Functional Neuroanatomy of Successful Paired Associate Learning in Alzheimer’s Disease
Abstract
OBJECTIVE: The purpose of the study was to develop a strategy for functional imaging of neurodegenerative disorders that overcomes confounds associated with differential performance between patient and comparison groups. METHOD: Functional magnetic resonance imaging was used to examine responses to increasing difficulty of visuospatial paired associate learning in 12 patients with mild probable Alzheimer’s disease and 12 age-matched healthy comparison subjects. Performance was matched across groups by only examining successful encoding and retrieval attempts. Adjustment for task difficulty was made on an individual basis so that the patients with Alzheimer’s disease and the comparison subjects performed at the same relative levels of difficulty. RESULTS: A network of lateral and medial frontoparietal and occipital regions was engaged in all subjects during successful associative learning. As task difficulty increased, blood-oxygen-level-dependent responses increased linearly in occipitoparietal regions during encoding and retrieval. Differential activations in patients with Alzheimer’s disease and comparison subjects were small and were found only when an uncorrected statistical threshold was used. CONCLUSIONS: By controlling for confounds of varying task difficulty and subsequent performance, remarkably similar brain activations were identified during successful paired associate learning in patients with Alzheimer’s disease and in healthy comparison subjects. The study methods provide a useful model for further applications of functional imaging involving cognitive activation paradigms in the study of neuropsychiatric disorders.
A major limitation of functional activation imaging studies in Alzheimer’s disease has been failure to control for differences in levels of task performance and effort across groups of patients and healthy subjects. Little consideration is typically given to such potential confounds, with differences in brain activity between patient and comparison groups often being interpreted as a reflection of intrinsic cognitive deficits. However, there is no reason to assume that the same cognitive processes are in operation when a task is performed at an 80% success level, compared to a 20% success level. Accordingly, brain activity under two such conditions may reflect fundamentally different cognitive operations.
Unfortunately, to date, most functional imaging studies of Alzheimer’s disease have failed to control for the effects of performance and task difficulty. For example, increased hippocampal activation was found in comparison subjects, relative to patients with Alzheimer’s disease, during encoding of color pictures (1). Subsequent mean correct picture recognition scores, however, were 63% in the comparison subjects and only 13% in the patients with Alzheimer’s disease. A similar study found greater medial temporal lobe activity during encoding of photographs in comparison subjects than in patients with Alzheimer’s disease, accompanied by significantly better performance in comparison subjects on measures of free recall (6.1 of 12 versus 1.75 of 12) and recognition (96.1% versus 85.7%) (2). During a task involving novel versus familiar face-name encoding, patients with Alzheimer’s disease were found to additionally activate the left precuneus, fusiform gyrus, posterior cingulate, and dorsolateral prefrontal cortex and bilateral middle temporal gyrus, while the comparison subjects showed greater activation within the right hippocampal formation (3). However, the comparison subjects were found to correctly recognize and name more faces than the patients with Alzheimer’s disease (face recognition: 78% versus 60%; name recall: 40% versus 12%). Finally, different networks of brain activity underlying semantic and episodic memory have been identified in patients with Alzheimer’s disease, relative to comparison subjects (4). However, patients with Alzheimer’s disease performed less well than the comparison subjects on the semantic and episodic tasks, with approximately one-fourth of the patients scoring below the score expected by chance. In all of these studies, variations in performance between the patient and comparison groups mean that observed functional differences could just as likely represent the effects of patients’ working harder at the tasks (because of greater subjective task difficulty) or performing less well than healthy comparison subjects.
One strategy to overcome nonequivalent task-related difficulty and performance involves manipulation of task difficulty such that performance is matched at an individual level. Although some studies have attempted to examine or control for performance differences between younger and older adults (5–7), this adjustment has rarely been attempted in Alzheimer’s disease imaging studies. In a study in which this adjustment was made by manipulating task difficulty so that both patients and comparison subjects achieved 75% accuracy, different brain networks were found to be active during serial verbal recognition learning in patients with Alzheimer’s disease, relative to the comparison subjects (8). Manipulation of task difficulty across groups in order to control for performance by using a task more sensitive to Alzheimer’s disease has yet to be tried, however.
In the current study, a visuospatial paired associate learning task was used, because this type of learning is known to be impaired early in the course of Alzheimer’s disease (9), and was combined with the experimental control of performance success and relative task difficulty across groups. We predicted that the patients with Alzheimer’s disease and comparison subjects would display similar functional responses during encoding and retrieval of object-location pairs if task performance were matched across groups. We further predicted that brain regions demonstrating linear and nonlinear relationships between task difficulty and the blood-oxygen-level-dependent (BOLD) response would be located in similar areas to those identified in our previous visuospatial paired associate learning study (10).
Method
Subjects
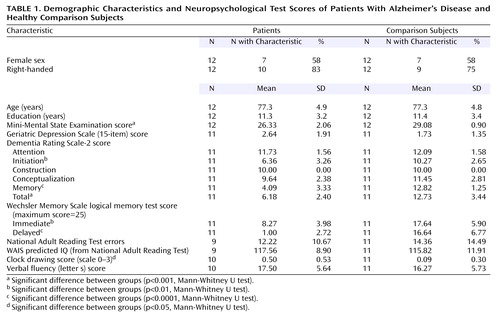
Twelve patients who met the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (11) for mild probable Alzheimer’s disease and 12 age-matched healthy comparison subjects were recruited. Seven patients were receiving treatment with cholinesterase inhibitors. Subjects were screened for concomitant serious medical diagnoses and previous psychiatric history and were assessed with a comprehensive battery of neuropsychological tests, including the Mini-Mental State Examination, Geriatric Depression Scale (12), Dementia Rating Scale-2 (13), logical memory test from the Wechsler Memory Scale (14), and National Adult Reading Test (15) (Table 1). All subjects provided written informed consent before participating in the study. The study had been approved by the Research Ethics Committee of the South London and Maudsley Trust.
Materials and Procedure
During the visuospatial paired associate learning task, subjects were required to remember the locations of objects that appeared on a computer screen (Figure 1). An object appeared in a white box for 5 seconds and was replaced after 500 msec by another object in a different location until all of the objects in that problem were presented (encoding phase). During encoding subjects heard the instruction “remember.” After 6 seconds, a previously presented object appeared in one of the boxes with the question “Was this here?” (retrieval phase). Subjects were required to make a recognition decision (yes/no) by pressing one of two response keys. If the subject made no response, then the object remained on the screen for 5 seconds. If a response was made, the object disappeared and all locations remained blank until 5 seconds had passed. After 500 msec another object was presented, and the process continued until all objects presented during encoding had been tested. At the end of each retrieval phase, there was a baseline rest period (8 seconds if unsuccessful, 11 seconds if successful or if five failed attempts had been made) before the next encoding phase. After an incorrect attempt, the same object-location pairings were repeated in a different order in the next attempt at the problem. This procedure continued until there was correct identification of all object-locations, or until five successive attempts had been failed, after which subjects were presented with new object-location pairings.
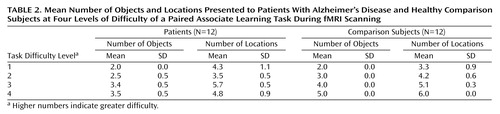
Before scanning, all subjects received off-line testing to identify individual levels of increasing difficulty that could be successfully performed in the scanner. Difficulty was manipulated by varying the number of objects and locations in each problem (Table 2). Problems were pseudorandomly presented at each difficulty level so that no more than two problems at the same level appeared consecutively. The comparison subjects completed three practice sessions, which took place 3–4 days before the first scan, 1 hour before the first scan, and 1 hour before the second scan. The patients with Alzheimer’s disease completed five practice sessions at 6–7 days, 3–4 days, and 1 hour before the first scan and at 3–4 days and 1 hour before the second scan. After scanning, the subjects rated the subjective difficulty at each level of the task.
Image Acquisition
Data were acquired on the 1.5-T General Electric Neuro-optimized Signa LX Horizon system (General Electric, Milwaukee) at the Maudsley Hospital. In each functional series, 150 T2*-weighted images depicting BOLD contrast were acquired by using an interleaved echo planar sequence at 16 axial slices (TR=2000 msec, TE=40 msec, flip angle=90°, matrix=64×64, field of view=240 mm2, slice thickness=7 mm, interslice distance=0.7 mm). Subjects received eight to 10 functional series in two scanning sessions (separated by 1 week), except for two patients who received five series in one session. A high-resolution, three-dimensional axial image that included the whole brain was also collected for each subject (TE=5.8 msec, TR=17.1 msec, flip angle=20°, matrix=256×256, thickness=1.5 mm).
Data Analyses
Two-way mixed analyses of variance with task difficulty as a within-subjects factor and group as a between-subjects factor were used to assess behavioral data. Mean response times to incorrect trials at each subject’s hardest level of difficulty were submitted to an independent samples t test. Neuropsychological test performance was assessed by using Mann-Whitney U tests.
By using statistical parametric mapping (16), functional data were slice-timing corrected, realigned to the first echo-planar imaging volume and unwarped to correct for motion-related variance, coregistered to the high resolution T1-weighted image, normalized by using affine transformations into standard space (17) based on the Montreal Neurological Institute reference brain, and spatially smoothed with an 8 mm full width at half maximum Gaussian kernel (18). Data were then parametrically analyzed to identify regions displaying differential responses to increasing task difficulty. Within the general linear model, task difficulty was regressed onto the BOLD response to the onset of stimuli, and data were modeled with an epoch design convolved with a canonical hemodynamic response function. Encoding epochs were calculated from the first object’s presentation onset to the last object’s offset, and retrieval epochs were calculated from the first object’s presentation onset to the last object’s response time onset (or presentation offset if no response was made). Epochs corresponding to two, three, four, and five objects lasted 11, 16.5, 22, and 27.5 seconds, respectively. A 128-second high-pass filter was used to remove low-frequency noise. Successful encoding and retrieval epochs were modeled as separate covariates of interest, and unsuccessful epochs and the 6-second interval between encoding and retrieval phases were covariates of no interest.
Load-independent and load-dependent relationships between task difficulty and BOLD responses were assessed by using polynomial regression in which zero-order (boxcar), first-order (linear), and second-order (nonlinear quadratic) terms were compared to a resting baseline. Using this method, we were able to identify both increases in activation and deactivation with increasing task difficulty. Contrast images generated from parameter estimates were entered into one- and two-sample t tests (accounting for subject-to-subject response variability at the random-effects level) to form statistical parametric maps of the z statistic. Voxel-level contrasts were thresholded at p<0.05 (false discovery rate corrected for multiple comparisons), unless stated otherwise, and activations were reported only if the clusters consisted of at least five contiguous voxels. Statistical parametric map coordinates were converted from Montreal Neurological Institute coordinates to standard space to better localize activations by using the Talairach and Tournoux atlas (19).
Encoding and retrieval epochs associated with success, compared to failure, were also assessed. Data were examined as for the parametric analyses with the following exceptions. Only epochs relating to the hardest level of difficulty were assessed to ensure approximately equal success versus failure epochs (in which there were 1 plus incorrect retrieval decisions). This procedure resulted in exclusion of data from two patients and two comparison subjects because of insufficient data associated with failure. All other epochs at easier levels of task difficulty and the encoding/retrieval interval were modeled as covariates of no interest. Contrast images of the difference between correct and incorrect epochs were generated for each subject. The statistical parametric map of this contrast was then masked with a statistical parametric map of the contrast (corresponding to correct plus incorrect attempts > rest) to separate differential activations from suppressions with respect to baseline.
Results
Behavioral Data
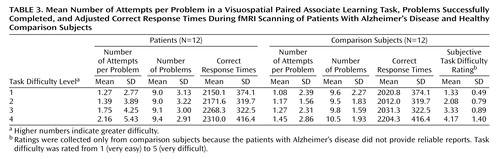
Behavioral data are presented in Table 1, Table 2, and Table 3. Subjective task difficulty ratings were collected only from comparison subjects because the patients with Alzheimer’s disease did not provide reliable reports. For the mean number of attempts per problem, all main effects and interaction terms were significant (task difficulty: F=20.08, df=3, 66, p<0.0005; group: F=11.12, df=1, 22, p<0.005; interaction: F=3.88, df=3, 66, p<0.05). Post hoc Tukey’s pairwise comparisons revealed that the patients with Alzheimer’s disease made a greater mean number of attempts per problem at the hardest level of difficulty. For the mean number of successful problems, the main effect of task difficulty was significant (task difficulty: F=3.11, df=3, 66, p<0.05), while the main effect of group and the interaction of task difficulty and group were not significant (group: F=0.54, df=1, 22, p=0.47; interaction: F=0.49, df=3, 66, p=0.81). The patients and the comparison subjects completed the same number of problems correctly at each level of difficulty. For subjective difficulty ratings, the main effect was significant (F=41.69, df=3, 33, p<0.0005), whereby subjective task difficulty ratings increased systematically across the four levels.
For mean correct response times (adjusted for mean number of attempts across all levels of difficulty) (Table 3), all main effects and the interaction of group and task difficulty were nonsignificant (task difficulty: F=0.69, df=3, 63, p=0.56; group: F=1.30, df=1, 21, p=0.27; interaction: F=0.40, df=3, 63, p=0.75). For incorrect response times, no significant difference was found between the patients (2972.8 msec, SD=278.5) and the comparison subjects (2859.9 msec, SD=374.0) (t=0.65, df=12, p=0.53).
Functional Data
Within-group analyses
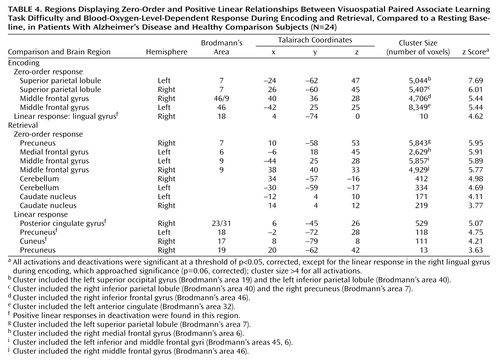
Characterization of the relationship between task difficulty and the BOLD response using a zero-order function revealed similar patterns of activation underlying successful visuospatial paired associate learning in all subjects (Figure 2, Table 4). During encoding, significant activations that were independent of task difficulty were observed bilaterally in the superior parietal lobule (Brodmann’s area 7, which extended to the occipital gyrus, inferior parietal lobule, and precuneus) and in the middle frontal gyrus (Brodmann’s area 46/9, Brodmann’s area 46, extending to the anterior cingulate and inferior frontal gyrus). During retrieval, significant activation changes were located in the right precuneus (Brodmann’s area 7, including the superior parietal lobule), left medial frontal gyrus (Brodmann’s area 6), and bilaterally in the middle frontal gyrus (Brodmann’s area 9, including the inferior frontal gyrus), cerebellum, and caudate nucleus.
The addition of a first-order linear component to the regression model better characterized the task difficulty/BOLD relationship in the occipitoparietal regions during successful encoding and retrieval (Figure 3, Table 4). During retrieval, a positive linear increase in activation with increasing task difficulty was found in the precuneus (Brodmann’s area 19), and positive linear increases in deactivation with increasing task difficulty were found in the right posterior cingulate gyrus (Brodmann’s area 23/31), bilateral precuneus (Brodmann’s area 18, Brodmann’s area 19), and right cuneus (Brodmann’s area 17). During encoding, a positive linear relationship between task difficulty and the BOLD deactivation response in the right lingual gyrus (Brodmann’s area 18) approached significance (p=0.06). Although positive linear increases in activation with increasing task difficulty did not survive correction for multiple comparisons, they were significant at an uncorrected height threshold of p<0.0005 in the left inferior parietal lobule (Brodmann’s area 40; x=–42, y=–41, z=43) and bilateral precuneus (Brodmann’s area 7; x=18, y=–62, z=47 and x=–10, y=–50, z=58). Positive nonlinear (quadratic) responses to increasing task difficulty during encoding and retrieval did not survive correction for multiple comparisons.
Dose-response curves of the association between task difficulty and BOLD responses were examined in the right posterior cingulate gyrus (x=6, y=–45, z=26) during retrieval (Figure 4). The main effect of task difficulty was significant (task difficulty: F=2.77, df=3, 66, p<0.05), and the main effect of group and the interaction of task difficulty and group were not significant (group: F=1.84, df=1, 22, p=0.19; interaction: F=0.14, df=3, 66, p=0.93). Furthermore, a comparison of the regression lines for the patient and comparison group revealed a nonsignificant difference between groups in the slope of change in BOLD response with increasing task difficulty (mean difference between slopes=0.00001, SD=0.0003) (t=0.16, df=92, p=0.87).
Between-group analyses
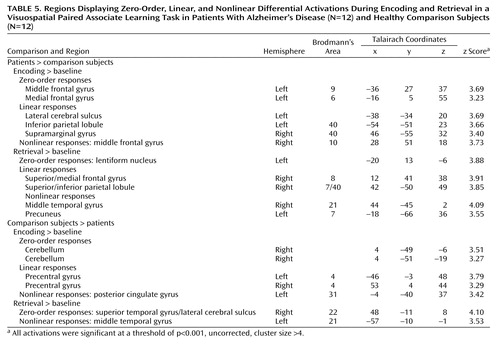
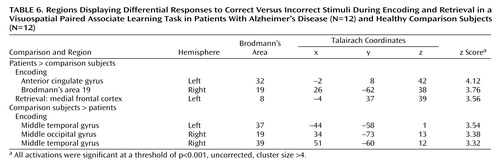
All differences in activation between the patients with Alzheimer’s disease and the comparison subjects during visuospatial paired associate learning failed to survive correction for multiple comparisons. It has been argued that correction for multiple comparisons should not be used “when asserting that a response is truly absent at a given location” (20, p. S85). Therefore, in order to avoid the possibility of making a type II statistical error, differences were assessed by using a threshold of p<0.001 (uncorrected for multiple comparisons). Even with this liberal threshold, very few significant differences in brain activation between the patients and comparison subjects were found (Figure 5, Table 5). Differences in the response to correct, compared to incorrect stimuli between the patients and the comparison subjects also failed to survive correction for multiple comparisons, with only a few differences being significant at an uncorrected threshold of p<0.001 (Table 6).
At p<0.001 (uncorrected), zero-order activations that were greater in the patients, relative to the comparison subjects, were located in the left middle frontal gyrus (Brodmann’s area 9) and the left medial frontal gyrus (Brodmann’s area 6) during encoding and in the left lentiform nucleus during retrieval. Linear signal intensity changes during encoding were observed in the left lateral cerebral sulcus, left inferior parietal lobule (Brodmann’s area 40), and right supramarginal gyrus (Brodmann’s area 40). During retrieval, linear responses were observed in the right superior/medial frontal gyrus (Brodmann’s area 8) and right superior/inferior parietal lobule (Brodmann’s area 7/40). Finally, brain regions demonstrating greater nonlinear responses in the patients than in the comparison subjects were located in the right middle frontal gyrus (Brodmann’s area 10) during encoding and in the right middle temporal gyrus (Brodmann’s area 21) and left precuneus (Brodmann’s area 7) during retrieval.
Differences in activation that were independent of task difficulty and that were greater in the comparison subjects than in the patients were located in the right cerebellum during encoding and in the right superior temporal gyrus/lateral cerebral sulcus (Brodmann’s area 22) during retrieval. Linear task difficulty-dependent activations were found in the precentral gyrus bilaterally (Brodmann’s area 4) during encoding, and no significant activations were observed during retrieval. Finally, nonlinear responses that were greater in the comparison subjects than in the patients were located in the left posterior cingulate gyrus (Brodmann’s area 31) during encoding and in the left middle temporal gyrus (Brodmann’s area 21) during retrieval.
Discussion
In this study, both similarities and differences in brain activations underlying paired associate learning were examined in patients with Alzheimer’s disease and comparison subjects, after adjustment for differences in performance and task difficulty. Independent of the level of difficulty, the majority of subjects in both groups activated a network of brain regions, including the anterior cingulate, lateral, and medial occipitoparietal and frontal cortices, during successful encoding and retrieval. Activations and deactivations in lateral and medial occipitoparietal areas were found to be better characterized by positive linear response functions, especially during retrieval rather than encoding. Greater linear responses during retrieval than encoding most likely reflect fewer cognitive demands during encoding, especially when multiple exposures to stimuli may have reduced the need for information to be encoded. The interactions of group and task difficulty revealed some small but significant differences in activation (albeit at an uncorrected threshold) in cerebellar, temporal, precentral, and posterior cingulate regions (comparison subjects > patients) and in frontoparietal regions (patients > comparison subjects).
Cognitive Function of Brain Regions Associated With Paired Associate Learning
Lateral parietal activations reported during episodic tasks are thought to reflect recognition processes (21) and retrieval processing of spatial information (22). Medial parietal activity has been proposed to underlie imagery (23) and retrieval success (24), and activity in the occipital cortex has been related to perceptual and recognition processes (25). Of the activations within the frontal regions, those in the anterior prefrontal cortex have been attributed to retrieval mode (26) and to postretrieval monitoring (27), while dorsolateral prefrontal cortex activations have been related to monitoring and verification processes and semantic production operations during episodic retrieval (28). Dorsolateral prefrontal cortex activity has also been associated with increasing task difficulty rather than mnemonic processes (29). Other studies have demonstrated increases in anterior cingulate activation during cognitively demanding tasks (30), and such activation has been attributed to inhibition of inappropriate responses (31) and initiation or willed control of behavior (32). Cerebellar activity has been commonly reported in studies of episodic retrieval and has been related to retrieval effort and mode (33) and self-initiation of retrieval processes (34). Finally, frontoparietal activity observed in the current study may also reflect practice-related effects. This interpretation is supported by a recent functional magnetic resonance imaging (fMRI) study in which activation in the lateral prefrontal, superior, and inferior parietal cortices increased after extensive training of visuospatial working memory over a 5-week period (35).
Between-Group Differences in Activation
Differences in activation between the patients with Alzheimer’s disease and the comparison subjects were found to result from activation in one group and deactivation in the other. Such differential patterns of activity may reflect the use of different mnemonic strategies across the two groups or may reflect functional compensation for neuropathological changes associated with Alzheimer’s disease. However, they may also reflect differences in effort between the patients and the comparison subjects at the hardest level of difficulty. Although we took as many steps as possible to control for relative difficulty of the task across four levels of difficulty included in the design, the behavioral data indicated that, on average, the patients needed an extra 0.71 attempt to successfully complete problems at the hardest level of difficulty. Given this difference, the findings of increased activation in the comparison subjects and decreased activation in the patients may have resulted from increasing item familiarity in the patient group (because of the additional attempts), while findings of activation in the patients and activation and deactivation in the comparison subjects may reflect increased effort at the hardest level of task difficulty in the patients with Alzheimer’s disease. This interpretation, however, is not supported by our observation that areas of increased activation in the patients and in the comparison subjects match neither brain regions previously identified as being involved in effort nor item familiarity. Instead, the greater signal changes in the lateral and medial parietal regions in the patients, relative to the comparison subjects, during encoding and retrieval appear to reflect additional activity within regions found to be active in comparison subjects during visuospatial paired associate learning. Thus, these small increases in activity in the patients with Alzheimer’s disease may reflect compensatory reallocation of neural resources to compensate for Alzheimer’s disease-related neuropathology. It has previously been argued that increases in activity in the right middle frontal gyrus, right precentral gyrus, and left cerebellum during overt rehearsal of word lists are associated with engagement of the articulatory loop within the white matter (36). Therefore, differential activations observed in the comparison group may be attributable to strategic effects such as greater engagement of subvocalization during encoding.
Alzheimer’s Disease Treatment Effects
In the current study seven patients were receiving acetylcholinesterase inhibitor treatment for cognitive impairment. Little is known about the effect of such treatment on the BOLD response in patients with Alzheimer’s disease, although findings of positron emission tomography and fMRI studies of cholinergic enhancement in healthy adults who performed explicit memory tasks suggest that cholinergic stimulation enhances activation in extrastriate regions (37), while cholinergic blockade decreases activation in these regions (38). The effect of cholinergic stimulation or blockade is less consistent in the frontal cortices, with frontal decreases in activation being reported with cholinergic stimulation and blockade during performance of explicit memory tasks (37, 38). Some evidence suggests that a single dose of rivastigmine increased activation in the fusiform gyrus bilaterally during face encoding, in the left superior frontal gyrus during a 1-back working memory task, and in the right inferior and superior frontal gyri during a 2-back working memory task in patients with Alzheimer’s disease (39). Decreases in activation were found in the right middle and superior frontal gyri in the 2-back working memory task alone. Therefore, it is possible that increased activation in frontal-occipital regions in patients receiving acetylcholinesterase treatment in the current study may have reduced any possible activation differences in these regions between the patients and comparison subjects. However, a report of decreases in right frontal activation with increasing memory load under rivastigmine administration (39) suggests that any possible differences in activation within this region between the patients and comparison subjects should have been enhanced. Therefore, the minimal activation differences between the patients and the comparison subjects in the current study are most likely attributable to our attempts to make adjustments for performance and relative task difficulty rather than to the effect of treatment.
 |
 |
 |
 |
 |
 |
Received March 30, 2004; revisions received July 16 and Aug. 4, 2004; accepted Aug. 19, 2004. From the Section of Old Age Psychiatry, the Department of Psychology, and the Neuroimaging Research Group, Institute of Psychiatry, King’s College London; Medical Research Council Cognition and Brain Sciences Unit, Cambridge, U.K.; and the Department of Psychiatry, University of Cambridge, Cambridge, U.K. Address correspondence and reprint requests to Dr. Gould, Section of Old Age Psychiatry, Box P070, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK; [email protected] (e-mail). Supported by the Wellcome Trust. The authors thank Dr. Catherine Foy for assistance with recruitment and the study subjects for their participation.
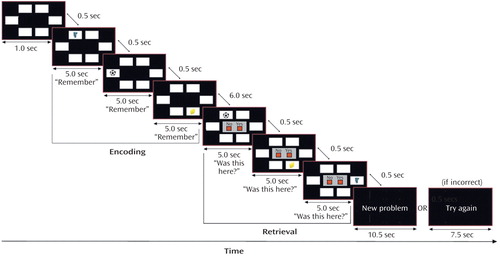
Figure 1. Visuospatial Paired Associate Learning Task Presented to Patients With Alzheimer’s Disease (N=12) and Healthy Comparison Subjects (N=12)a
aSubjects were required to remember objects and the locations in which they appeared. Task difficulty was varied by increasing the number of objects and locations presented in each problem. The example problem shown has three objects and six locations.
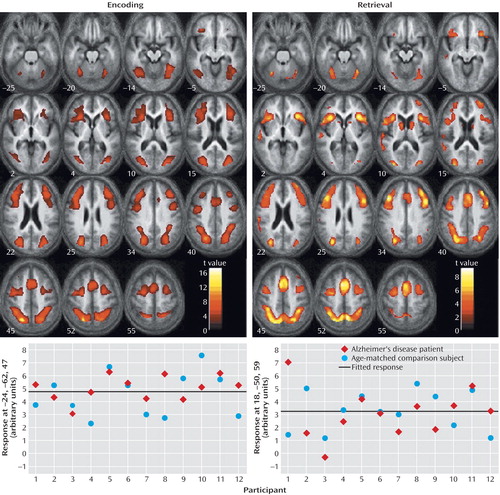
Figure 2. Regions Demonstrating a Zero-Order Response During Encoding and Retrieval in a Visuospatial Paired Associate Learning Task in Patients With Alzheimer’s Disease and Healthy Comparison Subjects (N=24)a
aIn the upper part of the figure, areas of activation are shown in overlay on the mean normalized structural image for all subjects. The graphs in the lower part of the figure represent mean responses in the peak voxel, compared to a resting baseline. Talairach coordinates for the peak voxel are given in the y axis label of each graph.
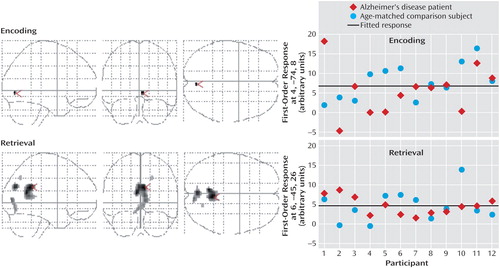
Figure 3. Regions Displaying a Positive Linear Response to Increasing Task Difficulty During Encoding and Retrieval in a Visuospatial Paired Associate Learning Task in Patients With Alzheimer’s Disease and Healthy Comparison Subjects (N=24)a
aThe graphs on the right side of the figure represent mean first-order responses in the peak voxel (indicated by a red arrowhead in the images on the left side of the figure), compared to a resting baseline. Talairach coordinates for the peak voxel are given in the y axis label of each graph.
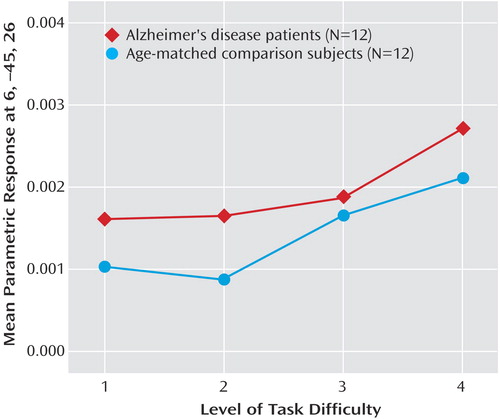
Figure 4. Mean Parametric Response in the Right Posterior Cingulate Gyrus During Retrieval at Four Levels of Task Difficulty of a Paired Associate Learning Task in Patients With Alzheimer’s Disease and Healthy Comparison Subjectsa
aTalairach coordinates for the peak voxel are given in the y axis label.
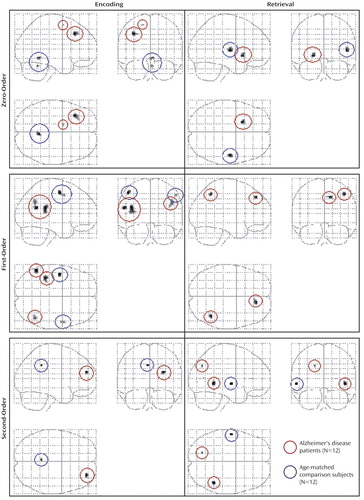
Figure 5. Differential Zero-Order, First-Order, and Second-Order Activations During Encoding and Retrieval in a Visuospatial Paired Associate Learning Task in Patients With Alzheimer’s Disease and Healthy Comparison Subjects
1. Rombouts S, Barkhof F, Veltman DJ, Machielsen WCM, Witter MP, Bierlaagh MA, Lazeron RHC, Valk J, Scheltens P: Functional MR imaging in Alzheimer’s disease during memory encoding. Am J Neuroradiol 2000; 21:1869–1875Medline, Google Scholar
2. Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR: Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 2003; 61:500–506Crossref, Medline, Google Scholar
3. Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS: fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003; 74:44–50Crossref, Medline, Google Scholar
4. Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE: Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci 2003; 23:986–993Crossref, Medline, Google Scholar
5. Hazlett EA, Buchsbaum MS, Mohs RC, Spiegel-Cohen J, Wei TC, Azueta R, Haznedar MM, Singer MB, Shihabuddin L, Luu-Hsia C, Harvey PD: Age-related shift in brain region activity during successful memory performance. Neurobiol Aging 1998; 19:437–445Crossref, Medline, Google Scholar
6. Morcom AM, Good CD, Frackowiak RS, Rugg MD: Age effects on the neural correlates of successful memory encoding. Brain 2003; 126:213–229Crossref, Medline, Google Scholar
7. Herholz K, Ehlen P, Kessler J, Strotmann T, Kalbe E, Markowitsch HJ: Learning face-name associations and the effect of age and performance: a PET activation study. Neuropsychologia 2001; 39:643–650Crossref, Medline, Google Scholar
8. Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, DiMauro AA, Park A, Campbell CE, Marder K, Bell K, Van Heertum R, Sackeim HA: Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology 2000; 55:1291–1297Crossref, Medline, Google Scholar
9. Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR: Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord 2004; 17:42–48Crossref, Medline, Google Scholar
10. Gould RL, Brown RG, Owen AM, ffytche DH, Howard RJ: fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 2003; 20:1006–1019Crossref, Medline, Google Scholar
11. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
12. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983; 17:37–49Google Scholar
13. Mattis S, Jurica PJ, Leitten C: DRS-2: Dementia Rating Scale-2. Lutz, Fla, Psychological Assessment Resources, 2001Google Scholar
14. Wechsler D: The Wechsler Memory Scale, 3rd ed. San Antonio, Tex, Psychological Corp (Harcourt), 1997Google Scholar
15. Nelson HE, Willison J: National Adult Reading Test (NART), Second Edition Test Manual. Windsor, UK, National Foundation for Educational Research-Nelson, 1991Google Scholar
16. Friston KJ, Holmes AP, Worsely KJ, Poline JB, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
17. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
18. SPM2. London, University College, Institute of Neurology, Wellcome Department of Cognitive Neurology, 2003Google Scholar
19. Brett M, Christoff K, Cusack R, Lancaster J: Using the Talairach atlas with the MNI template. Neuroimage 2001; 13:S85-S85Google Scholar
20. Woods RP: Modeling for intergroup comparisons of imaging data. Neuroimage 1996; 4:S84-S94Google Scholar
21. Cabeza R, Kapur S, Craik FIM, McIntosh AR, Houle S, Tulving E: Functional neuroanatomy of recall and recognition: a PET study of episodic memory. J Cogn Neurosci 1997; 9:254–265Crossref, Medline, Google Scholar
22. Moscovitch M, Kapur S, Kohler S, Houle S: Distinct neural correlates of visual long-term-memory for spatial location and object identity: a positron emission tomography study in humans. Proc Natl Acad Sci USA 1995; 92:3721–3725Crossref, Medline, Google Scholar
23. Fletcher PC, Shallice T, Frith CD, Frackowiak RSJ, Dolan RJ: Brain activity during memory retrieval: the influence of imagery and semantic cueing. Brain 1996; 119:1587–1596Crossref, Medline, Google Scholar
24. Kapur S, Craik FIM, Jones C, Brown GM, Houle S, Tulving E: Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport 1995; 6:1880–1884Crossref, Medline, Google Scholar
25. Ungerleider LG, Mishkin M: Two cortical visual systems, in Analysis of Visual Behavior. Edited by Ingle DJ, Goodale MA, Mansfield RJW. Cambridge, Mass, MIT Press, 1982, pp 549–586Google Scholar
26. Lepage M, Ghaffar O, Nyberg L, Tulving E: Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA 2000; 97:506–511Crossref, Medline, Google Scholar
27. Allan K, Dolan RJ, Fletcher PC, Rugg MD: The role of the right anterior prefrontal cortex in episodic retrieval. Neuroimage 2000; 11:217–227Crossref, Medline, Google Scholar
28. Cabeza R, Locantore JK, Anderson ND: Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci 2003; 15:249–259Crossref, Medline, Google Scholar
29. Duncan J, Owen AM: Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 2000; 23:475–483Crossref, Medline, Google Scholar
30. Paus T, Koski L, Caramanos Z, Westbury C: Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 1998; 9:R37-R47Google Scholar
31. George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post SM: Regional brain activity when selecting a response despite interference: an H215O PET study of the Stroop and an emotional Stroop. Hum Brain Mapp 1994; 1:194–209Crossref, Medline, Google Scholar
32. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2001; 2:417–424Crossref, Medline, Google Scholar
33. Cabeza R, Nyberg L: Imaging cognition, II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000; 12:1–47Crossref, Medline, Google Scholar
34. Backman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Langstrom B: Brain activation in young and older adults during implicit and explicit retrieval. J Cogn Neurosci 1997; 9:378–391Crossref, Medline, Google Scholar
35. Olesen PJ, Westerberg H, Klingberg T: Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 2004; 7:75–79Crossref, Medline, Google Scholar
36. Woodard JL, Grafton ST, Votaw JR, Green RC, Dobraski ME, Hoffman JM: Compensatory recruitment of neural resources during overt rehearsal of word lists in Alzheimer’s disease. Neuropsychology 1998; 12:491–504Crossref, Medline, Google Scholar
37. Furey ML, Pietrini P, Haxby JV: Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 2000; 290:2315–2319Crossref, Medline, Google Scholar
38. Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M: Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA 2002; 99:455–460Crossref, Medline, Google Scholar
39. Rombouts SA, Barkhof F, Van Meel CS, Scheltens P: Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2002; 73:665–671Crossref, Medline, Google Scholar



