinhandler DA: Alteration of ethanol self-administration by naltrexone. Life Sci 1980; 26:679–688Crossref, Medline, Google Scholar
16. Volpicelli JR, O’Brien CP, Alterman AI, Hayashida M: Naltrexone and the treatment of alcohol dependence: initial observations, in Opioids, Bulimia, Alcohol Abuse and Alcoholism. Edited by Reid LB. New York, Springer-Verlag, 1990, pp 195–214Google Scholar
17. O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B: Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry 1992; 49:881–887Crossref, Medline, Google Scholar
18. Gonzales RA, Weiss F: Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 1998; 18:10663–10671Crossref, Medline, Google Scholar
19. Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH: mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther 2000; 293:1002–1008Medline, Google Scholar
20. Gianoulakis CB, Krishnan B, Thavundayil J: Enhanced sensitivity of pituitary-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry 1996; 53:250–257Crossref, Medline, Google Scholar
21. King AC, Volpicelli JR, Frazer A, O’Brien CP: Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997; 129:15–22Crossref, Medline, Google Scholar
22. Volpicelli JR, Clay KL, Watson NT, O’Brien CP: Naltrexone in the treatment of alcoholism: predicting response to naltrexone. J Clin Psychiatry 1995; 56:39–44Medline, Google Scholar
23. O’Malley SS, Jaffe AJ, Rode S, Rounsaville BJ: Experience of a “slip” among alcoholics treated with naltrexone or placebo. Am J Psychiatry 1996; 153:281–283Link, Google Scholar
24. Liu X, Weiss F: Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 2002; 22:7856–7861Crossref, Medline, Google Scholar
25. Rohsenow DJ, Colby SM, Monti PM, Swift RM, Martin RA, Mueller TI, Gordon A, Eaton CA: Predictors of compliance with naltrexone among alcoholics. Alcohol Clin Exp Res 2000; 24:1542–1549Crossref, Medline, Google Scholar
26. Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK: Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res 2001; 25:1634–1647Crossref, Medline, Google Scholar
27. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP: Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156:11–18Link, Google Scholar
28. Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Flor H, Braus DF, Buchholz HG, Gründer G, Schreckenberger M, Smolka MN, Rösch F, Mann K, Bartenstein P: Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 2004; 161:1783–1789; correction, 161:2344Link, Google Scholar
29. Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK: Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 1999; 156:1758–1764Abstract, Google Scholar
30. O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J: Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002; 160:19–29Crossref, Medline, Google Scholar
31. Sinha R, Catapano D, O’Malley SA: Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999; 142:343–351Crossref, Medline, Google Scholar
32. Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF: Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 1988; 29:1207–1211Medline, Google Scholar
33. Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP: A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol 1998; 15:281–289Crossref, Medline, Google Scholar
34. Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP: Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry 1997; 54:737–742Crossref, Medline, Google Scholar
35. Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B: A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol 2000; 35:587–593Crossref, Medline, Google Scholar
36. Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L: Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet 1996; 67:473–477Crossref, Medline, Google Scholar
37. Higuchi S, Matsushita S, Muramatsu T, Murayama M, Hayashida M: Alcohol and aldehyde dehydrogenase genotypes and drinking behavior in Japanese. Alcohol Clin Exp Res 1996; 20:493–497Crossref, Medline, Google Scholar
38. Schuckit MA: Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 1994; 151:184–189Link, Google Scholar
39. Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O’Malley SS: Naltrexone, relapse prevention, and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol 1996; 64:1044–1053Crossref, Medline, Google Scholar
40. Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, Volpicelli JR: Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict 2001; 10:258–268Crossref, Medline, Google Scholar
41. Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B: Types of alcoholics, I: evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry 1992; 49:599–608Crossref, Medline, Google Scholar
42. Bohman M, Cloninger R, Sigvardsson S, von Knorring AL: The genetics of alcoholisms and related disorders: J Psychiatr Res 1987; 21:447–452Google Scholar
43. Lesch OM, Walter H: Subtypes of alcoholism and their role in therapy. Alcohol Alcohol 1996; 31:63–67Crossref, Medline, Google Scholar
44. Gianoulakis C: Influence of the endogenous opioid system on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci 2001; 26:304–318Medline, Google Scholar
45. Bond C, LaForge K, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L: Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA 1998; 95:9608–9613Crossref, Medline, Google Scholar
46. Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A: The mu-opioid receptor gene polymorphism (All8G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology 2002; 26:106–114Crossref, Medline, Google Scholar
47. Hernandez-Avila C, Wand G, Luo X, Gelernter J, Kranzler HR: Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1). Am J Med Genet B Neuropsychiatr Genet 2003; 118:60–65Crossref, Google Scholar
48. Oslin DW, Berrettini W, Kranzler H, Pettinati HM, Gelernter J, Volpicelli J, O’Brien CP: A functional polymorphism of the mu opioid receptor gene is associated with naltrexone response in alcohol dependent patients. Neuropsychopharmacology 2003; 28:1546–1552Crossref, Medline, Google Scholar
49. Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J: The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology 2001; 40:749–760Crossref, Medline, Google Scholar
50. Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Widemann K: Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2003; 60:92–99Crossref, Medline, Google Scholar
51. Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I: Disulfiram treatment of alcoholism: a Veterans Administration Cooperation Study. JAMA 1986; 256:1449–1455Crossref, Medline, Google Scholar
52. Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ: Oral topiramate for treatment of alcohol dependence: a randomized controlled trial. Lancet 2003; 361:1666–1667Crossref, Medline, Google Scholar
53. Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J: Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled study. JAMA 2000; 284:963–971Crossref, Medline, Google Scholar
54. Ferry LH, Pettis JL, Loma L, Burchette RJ: Evaluation of bupropion versus placebo for treatment of nicotine dependence, in 1994 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, pp 199–200Google Scholar
55. De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN: A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 2001; 7:1151–1154Crossref, Medline, Google Scholar
56. Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA: Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003; 167:103–111Crossref, Medline, Google Scholar
57. Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G: Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997; 132:104–106Crossref, Medline, Google Scholar
58. Robinson TE, Berridge KC: Incentive sensitization and addiction. Addiction 2001; 96:103–114Crossref, Medline, Google Scholar
59. Anthony JC, Warner LA, Kessler RC: Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Experimental Clin Psychopharmacol 1994; 2:244–268Crossref, Google Scholar
60. Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ: Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 2004; 61:264–272Crossref, Medline, Google Scholar
61. McCance-Katz EF, Kosten TR, Jatlow P: Disulfiram effects on acute cocaine administration. Drug Alcohol Depend 1998; 52:27–39Crossref, Medline, Google Scholar
62. Vaccari A, Saba PL, Ruiu S, Collu M, Devoto P: Disulfiram and diethyldithiocarbamate intoxication affects the storage and release of striatal dopamine. Toxicol Appl Pharmacol 1996; 139:102–108Crossref, Medline, Google Scholar
63. Dackis CA, O’Brien CP: Glutamatergic agents for cocaine dependence. Ann NY Acad Sci 2003; 1003:1–18Crossref, Medline, Google Scholar
64. Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP: A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005; 30:205–211Crossref, Medline, Google Scholar
65. Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D: Glutamate transmission and addiction to cocaine. Ann NY Acad Sci 2003; 1003:169–175Crossref, Medline, Google Scholar
66. Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta R, Luce D, O’Brien CP: Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend 2002:63:69–78Google Scholar
67. Kampman KM, Pettinati HM, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP: A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend 2004; 75:233–240Crossref, Medline, Google Scholar
68. Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP, Dewey SL: The effect of gamma-vinyl-GABA on the consumption of concurrently available oral cocaine and ethanol in the rat. Pharmacol Biochem Behav 2001; 68:291–299Crossref, Medline, Google Scholar
69. Brodie JD, Figueroa E, Dewey SL: Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse 2003; 50:261–265Crossref, Medline, Google Scholar
70. Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W: Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry 2003; 64:1440–1448Crossref, Medline, Google Scholar



