Association Between Catechol-O-Methyltransferase and Phobic Anxiety
Abstract
OBJECTIVE: The authors assessed the association between the catechol-O-methyltransferase (COMT)Val158Met polymorphism and scores on the phobic anxiety scale of the Crown-Crisp Experimental Index. METHOD: A total of 1,234 women completed the Crown-Crisp Experimental Index phobic anxiety scale and were genotyped for the COMT polymorphism. The authors used unconditional logistic regression to compute odds ratios and 95% confidence intervals (CIs) to evaluate the association between the COMT genotype and phobic anxiety. RESULTS: The mean scores for the three genotypes were statistically significantly different. Compared to the COMTMet/Met genotype, the age-adjusted odds ratio for scoring ≥6 compared to scoring 0 or 1 were 1.15 (95% CI=0.71–1.85) and 1.99 (95% CI=1.17–3.40) for the COMTVal/Met and COMTVal/Val genotypes, respectively; a significant gene dosage effect was observed. CONCLUSIONS: Our results suggest that the functional COMT polymorphism is associated with the development of phobic anxiety.
Prefrontal dopamine levels are believed to be important in the etiology and expression of anxiety. Catechol-O-methyltransferase (COMT) catalyzes the degradation of dopamine (1). Lachman et al. (2) identified a functional polymorphism in the COMT gene leading to a substitution of methionine for valine, encoding for an enzyme with decreased activity. The high-activity Val allele is hypothesized to impair prefrontal cognition and physiology by increasing prefrontal dopamine catabolism (3) and therefore would increase the risk of phobic anxiety. Differences in the risk of phobic anxiety may be due to individual differences in catecholamine inactivation by COMT. We assessed the association between COMT genotypes and scores on the phobic anxiety scale of the Crown-Crisp Experimental Index (4) among 1,234 women in the Nurses’ Health Study.
Method
The Nurses’ Health Study began in 1976, when 121,700 U.S. nurses returned an initial questionnaire reporting medical histories and baseline health-related exposures. We used genotyping data available from control subjects from previously conducted studies of breast cancer, endometrial cancer, and Parkinson’s disease to assemble a series of 1,234 subjects who were free of cancer other than nonmelanoma skin cancer. This study was approved by the Human Subjects Committee of Brigham and Women’s Hospital.
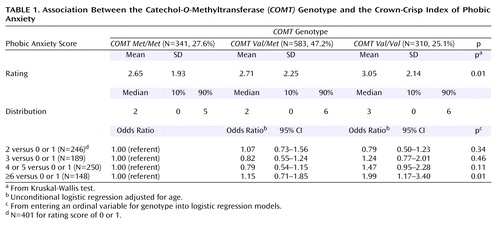
The 1988 Nurses’ Health Study questionnaire asked participants to complete the phobic anxiety scale of the Crown-Crisp Experimental Index. The phobic anxiety scale inquired about recent events and consists of eight questions measuring personality traits and symptoms of phobic anxiety, including fear of heights, crowds, and enclosed spaces. Each question has two to three levels of possible responses, with scores on the scale ranging from 0 (no symptoms) to 16 (higher scores indicating higher levels of phobic anxiety). The instrument has been validated in a psychiatric outpatient setting and was found to discriminate patients with anxiety disorders from healthy subjects (4). Similar to a previous study, we categorized respondents into groups according to total score: 0 or 1 (reference), 2, 3, 4 or 5, and ≥6 (highest level of anxiety) (5).
Genotyping was performed by restriction fragment length polymorphism assays based on polymerase chain reaction, as previously described (2). All genotyping was performed by laboratory personnel who were blind to phobic anxiety score. For quality control, a random 10% of the samples was inserted to validate genotyping identification procedures; concordance for blinded samples was 100%.
The chi-square test and Kruskal-Wallis test were used to compare COMT genotypes and distribution of phobic anxiety scores. We used unconditional logistic regression to compute age-adjusted odds ratios and 95% confidence intervals (CIs) to evaluate the association between COMT genotype and phobic anxiety scores. Indicator variables for the COMT genotype were created by using the individual’s homozygote for the Met allele as the referent category. Gene dosage effects were modeled by assigning the values of 0, 1, and 2 to a genotype trend variable, according to the subject’s number of Val alleles. All p values were two-tailed.
Results
The scores on the phobic anxiety index ranged from 0 to 13. Total mean index scores were statistically significantly different for the three genotypes (χ2=9.05, df=2, p=0.01). One individual item on the phobic anxiety index—“feel panicky in crowds”—was marginally associated with the COMT genotype (F=2.85, df=2, p=0.06). We categorized women into the following groups: 0 or 1 (32.5% of the population), 2 (19.9%), 3 (15.3%), 4 or 5 (20.3%), and ≥6 (12.0%). There was a marginally significant difference in genotype distribution, according to categorical Crown-Crisp score (χ2=13.91, df=8, p=0.08). Compared to the COMTMet/Met genotype, the odds ratios for scoring ≥6 on the Crown-Crisp Experimental Index compared to scoring 0 or 1 were 1.15 (95% CI=0.71–1.85) and 1.99 (95% CI=1.17–3.40) for the COMTVal/Met and the COMTVal/Val genotypes, respectively (Table 1).
Discussion
Prefrontal cortical dopamine depletion in rats causes an enhanced anxiety level, implicating dopamine in the modulation of anxiety and a necessary component to cope with an anxiogenic event (6). A recent linkage analysis (7) provided evidence for a susceptibility locus for panic disorder either within or near the COMT gene. Given the important role COMT has in dopamine metabolism, we evaluated the association between COMT genotypes and phobic anxiety.
Our results suggest that the COMTVal/Val genotype was associated with an increased risk of phobic anxiety (odds ratio=1.99, 95% CI=1.17–3.40). We observed significant differences in phobic anxiety scores among the three genotypes.
Previous epidemiological studies assessing COMT and anxiety and panic disorder have not been consistent. A Japanese study observed no significant differences in genotype distribution among 108 patients with anxiety compared to 135 comparison subjects (8). Woo et al. (9) observed a higher proportion of the COMTMet allele in 51 Korean patients with panic disorder compared to 45 healthy comparison subjects, and Enoch et al. (10) observed an association with two harm avoidance subscales and low-voltage alpha ECG and COMT when comparing the Met/Met genotype to the combined genotype of Val/Met and Met/Met among 75–92 predominantly Caucasian women. The associations observed in these studies may differ from what we observed among our 1,234 Caucasian women potentially because of ethnic differences in genotype distribution, gender, sample size, and differences in outcome assessment.
Panic disorder is twice as frequent in women as in men in the general population (11). Estrogen has been observed to inhibit COMT gene transcription (12), and Gogos et al. (13) suggested that COMT has an important sex- and region-specific contribution in the maintenance of catecholamines in the brain. Therefore, COMT is a candidate gene that can potentially explain the differences in susceptibility and the prevalence of anxiety disorders between men and women. Recent evidence has suggested that the variation at Val158Met may not be sufficient to identify all genetic variations in COMT and that the COMT haplotypes should be used in association studies to ascertain the contribution of COMT in disease etiology (14). Further studies stratified by gender are warranted to confirm the association between COMT and phobic anxiety.
 |
Received July 15, 2003; revisions received Oct. 8, 2003, and Feb. 28, 2004; accepted March 17, 2004. From the Departments of Epidemiology, Nutrition, and Health and Social Behavior and the Program in Molecular and Genetic Epidemiology, Harvard School of Public Health, Boston; and the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School. Address reprint requests to Dr. McGrath, Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 181 Longwood Ave., Boston, MA 02115; [email protected] (e-mail). Supported by NIH grants from the Division of Extramural Activities of the National Cancer Institute T32 CA-09001-27 (to Dr. McGrath), CA-82838 (to Dr. De Vivo), and CA-87969 (to Dr. Hunter), grant NS-35624 from the National Institute of Neurological and Communicative Disorders and Stroke (to Dr. Ascherio), and grant RSG-00-061-04-CCE from the American Cancer Society (to Dr. De Vivo). The authors thank Rong Chen and Robert O’Brien for technical assistance and the participants in the Nurses’ Health Study for their dedication.
1. Axelrod J, Tomchick R: Enzymatic O–methylation of epinephrine and other catechols. J Biol Chem 1958; 233:702–705Medline, Google Scholar
2. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM: Human catechol-O–methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996; 6:243–250Crossref, Medline, Google Scholar
3. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Crossref, Medline, Google Scholar
4. Crown S, Crisp AH: A short clinical diagnostic self-rating scale for psychoneurotic patients: the Middlesex Hospital Questionnaire (MHQ). Br J Psychiatry 1966; 112:917–923Crossref, Medline, Google Scholar
5. Haines A, Cooper J, Meade TW: Psychological characteristics and fatal ischaemic heart disease. Heart 2001; 85:385–389Crossref, Medline, Google Scholar
6. Espejo EF: Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res 1997; 762:281–284Crossref, Medline, Google Scholar
7. Hamilton SP, Slager SL, Heiman GA, Deng Z, Haghighi F, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA: Evidence for a susceptibility locus for panic disorder near the catechol-O–methyltransferase gene on chromosome 22. Biol Psychiatry 2002; 51:591–601Crossref, Medline, Google Scholar
8. Ohara K, Nagai M, Suzuki Y, Ochiai M: No association between anxiety disorders and catechol-O–methyltransferase polymorphism. Psychiatry Res 1998; 80:145–148Crossref, Medline, Google Scholar
9. Woo J-M, Yoon K-S, Yu B-H: Catechol O–methyltransferase genetic polymorphism in panic disorder. Am J Psychiatry 2002; 159:1785–1787Link, Google Scholar
10. Enoch MA, Xu K, Ferro E, Harris CR, Goldman D: Genetic origins of anxiety in women: a role for a functional catechol-O–methyltransferase polymorphism. Psychiatr Genet 2003; 13:33–41Crossref, Medline, Google Scholar
11. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen HU, Yeh EK: The cross-national epidemiology of panic disorder. Arch Gen Psychiatry 1997; 54:305–309Crossref, Medline, Google Scholar
12. Xie T, Ho SL, Ramsden D: Characterization and implications of estrogenic down-regulation of human catechol-O–methyltransferase gene transcription. Mol Pharmacol 1999; 56:31–38Crossref, Medline, Google Scholar
13. Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M: Catechol-O–methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 1998; 95:9991–9996Crossref, Medline, Google Scholar
14. Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O’Donovan MC: A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet 2003; 73:152–161Crossref, Medline, Google Scholar



