Additive Effects of HIV and Chronic Methamphetamine Use on Brain Metabolite Abnormalities
Abstract
OBJECTIVE: Proton magnetic resonance spectroscopy (1H-MRS) showed decreased neuronal marker N-acetylaspartate and increased glial marker myo-inositol in subjects with chronic methamphetamine use and in subjects infected with HIV. The authors sought to determine whether HIV and a history of chronic methamphetamine use might have additive or interactive effects on brain metabolite abnormalities. METHOD: 1H-MRS was performed in 68 HIV-positive subjects (24 with a history of chronic methamphetamine use with a lifetime exposure of a mean of 2,167 g [SD=2,788] and last use a mean of 4.9 months earlier [SD=6.0]; 44 with no history of drug abuse) and 75 HIV-negative subjects (36 with a history of chronic methamphetamine use with a lifetime exposure of a mean of 8,241 g [SD=16,850] and last use a mean of 6.3 months earlier [SD=7.8]; 39 with no history of drug abuse). Concentrations of N-acetylaspartate, creatine, choline, and myo-inositol were measured in the frontal cortex, frontal white matter, and basal ganglia. RESULTS: HIV-negative subjects with a history of chronic methamphetamine use showed lower concentrations of the neuronal marker N-acetylaspartate in the frontal white matter and basal ganglia and higher concentrations of choline compounds and the glial marker myo-inositol in the frontal cortex, relative to subjects with no history of drug abuse. HIV-positive status was associated with lower concentrations of N-acetylaspartate and creatine in the frontal cortex and higher concentrations of myo-inositol in the white matter, compared with HIV-negative status. Compared to the mean concentrations of metabolites in HIV-negative subjects with no history of drug abuse, the mean concentrations in subjects with HIV and chronic methamphetamine use showed additive effects on N-acetylaspartate in all three regions (–9% in the basal ganglia, –7% in the frontal white matter, and –6% in the frontal gray matter), on creatine in the basal ganglia (–7%), and on myo-inositol in the frontal white matter (+11%). CONCLUSIONS: The combined effects of HIV and chronic methamphetamine use were consistent with an additive model, suggesting additional neuronal injury and glial activation due to the comorbid conditions.
Methamphetamine, a psychostimulant with reinforcing effects similar to those of cocaine (1), has reemerged as a major drug of abuse worldwide and is commonly used among HIV-infected individuals. In Thailand, both inhalation and injection of methamphetamine are associated with higher risk for HIV infection (2). Similarly, studies and large surveys in the United States (California and New York) consistently found that even noninjection methamphetamine use contributes to higher rates of unprotected sexual activity in both men and women and to the risk of contracting sexually transmitted diseases, including HIV (3, 4). Conversely, the rate of stimulant use is four times higher in men who have sex with men (who are at high risk for HIV), compared to heterosexual men, according to the U.S. National Household Survey on Drug Abuse (5). Therefore, HIV and use of methamphetamine have become a “double epidemic” over the past decade (4).
Like individuals with HIV-associated dementia, methamphetamine users have been reported to have slower reaction times, poorer decision-making abilities, and poorer performance on measures of memory, attention, and concentration, compared to non-drug users (6, 7). A postmortem study (8) and positron emission tomography (PET) studies (9, 10) consistently found lower concentrations of dopamine transporter in methamphetamine users, compared to subjects with no history of methamphetamine use, but the differences in dopamine transporter levels were smaller (20%–30%) than those observed in animals treated with methamphetamine (>50%) or in patients with Parkinson’s disease (36%–71%) (11). Although methamphetamine users typically do not have clinically evident extrapyramidal signs, one study found them to have slower performance on computerized tests that measure psychomotor speed and working memory, relative to age- and gender-matched comparison subjects (12). The lower concentrations of dopamine transporter measured with PET were also associated with slower motor function (timed gait) and poorer recall (Auditory Verbal Learning Test scores) (10). Because HIV viral burden is often highest in the basal ganglia, where the highest density of dopaminergic terminals is located, it is reasonable to hypothesize that the toxic effects of methamphetamine on the dopaminergic terminals may exacerbate HIV-associated brain injury in the basal ganglia.
Proton magnetic resonance spectroscopy (1H-MRS) documented lower concentrations of N-acetyl compounds (N-acetylaspartate) and total creatine in the basal ganglia (13, 14) and higher concentrations of choline compounds and myo-inositol in the frontal cortex in abstinent methamphetamine-dependent subjects (13), compared with healthy non-drug users. These findings suggested long-term neuronal damage (with lower concentrations of N-acetylaspartate) and glial abnormalities (with higher concentrations of myo-inositol and choline compounds) in abstinent methamphetamine users. Numerous studies in patients with HIV dementia also found lower concentrations of the neuronal marker N-acetylaspartate, especially in those with moderate to severe dementia, and higher concentrations of the glial marker myo-inositol or choline compounds that are related to dementia severity (15, 16). Therefore, 1H-MRS may provide useful surrogate markers to assess the combined effects of methamphetamine and HIV on the brain.
To determine whether HIV and methamphetamine have an additive effect on brain metabolite abnormalities, we performed 1H-MRS in four groups of subjects: HIV-positive subjects with no history of drug dependence, HIV-positive subjects with a history of methamphetamine dependence or abuse, HIV-negative subjects with a history of methamphetamine dependence or abuse, and HIV-negative subjects with no history of drug dependence. We hypothesized that 1) neurochemical abnormalities, particularly lower concentrations of N-acetyl compounds (N-acetylaspartate), would be more evident in HIV-positive subjects with a history of chronic methamphetamine use, compared to subjects with either HIV or chronic methamphetamine use alone, and 2) that the basal ganglia brain region would be affected most severely (lowest N-acetylaspartate concentrations), because this region contains the highest concentrations of dopaminergic nerve terminals.
Method
Subjects
Sixty-eight patients seropositive for HIV (24 with a history of methamphetamine dependence or abuse and 44 with no history of methamphetamine dependence or abuse) and 75 HIV-negative subjects (36 with a history of methamphetamine dependence or abuse and 39 with no history of methamphetamine dependence or abuse) were assessed. The subjects with methamphetamine dependence or abuse were recruited from several local drug rehabilitation centers, and the HIV-positive subjects were recruited from local HIV clinics and the community. The HIV-negative subjects were recruited from the same local communities by advertisement and through word of mouth; some were friends or relatives of subjects already enrolled. Before the study, each subject was verbally informed of the study protocol and signed a consent form approved by the researchers’ institution. Each subject was evaluated with a detailed medical and neuropsychiatric history, screening blood tests, and urine toxicology screens. The screening blood tests included routine chemistry profiles, routine blood count, thyroid panel, and HIV test, if the subject’s serostatus was unknown. In addition, the HIV-positive subjects were evaluated with syphilis serology, CD4 count, and plasma viral load measurements. Each subject’s history of methamphetamine and other drug use was recorded. On the day of the 1H-MRS scan, Karnofsky Performance Scale (17) and HIV Dementia Scale (18) ratings were obtained and the AIDS dementia complex stage was assessed in each HIV-positive subject.
On the basis of the screening evaluation, HIV-positive subjects (with or without a history of chronic methamphetamine use) were enrolled if they met the following inclusion criteria: 1) age 18–65 years; 2) AIDS dementia complex stage ≤2, diagnosed according to the Memorial Sloan Kettering system (19); 3) CD4 count <500/mm3; and 4) ability to provide consent and willingness to participate in the study. Methamphetamine users (with or without HIV) needed to fulfill the following criteria: 1) history of methamphetamine dependence or abuse according to the DSM-IV criteria; 2) regular methamphetamine use for at least 12 months, at least 2 days/week; 3) intake of at least 0.25 g of methamphetamine per day; 4) use of methamphetamine as the primary drug of abuse; 5) last methamphetamine use more than 1 week before enrollment; and 6) HIV-negative status for subjects in the group with a history of chronic methamphetamine use only. HIV-negative comparison subjects with no history of methamphetamine dependence or abuse had to be 1) healthy individuals age 18–65 years, 2) seronegative for HIV within the past 6 months, and 3) taking no medications except for vitamins. Subjects who met any of the following criteria were excluded: 1) on the day of the scans, a positive urine toxicology screen for illicit drugs (cocaine, marijuana, benzodiazepine, barbiturates, and opiates), except for amphetamines in the methamphetamine users; 2) history of head trauma with loss of consciousness >30 minutes; 3) history of alcohol or other substance dependence, except for methamphetamine dependence or abuse (for the methamphetamine user groups) and nicotine dependence (daily marijuana users were also excluded); 4) chronic medical, neurological, or psychiatric illnesses (e.g., seizure disorders, depression, schizophrenia, infarcts, tumors, hypertension, or diabetes) other than HIV (for the HIV groups); 5) opportunistic focal brain lesions in the HIV-positive subjects; 6) pregnancy; or 7) ferromagnetic implants or other contraindications for magnetic resonance imaging (MRI) studies.
Proton Magnetic Resonance Spectroscopy (1H-MRS)
Subjects were studied with MRI and localized 1H-MRS by using a 1.5-T scanner (GE Medical Systems, Waukesha, Wis.). The imaging protocol consisted of 1) a sagittal T1-weighted localizer (TE/TR=11/500 msec, 4-mm slice thickness, 1-mm gap), 2) an axial fast inversion recovery scan (TE/TI/TR=32/120/4000 msec, 3.5-mm contiguous slices), and 3) a coronal fast spin echo scan (TE/TR=102/4000 msec, 5-mm contiguous slices). 1H-MRS data were acquired in three brain regions (voxels): the mid-frontal gray matter, right frontal white matter, and right basal ganglia (including the caudate and putamen) (Figure 1). Voxel sizes ranged between 3 and 5 cm3, depending on the subjects’ anatomy. The voxel sizes and water line widths were not significantly different between the four subject groups. Data were acquired by using an optimized point resolved spectroscopy sequence (20). The acquisition parameters were TE/TR=30/3000 msec; 64 averages were acquired. Metabolite concentrations were determined with a well-validated technique (21, 22) that used the brain water signal as an internal reference and corrected for the CSF in each voxel; brain water and CSF signals were calculated by least-squares fitting from the amplitude of the unsuppressed water signal at 10 different echo times (21). The data were processed with a semiautomatic program (21, 22) that yielded metabolite concentrations in institutional units that were converted to molar concentrations (mmol/kg) with prior measurements (22). Typical interindividual variation in the metabolite concentrations was about 10%, and intrasubject variation ranged from 3% to 8%.
Statistical analyses were performed in StatView (SAS Institute Inc., Cary, N.C.). Two-way analysis of variance (ANOVA) was performed with the data for each metabolite, with methamphetamine use history and HIV serostatus as the two factors. In addition, analysis of covariance (ANCOVA) (with adjustment for age and education) was performed with the metabolite data that showed age-dependent or education-dependent effects. For variables that showed significant ANOVA main effects of HIV serostatus, methamphetamine use history, or interactions between the two, post hoc tests were performed with Fisher’s protected least significant difference (PLSD) test. A type I error probability of ≤0.05 was used to determine statistical significance.
Results
Clinical and Demographic Variables
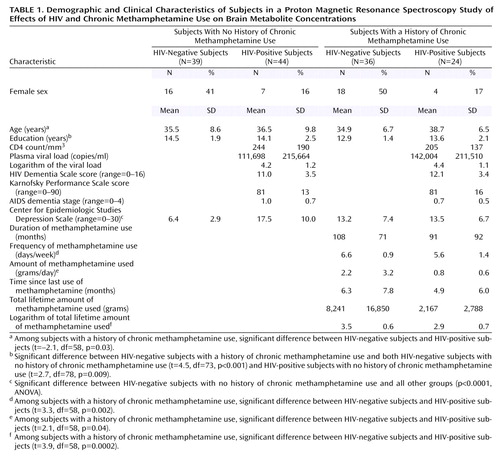
Table 1 summarizes the demographic characteristics and clinical variables related to HIV disease severity or methamphetamine use in the respective subject groups. No significant difference in age was found between the groups. The two HIV-positive groups included fewer female subjects, compared to the two HIV-negative groups. However, the two HIV-positive groups had similar HIV disease severity, as measured by CD4 count, plasma viral loads, Karnofsky Performance Scale scores, and AIDS dementia stage. The HIV-negative subjects with no history of chronic methamphetamine use had significantly lower scores for depressive symptoms, as measured by the Center for Epidemiologic Studies Depression Scale, compared to the other three groups (p<0.0001, Fisher’s PSLD test). A significant difference in educational level was found between groups (p=0.002, ANOVA); the difference was due to the lower level of education in the HIV-negative subjects with a history of chronic methamphetamine use, compared to the two groups with no history of chronic methamphetamine use, but the educational levels of two groups with a methamphetamine use history were not significantly different.
Methamphetamine and Other Drug Use
The two groups with a history of chronic methamphetamine use had similar durations of methamphetamine use. However, because the HIV-positive subjects with a methamphetamine use history used the drug in smaller amounts (t=2.1, df=58, p=0.04) and at a lower frequency (t=3.3, df=58, p=0.002) than the HIV-negative subjects, their total lifetime methamphetamine intake was also lower (log transformed t=3.9, df=58, p=0.0002). Significantly greater nicotine use (p≤0.001, Fisher’s PLSD test) was observed in both groups with a history of chronic methamphetamine use (HIV-negative subjects: mean=14.6 pack-years, SD=15; HIV-positive subjects: mean=13.5 pack-years, SD=12), compared to the non-drug-user groups (HIV-negative subjects: mean=3.8 pack-years, SD=10; HIV-positive subjects: mean=4.7 pack-years, SD=9). Although subjects with a history of other drug dependence, including alcohol dependence, were excluded, 20 (83%) HIV-positive subjects with a history of chronic methamphetamine use, 16 (44%) HIV-negative subjects with a history of chronic methamphetamine use, and four (9%) HIV-positive subjects with no history of chronic methamphetamine use also admitted to past recreational or occasional use of cocaine. In addition, 20 (55%) HIV-negative subjects with a history of chronic methamphetamine use, 13 (54%) HIV-positive subjects with a history of chronic methamphetamine use, 18 (41%) HIV-positive subjects with no history of methamphetamine use, and 11 (28%) HIV-negative subjects with no history of methamphetamine use also used marijuana occasionally. The amount of alcohol used was less than 7 drinks/week in all subjects except two HIV-negative subjects with a history of chronic methamphetamine use. All subjects with a history of chronic methamphetamine use indicated that methamphetamine was their primary drug of abuse and their drug of choice and that their use of other drugs was minimal, compared to the use of methamphetamine.
Group Differences in Brain Metabolites
HIV infection (independent of methamphetamine use), compared with seronegative status, was associated with mildly lower concentrations of N-acetylaspartate in the frontal gray matter (–5.3% difference in concentration; F=11.1, df=1, 121, p=0.001) and basal ganglia (–5.0%; F=8.6, df=1, 128, p=0.004) but not in the frontal white matter (–2.6%; F=2.2, df=1, 128, p=0.10), mildly lower concentrations of creatine in the basal ganglia (–3.7%; F=4.6, df=1, 127, p=0.03) and the frontal gray matter (–3.9%; F=4.7, df=1, 121, p=0.03), and higher concentrations of choline compounds (7.1%; F=9.3, df=1, 129, p=0.003) and myo-inositol (7.1%; F=6.1, df=1, 129, p=0.01) in the frontal white matter. In contrast, a history of chronic methamphetamine use (independent of HIV serostatus), compared with no history of chronic methamphetamine use, was associated with a mildly lower concentration of N-acetylaspartate in the basal ganglia (–3.5% difference in concentration; F=4.6, df=1, 128, p=0.03), lower concentrations (approaching significance) of creatine in the basal ganglia (–3.1%; F=3.3, df=1, 127, p=0.07) and of N-acetylaspartate in the frontal white matter (–3.8%; F=2.7, df=1, 128, p=0.1), higher concentrations of choline compounds in both the frontal white matter (4.5%; F=4.9, df=1, 129, p=0.03) and the frontal gray matter (9.8%; F=7.2, df=1, 121, p=0.008), and a higher concentration of myo-inositol in the frontal gray matter (9.0%; F=7.0, df=1, 120, p=0.009). ANOVA showed no significant interactions on any of the 1H-MRS variables.
Because the concentrations of several metabolites showed education effects (basal ganglia choline compounds, frontal white matter choline compounds, and basal ganglia myo-inositol) and age effects (frontal white matter choline compounds and basal ganglia myo-inositol), we adjusted for these variables in further analyses. After adjustments for age and education with ANCOVA, the higher concentration of frontal white matter choline compounds initially observed in the HIV-positive subjects and the subjects with a history of chronic methamphetamine use was no longer significant; however, the other results were unaffected. The greater proportion of men in the HIV-positive groups might have further contributed to the higher concentration of frontal white matter choline compounds in those groups, as the concentration of choline compounds was 7% higher in men than in women among the HIV-negative subjects with no history of chronic methamphetamine use (p=0.08, Fisher’s PLSD test).
The combined effects of HIV infection and chronic methamphetamine use were generally consistent with an additive model. As a result, metabolite abnormalities commonly were greatest in HIV-positive subjects with a history of chronic methamphetamine use (Figure 2). The synergistic effect was particularly pronounced for the neuronal marker N-acetylaspartate, in that HIV-positive drug users consistently had the lowest concentration of N-acetylaspartate of all four subject groups. Post hoc test findings for the HIV-positive methamphetamine users, compared to the HIV-negative subjects with no history of chronic methamphetamine use, showed lower concentrations of N-acetylaspartate in the frontal white matter (–6.1% difference in concentration; t=2.3, df=57, p=0.02), the frontal gray matter (–5.7%; t=2.2, df=60, p=0.03), and the basal ganglia (–9%; t=4.2, df=60, p=0.0001). In addition, the HIV-positive subjects with a history of chronic methamphetamine use had the lowest concentration of creatine in the basal ganglia (–7.4%; t=2.8, df=60, p=0.007) and the highest concentrations of choline compounds (14.5%; t=3.5, df=57, p=0.0009) and myo-inositol (12.2%; t=2.4, df=56, p=0.02) in the frontal white matter. However, the higher concentration of frontal white matter choline compounds was no longer significant after adjustment for age and sex differences between the groups.
Correlations Between Metabolite Concentrations, Drug Use, and HIV Disease Severity
Metabolite concentrations did not correlate with methamphetamine use variables (frequency of use, amount used, duration of use, or cumulative dose) or with other drug use variables (pack-years of cigarettes, cumulative lifetime amount of alcoholic drinks, lifetime amount of cocaine, or lifetime joints of marijuana). Most of the HIV disease severity measures correlated with 1H-MRS metabolite concentrations. CD4 count correlated with concentrations of choline compounds in the frontal gray matter (r=–0.38, p=0.006, N=56), choline compounds in the frontal white matter (r=–0.26, p=0.05, N=56), myo-inositol in the frontal gray matter (r=–0.41, p=0.003, N=51) and myo-inositol in the frontal white matter (r=0.55, p<0.0001, N=56). The correlation of the logarithm of the viral load with the concentration of N-acetylaspartate in the basal ganglia approached significance (r=–0.25, p=0.08, N=50), and Karnofsky Performance Scale scores correlated inversely with concentrations of choline compounds in all three brain regions (frontal cortex: r=–0.48, p=0.0003, N=52; frontal white matter: r=–0.34, p=0.01, N=55; basal ganglia: r=–0.33, p=0.01, N=57), as well as inversely with concentrations of myo-inositol in all three brain regions (frontal cortex: r=–0.56, p<0.0001, N=51; frontal white matter: r=–0.35, p=0.009, N=55; basal ganglia: r=–0.3, p=0.03, N=56). In addition, AIDS dementia stage correlated with concentrations of creatine in the frontal white matter (r=0.27, p=0.04, N=60), myo-inositol in the basal ganglia (r=0.33, p=0.01, N=60), and myo-inositol in the frontal gray matter (r=0.36, p=0.009, N=51). These clinical variables, however, did not correlate with any of the self-reported drug use variables.
Discussion
This study shows that the injurious effects of HIV and chronic methamphetamine use on the brain are generally additive, with respect to both neuronal and glial 1H-MRS markers. First, the HIV-positive subjects with a history of chronic methamphetamine use had the lowest concentration of N-acetylaspartate in all three brain regions studied, compared to the other three subject groups; this finding suggests significant neuronal loss or dysfunction in these brain regions. As predicted, the region most affected was the basal ganglia, which has the highest density of dopaminergic nerve terminals. Although a lower concentration of N-acetylaspartate was detected in a group of subjects with methamphetamine use alone (13), prior 1H-MRS studies typically detected minimal or no differences in N-acetylaspartate concentration in HIV patients who had mild dementia or were neuroasymptomatic (16, 23). In the current study, the HIV-positive subjects with and those without a history of chronic methamphetamine use both had mild dementia; therefore, any differences in N-acetylaspartate concentrations in those groups, compared with the HIV-negative subjects, should have been minimal. However, the N-acetylaspartate concentration in the HIV-positive subjects with a history of chronic methamphetamine use was almost twofold smaller than that in subjects with HIV or chronic methamphetamine use alone, despite similar dementia severity in the HIV-positive subjects with and without a history of chronic methamphetamine use and the lower level of methamphetamine use in the HIV-positive subjects, compared with the HIV-negative subjects. Possibly because of the variability in the dementia status of the HIV-positive subjects, however, the difference between the HIV-positive subjects with no history of chronic methamphetamine use and those with a history of chronic methamphetamine use did not reach statistical significance. We also found a lower concentration of creatine in the basal ganglia in the HIV-positive subjects with a history of chronic methamphetamine use, which parallels the finding of a lower concentration of N-acetylaspartate and is also consistent with possible neuronal injury, because creatine is present in both neurons and glia. The findings for N-acetylaspartate in the HIV-positive subjects with a history of chronic methamphetamine use are consistent with reports from a prior, but preliminary 1H-MRS study in which lower concentrations of N-acetylaspartate only and no differences in other metabolites were found in the frontal lobes of HIV-infected and stimulant-dependent individuals, compared to subjects with either condition alone (24). In that report, however, the stimulant drug(s) and the amounts used were not specified and the number of subjects was substantially smaller (three to seven subjects per group) than in the current study.
Neuropathological evidence for neuronal loss or injury is available only from studies performed in subjects with either methamphetamine use only or HIV only. A postmortem study of relatively young methamphetamine users found lower concentrations of dopamine nerve terminal markers (dopamine, tyrosine hydroxylase, and the dopamine transporter) and normal levels of dopa decarboxylase and vesicular monoamine transporter in the striatum (8). In HIV-infected brains, neuronal apoptosis and consequent neuronal loss are well described (25). Therefore, combined neuronal injury related to methamphetamine use and HIV, as observed in additive effects on N-acetylaspartate concentration, would be expected.
Although the exact mechanisms of brain injury underlying the interaction between HIV and methamphetamine are unclear, HIV-1 Tat protein and methamphetamine synergistically interacted to reduce striatal dopaminergic function (up to a fivefold reduction) in an in vivo rodent model (26). One possible pathway to the combined neurotoxicity might be a methamphetamine-induced increase in extracellular dopamine, which in turn would activate HIV replication. Dopamine can increase viral replication in HIV-infected T-lymphoblasts (27), and dopaminergic agents (selegiline and l-dopa) might enhance CNS pathology and viral replication, as shown in a simian immunodeficiency virus model (28). Methamphetamine itself also increased replication of feline immunodeficiency virus in cultured feline astrocytes (29). Likewise, cocaine, which was abused occasionally by the majority of the HIV-positive subjects with a history of chronic methamphetamine use in the current study, might further enhance HIV replication (30), although cocaine’s direct effect on N-acetylaspartate would be minimal (31). Thus, the intermittently increased viral burden in the combined condition might exacerbate brain injury.
Data regarding the combined effects of HIV and psychostimulant abuse on the dopaminergic system in humans are extremely limited. Because many antiretrovirals are potent inhibitors of cytochrome P450 systems, HIV patients taking protease inhibitors have experienced prolonged effects of psychostimulants, fatal interactions with 3,4-methylenedioxymethamphetamine (32), and possible fatal interactions with methamphetamine (33). In a case report, an HIV-positive psychostimulant (methamphetamine and cocaine) user showed rapid decline of cognitive function and an unusual movement disorder; only the motor disorder responded to dopamine replacement (34). No human studies have specifically evaluated the interactive effects of HIV and chronic methamphetamine use on the dopaminergic system.
In this study, the HIV-negative subjects with a history of chronic methamphetamine use (who had an average of 6 months of abstinence) had higher concentrations of choline compounds and myo-inositol in the frontal cortex (gray matter). These findings suggest an inflammatory response, or glial activation, because these metabolites are found in much higher concentrations in glial cells than in neurons (35), and are consistent with findings of acute methamphetamine-induced glial activation in rodents (36) and monkeys (37), as well as findings of chronic effects in monkeys up to 1.5 years after methamphetamine exposure (37). Methamphetamine, like HIV, has been reported to stimulate the production of the inflammatory cytokine, tumor necrosis factor α (38), and to increase binding and activation of the redox-responsive transcription factors, AP-1 and NF-kappaB (39). These factors may be associated with the inflammatory findings (higher concentrations of choline compounds and myo-inositol) observed with 1H-MRS.
In the HIV-positive subjects with a history of chronic methamphetamine use in the current study, the frontal white matter showed additive effects of higher concentrations of the glial metabolite myo-inositol, compared to the subjects with either HIV or chronic methamphetamine use alone. This finding suggests a synergistic effect of HIV and methamphetamine on reactive glial processes in the frontal white matter, as higher concentrations of glial metabolites were observed both in the current study and in prior 1H-MRS studies of HIV patients (16) and of abstinent methamphetamine-dependent subjects (13). Microglial activation (40) and neuronal apoptosis (41) in HIV encephalitis, as well as the effects of the synergistic interaction between drug use (primarily opiates) and advanced HIV infection on inflammatory infiltrates (40) and microglial activation (42), have been well documented. However, little is known about how the HIV-infected brain responds to chronic methamphetamine use. Because excessive levels of dopamine related to chronic methamphetamine use may stimulate HIV replication (27), increased concentrations of inflammatory glial markers may be a response to the intermittently higher levels of viral burden in the HIV-positive subjects with a history of chronic methamphetamine use, as well as a direct effect of methamphetamine use. In the basal ganglia, however, no differences between study groups in concentrations of choline compounds or myo-inositol were observed. These findings suggest the lack of inflammatory or repair responses in the basal ganglia, which might lead to the lower concentration of N-acetylaspartate.
Preclinical studies indicate that methamphetamine affects the dopaminergic system, causing massive release of dopamine acutely, whereas chronic methamphetamine abusers showed down-regulation of dopamine transporters and postsynaptic D2 receptors, as observed during early abstinence (10, 43). However, significant recovery of both the dopamine transporter and D2 receptors occurs in protracted abstinence in nonhuman primates (37) and in humans (43). This recovery may be mediated by activated macrophages and more so by microglia, which express brain-derived neurotrophic factor mRNA to stimulate dopaminergic sprouting, as shown in response to striatal injury in a lesioned mouse model (44). Both macrophages and microglia quickly accumulated near the wound site after striatal injury and may persist for the long term (44). Therefore, subjects with a history of chronic methamphetamine use may demonstrate an increased glial response even during protracted abstinence. The current study found higher concentrations of glial metabolites primarily in the frontal cortex of HIV-negative subjects with a history of chronic methamphetamine use and in the frontal white matter of the HIV-positive subjects with a history of chronic methamphetamine use.
These findings are similar to those of some preclinical MRS studies. An ex vivo 1H-MRS study that evaluated the effects of feline immunodeficiency virus and methamphetamine in the brain found that higher concentrations of choline compounds in the frontal white matter and higher levels of γ-aminobutyric acid were associated with methamphetamine, but lower concentrations of choline compounds were associated with feline immunodeficiency virus, and no additive effects resulting in lower concentrations of N-acetylaspartate were associated with both methamphetamine and feline immunodeficiency virus (45). Lower levels of glutamate were found in one ex vivo MRS study of feline immunodeficiency virus (45), but higher levels were found in another (46). However, the two studies differed in viral strain, route of infection, time of infection, and possibly differences in the brain region(s) that were measured. Such animal models can measure the exact doses of drugs used and the duration of HIV infection, which are difficult to assess precisely in humans. This lack of precision might be one reason we did not find correlations between 1H-MRS metabolite levels and self-reported drug use. However, most preclinical studies cannot model the longer duration of HIV infection or methamphetamine exposure in humans. Furthermore, because the metabolism of methamphetamine is significantly different among species and because there are differences in pathogenesis between the lentiviruses in the preclinical models (feline and simian immunodeficiency virus) and HIV, studies such as the current study are needed to assess the combined effects of methamphetamine and HIV in humans.
Postmortem human studies are also valuable, but the ability to study a large group of subjects in postmortem studies is extremely limited, and much of the in vivo pathophysiology may not be evident or may be altered postmortem. This study demonstrates that 1H-MRS is a robust and sensitive method for assessing in vivo pathophysiology. Future studies using other noninvasive neuroimaging techniques, such as dopamine markers on PET and functional MRI, may provide further insights into the in vivo pathophysiological changes associated with the combined effects of HIV and methamphetamine and may help to determine whether treatment with medications or cognitive behavior therapy alters brain function and pathology. The current findings of metabolite abnormalities support an additive effect of HIV and methamphetamine on brain injury, especially in the striatal and frontal brain regions that have the highest density of dopaminergic nerve terminals.
 |
Received Oct. 1. 2003; revision received Jan. 6. 2004; accepted April 13, 2004. From the Department of Medicine, John A. Burns School of Medicine, University of Hawaii; the Department of Radiology, University of Freiburg, Freiburg, Germany; and Harbor-UCLA Medical Center, Torrance, Calif. Address correspondence and reprint requests to Dr. Chang, Department of Medicine, John A. Burns School of Medicine, University of Hawaii, 1356 Lusitana St., 7th Floor, Honolulu, HI 96813; [email protected] (e-mail). Supported in part by NIH grants K20-DA-00280, K24-DA-16170, R01-NS-38834, and MO1-RR-00425. The authors thank the HIV patients for volunteering for this study, Dr. Mallory Witt for referring patients to the study, Dr. M. Leonido-Yee for subject recruitment, and the staff of the Harbor-UCLA Imaging Center for technical support.
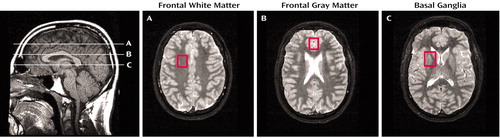
Figure 1. Sagittal T1-Weighted MRI Scan Showing the Locations of Center Slices and Axial Inversion Recovery Images Showing Voxels in Three Brain Regions Examined With Proton Magnetic Resonance Spectroscopy
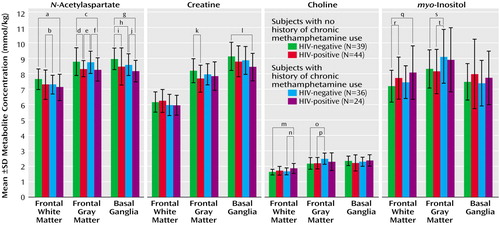
Figure 2. Concentrations of N-Acetylaspartate, Creatine, Choline, and myo-Inositol in Three Brain Regions in HIV-Positive and HIV-Negative Subjects With and Without a History of Chronic Methamphetamine Use
aSignificant –6% difference between groups (t=2.37, df=57, p=0.02).
bSignificant –4% difference between groups (t=1.92, df=70, p=0.05).
cSignificant –6% difference between groups (t=2.21, df=60, p=0.03).
dSignificant –5% difference between groups (t=2.39, df=66, p=0.02).
eSignificant –5% difference between groups (t=2.59, df=57, p=0.01).
fSignificant –5% difference between groups (t=2.35, df=57, p=0.02).
gSignificant –9% difference between groups (t=4.16, df=60, p=0.0001).
hSignificant –4% difference between groups (t=2.21, df=70, p=0.03).
iSignificant –6% difference between groups (t=2.23, df=75, p=0.02).
jSignificant –5% difference between groups (t=2.11, df=55, p=0.04).
kSignificant –6% difference between groups (t=2.51, df=66, p=0.01).
lSignificant –7% difference between groups (t=2.80, df=60, p=0.007).
mSignificant 15% difference between groups (t=3.51, df=57, p=0.0009).
nSignificant 11% difference between groups (t=2.45, df=56, p<0.02).
oSignificant 14% difference between groups (t=3.49, df=72, p=0.0008).
pSignificant 14% difference between groups (t=3.20, df=63, p=0.002).
qSignificant 12% difference between groups (t=2.41, df=56, p=0.02).
rSignificant 8% difference between groups (t=1.96, df=75, p=0.05).
sSignificant 10% difference between groups (t=2.27, df=72, p=0.02).
tSignificant 10% difference between groups (t=2.37, df=62, p=0.02).
1. Peltier R, Li D, Lytle D, Taylor C, Emmett-Oglesby M: Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther 1996; 277:212–218Medline, Google Scholar
2. Vongsheree S, Sri-Ngam P, Ruchusatsawat N, Thaisri H, Puangtabtim W, Phutiprawan T, Sawanpanyalert P: High HIV-1 prevalence among methamphetamine users in central Thailand, 1999–2000. J Med Assoc Thai 2001; 84:1263–1267Medline, Google Scholar
3. Molitor F, Ruiz J, Flynn N, Mikanda J, Sun R, Anderson R: Methamphetamine use and sexual and injection risk behaviors among out-of-treatment injection drug users. Am J Drug Alcohol Abuse 1999; 25:475–493Crossref, Medline, Google Scholar
4. Halkitis P, Parsons J, Stirratt M: A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex 2001; 41:17–35Crossref, Medline, Google Scholar
5. Woody GE, VanEtten-Lee ML, McKirnan D, Donnell D, Metzger D, Seage G III, Gross M (HIVNET VPS 001 Protocol Team): Substance use among men who have sex with men: comparison with a national household survey. J Acquir Immune Defic Syndr 2001; 21:86–90Crossref, Google Scholar
6. McKetin R, Mattick R: Attention and memory in illicit amphetamine users: comparison with non-drug-using controls. Drug Alcohol Depend 1998; 50:181–184Crossref, Medline, Google Scholar
7. Simon S, Domier C, Sim T, Richardson K, Rawson R, Ling W: Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis 2002; 21:61–74Crossref, Medline, Google Scholar
8. Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ: Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996; 2:699–703Crossref, Medline, Google Scholar
9. McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA: Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography. J Neurosci 1998; 18:8417–8422Crossref, Medline, Google Scholar
10. Volkow N, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 2001; 158:377–382Link, Google Scholar
11. Frost J, Rosier A, Reich S, Smith J, Ehlers M, Snyder S, Ravert H, Dannals R: Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson’s disease. Ann Neurol 1993:423–431Google Scholar
12. Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller E: Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Res 2002; 114:65–79Crossref, Medline, Google Scholar
13. Ernst T, Chang L, Leonido-Yee M, Speck O: Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 2000; 54:1344–1349Crossref, Medline, Google Scholar
14. Sekine Y, Minabe Y, Kawai M, Suzuki K, Iyo M, Isoda H, Sakahara H, Ashby CJ, Takei N, Mori N: Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms: a proton MRS study. Neuropsychopharmacology 2002; 27:453–461Crossref, Medline, Google Scholar
15. Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G: Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology 1993; 43:509–515Crossref, Medline, Google Scholar
16. Chang L, Ernst T, Witt M, Ames N, Jocivich J, Speck O, Gaiefsky M, Walot I, Miller E: Relationships among cerebral metabolites, cognitive function and viral loads in antiretroviral-naive HIV patients. Neuroimage 2002; 17:1638–1648Crossref, Medline, Google Scholar
17. Karnofsky DA, Burchenal, JH: The clinical evaluation of chemotherapeutic agents in cancer, in Evaluation of Chemotherapeutic Agents. Edited by MacLeod CM. New York, Columbia University Press, 1949, pp 191–205Google Scholar
18. Power C, Selnes OA, Grim JA, McArthur JC: HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8:273–278Crossref, Medline, Google Scholar
19. Aronow HA, Brew BJ, Price RW: The management of the neurological complications of HIV infection and AIDS. AIDS 1988; 2:151–159Crossref, Medline, Google Scholar
20. Ernst T, Chang L: Elimination of artifacts in short echo time 1H MR spectroscopy of the frontal lobe. Magn Reson Med 1996; 36:462–468Crossref, Medline, Google Scholar
21. Ernst T, Kreis R, Ross BD: Absolute quantitation of water and metabolites in the human brain, part I: compartments and water. J Magn Reson 1993; 102:1–8Crossref, Google Scholar
22. Kreis R, Ernst T, Ross BD: Absolute quantitation of water and metabolites in the human brain, part II: metabolite concentrations. J Magn Reson 1993; 102:9–19Crossref, Google Scholar
23. Meyerhoff D, Bloomer C, Cardenas V, Norman D, Weiner M, Fein G: Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV plus patients. Neurology 1999; 52:995–1003Crossref, Medline, Google Scholar
24. Taylor M, Alhassoon O, Schweinsburg B, Videen J, Grant I (HNRC [HIV Neurobehavioral Research Center] Group, HIV Neurobehavioral Research Center): MR spectroscopy in HIV and stimulant dependence. J Int Neuropsychol Soc 2000; 6:83–85Crossref, Medline, Google Scholar
25. Gray F, Adle-Biassette H, Brion F, Ereau T, le Maner I, Levy V, Corcket G: Neuronal apoptosis in human immunodeficiency virus infection. J Neurovirol 2000; 6(suppl 1):38–43Google Scholar
26. Maragos W, Young K, Turchan J, Guseva M, Pauly J, Nath A, Cass W: Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem 2002; 83:955–963Crossref, Medline, Google Scholar
27. Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E: Dopamine activates HIV in chronically infected T lymphoblasts. J Neural Transm 2000; 107:1483–1489Crossref, Medline, Google Scholar
28. Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller J, Pedersen V, Gsell W, Heeney J, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V: Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol (Berl) 2001; 101:85–91Medline, Google Scholar
29. Gavrilin M, Mathes L, Podell M: Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol 2002; 8:240–249Crossref, Medline, Google Scholar
30. Roth M, Tashkin D, Choi R, Jamieson B, Zack J, Baldwin G: Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis 2002; 185:701–705Crossref, Medline, Google Scholar
31. Chang L, Ernst T, Strickland T, Mehringer CM: Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry 1999; 156:716–722Abstract, Google Scholar
32. Henry JA, Hill IR: Fatal interaction between ritonavir and MDMA (letter). Lancet 1998; 352:1751–1752Crossref, Medline, Google Scholar
33. Hales G, Roth N, Smith D: Possible fatal interaction between protease inhibitors and methamphetamine (letter). Antivir Ther 2000; 5:19Medline, Google Scholar
34. Nath A, Maragos W, Avison M, Schmitt F, Berger J: Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol 2001; 7:66–71Crossref, Medline, Google Scholar
35. Brand A, Richter-Landsberg C, Leibfritz D: Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15:289–298Crossref, Medline, Google Scholar
36. Hebert M, O’Callaghan J: Protein phosphorylation cascades associated with methamphetamine-induced glial activation. Ann NY Acad Sci 2000; 914:238–262Crossref, Medline, Google Scholar
37. Harvey D, Lacan G, Tanious S, Melega W: Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 2000; 871:259–270Crossref, Medline, Google Scholar
38. Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT: Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 2002; 31(suppl 2):62–69Google Scholar
39. Lee Y, Hennig B, Yao J, Toborek M: Methamphetamine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells. J Neurosci Res 2001; 66:583–591Crossref, Medline, Google Scholar
40. Bell J, Brettle R, Chiswick A, Simmonds P: HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS: effect of neocortical involvement. Brain 1998; 121(part 11):2043–2052Google Scholar
41. Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F: Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 1999; 25:123–133Crossref, Medline, Google Scholar
42. Arango J, Simmonds P, Brettle RP, Bell JE: Dose drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS 2004; 18(suppl 1): S69-S74Google Scholar
43. Volkow N, Chang L, Wang G-J, Fowler J, Franceschi D, Sedler M, Gatley S, Miller E, Hitzemann R, Ding Y-S, Logan J: Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 2001; 21:9414–9418Crossref, Medline, Google Scholar
44. Batchelor P, Liberatore G, Wong J, Porritt M, Frerichs F, Donnan G, Howells D: Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci 1999; 19:1708–1716Crossref, Medline, Google Scholar
45. Cloak CC, Chang L, Ernst T, Barr MC, Huitron-Resendiz S, Sanchez-Alavez M, Phillips TR, Henriksen S: Methamphetamine and AIDS: 1HMRS studies in a feline model of human disease. J Neuroimmunol 2004; 147:16–20Crossref, Medline, Google Scholar
46. Power C, Moench T, Peeling J, Kong PA, Langelier T: Feline immunodeficiency virus causes increased glutamate levels and neuronal loss in brain. Neuroscience 1997; 77:1175–1185Crossref, Medline, Google Scholar



