Smoking-Induced Ventral Striatum Dopamine Release
Abstract
OBJECTIVE: Substantial evidence from animal models demonstrates that dopamine release in the ventral striatum underlies the reinforcing properties of nicotine. The authors used [11C]raclopride bolus-plus-continuous-infusion positron emission tomography (PET) to determine smoking-induced ventral striatum dopamine release in humans. METHOD: Twenty nicotine-dependent smokers (who smoked ≥15 cigarettes/day) underwent a [11C]raclopride bolus-plus-continuous-infusion PET session. During the session, subjects had a 10-minute break outside the PET apparatus during which 10 subjects smoked a cigarette and 10 did not smoke (as a control condition). RESULTS: The group that smoked had greater reductions in [11C]raclopride binding potential in ventral striatum regions of interest than the group that did not smoke, particularly in the left ventral caudate/nucleus accumbens and left ventral putamen (range for smoking group=–25.9% to –36.6% reduction). Significant correlations were found between change from before to after the smoking break in craving ratings and change from before to after the break in binding potential for these two regions. CONCLUSIONS: Nicotine-dependent subjects who smoked during a break in PET scanning had greater reductions in [11C]raclopride binding potential (an indirect measure of dopamine release) than nicotine-dependent subjects who did not smoke. The magnitude of binding potential changes was comparable to that found in studies that used similar methods to examine the effects of other addictive drugs.
Substantial evidence from laboratory studies of animals demonstrates that dopamine release in the ventral striatum underlies the reinforcing properties of nicotine (1, 2). Microdialysis (3–6), lesion (7), and imaging (8) studies in rats indicate that nicotine-induced dopamine release is strongest in this region and is more robust than the dopamine release found in associated structures receiving dopaminergic input, such as the dorsal striatum (3). Several of these studies used nicotine doses that simulated human cigarette smoking (4, 7, 8), and it has been reported that nicotine-induced dopamine release is comparable in magnitude to that induced by other addictive drugs (4). A study of nonhuman primates (9) that used the [11C]raclopride positron emission tomography (PET) method also found nicotine-induced ventral striatum dopamine release. In addition, one report (10) described a difference between smokers and nonsmokers in 18F-fluorodopa uptake (a measure of presynaptic dopamine activity) during PET imaging. Smokers had greater tracer uptake in the caudate and putamen than nonsmokers, suggesting greater dopaminergic turnover in smokers. However, to our knowledge, there have been no published reports of smoking-induced ventral striatum dopamine release in humans.
In recent years, the [11C]raclopride bolus-plus-continuous-infusion PET method has been used increasingly for the (indirect) measurement of dopamine release in response to addictive drugs (11). [11C]Raclopride binds with relative specificity to dopamine D2 (and D2-like) receptors and has low affinity for D1 receptors (11, 12). [11C]Raclopride binding potential (a measure of D2 receptor occupancy) has been demonstrated to have an inverse linear relationship with extracellular dopamine concentration, as measured with microdialysis in nonhuman primates (13). Because synaptic dopamine competes with [11C]raclopride for D2 receptor binding, dopamine release is thought to decrease [11C]raclopride binding potential directly (11). This method has been used to demonstrate changes in synaptic dopamine concentration in response to cocaine (14), amphetamine (15–17), and methylphenidate (18) administration in humans, as well as to nicotine (9, 19), cocaine (20, 21), and amphetamine (22, 23) administration in animals. Decreases in [11C]raclopride binding potential have also been demonstrated in response to nonpharmacological stimuli, such as placebo administration (24), meditation (25), and playing a video game (26). Test-retest reliability of the [11C]raclopride method is strong, especially for binding potential (27, 28), the measure of [11C]raclopride binding used here.
We sought to determine ventral striatum dopamine release in response to cigarette smoking in nicotine-dependent human subjects by using [11C]raclopride PET. We also sought to determine if smoking-induced changes in dopamine concentration were associated with changes in smoking-related clinical states, such as craving and anxiety.
Method
Subjects
Twenty smokers who smoked ≥15 cigarettes/day and who met the DSM-IV criteria for nicotine dependence completed the study. The subjects were adults (age 21–65 years) and were screened initially during a telephone interview in which medical, psychiatric, and substance abuse histories were obtained. All subjects passing this screening were assessed in person by using screening questions from the Structured Clinical Interview for DSM-IV (29). Subjects were excluded for any history of an axis I psychiatric diagnosis other than nicotine dependence. Subjects were also excluded if they were currently taking medication or had any history of a medical condition that might affect the central nervous system at the time of scanning (e.g., current treatment with a beta-blocker or analgesic medication, history of head trauma with loss of consciousness, history of epilepsy). Pregnancy was an exclusion criterion because of the potential risk to a fetus of radiation exposure. Subjects who occasionally used alcohol, caffeine, or other drugs but did not meet the criteria for abuse or dependence were allowed to participate in the study but were instructed to abstain from using these substances for 24 hours before scanning (72 hours for marijuana; verified by urine toxicology screen). Subjects who drank more than the equivalent of two cups of coffee per day (200 to 300 mg/day of caffeine) were also excluded, as were subjects who experienced caffeine withdrawal symptoms (such as irritability, flushing, or headache) temporally associated with caffeine ingestion. After complete description of the study to the subjects, written informed consent was obtained.
[11C]Raclopride PET Scanning
The general procedures of the study were that subjects underwent a single [11C]raclopride bolus-plus-continuous-infusion PET session, along with a structural magnetic resonance imaging (MRI) session within 1 week of the PET session. For the PET session, subjects were assigned (alternately) either to smoke or not smoke during the scanning session, as described in more detail later in this section.
All study subjects were instructed to smoke as per their usual habit on the morning of the PET session and to smoke a cigarette immediately before the session, which began at noon. Rating scales were then administered, including screening questions from the Structured Clinical Interview for DSM-IV (29), the Urge to Smoke Scale (30), state anxiety questions from the Spielberger State-Trait Anxiety Inventory (31), and the Hamilton Depression Rating Scale (32). The subjects’ exhaled carbon monoxide (CO) level, a rough estimate of recent smoking intensity, was measured before [11C]raclopride injection.
Subjects had a 20-gauge intravenous catheter placed at 1:45 p.m., and were positioned (for acquisition of planes parallel to the orbital-meatal line) on the PET scanner at 2:00 p.m. Scanning was initiated at 2:10 p.m. with a slow bolus injection of 5 mCi [11C]raclopride in a 20-ml normal saline solution over a 60-second period, followed by continuous infusion of the tracer (3 mCi/hour) for the remainder of the testing session. This bolus-plus-continuous-infusion method was performed as described in prior studies (23, 33). Brain scans were acquired continuously for the next 50 minutes.
At 3:00 p.m., subjects were taken off the scanner with the infusion still continuing and had a 10-minute break in an outdoor area adjacent to the PET scanning room. Ten of the 20 subjects smoked one cigarette (their favorite brand) during this period, while the remaining subjects stood in the same outdoor area but did not smoke, as a control condition. The 3-hour period of abstinence before this break was chosen on the basis of a previous study showing that craving peaks at about this time in nicotine-dependent smokers (34). Smoking a cigarette (rather than receiving nicotine administration) was chosen as the test condition because it was felt to have a greater likelihood of providing reward for smokers in this initial study. Six subjects smoked regular cigarettes (nicotine content: 1.1 mg), and the remaining four smoked light cigarettes (nicotine content: 0.7 mg [two subjects] or 0.8 mg [two subjects]). A study investigator (A.L.B. or R.E.O.) monitored subjects continuously during the break. After the break, subjects were quickly repositioned (less than a minute) in the scanner by using a preplaced mark on their forehead and a laser light from the scanner. Subjects were then scanned for 30 more minutes.
Cigarette craving and anxiety levels were monitored with the Urge to Smoke Scale and State-Trait Anxiety Inventory, respectively, at four time points: before bolus injection, at the beginning and end of the break, and after the postbreak scan. In addition to the 20 subjects reported on here, three additional subjects were scanned, but one subject had a Hamilton depression scale score greater than 10 (indicating significant symptoms of depression), while two others admitted to daily drug use (one with marijuana and the other with 3,4-methylenedioxymethamphetamine). Data from these three subjects were omitted from this analysis.
PET scans were acquired with an ECAT 953 scanner (CTI-Siemens, Knoxville, Tenn.) with 31 slices in the two-dimensional mode. The average transaxial resolution of this camera is 5.6 mm full width at half maximum, with plane spacing of 3.4 mm. Scans were acquired as 10 prebreak and six postbreak 5-minute frames. [11C]Raclopride was prepared by an established procedure (35, 36).
MRI Scanning
An MRI scan of the brain was obtained from each research participant. The MRI had the following specifications: three-dimensional Fourier-transform spoiled-gradient-recalled acquisition with TR=30 msec, TE=7 msec, 30° angle, two acquisitions, 256×192 view matrix. The acquired volume was reconstructed as roughly 90 contiguous 1.5-mm thick transaxial slices. MRI-to-PET coregistration was performed by using the automated image registration method (37). These coregistered images were visually assessed and then manually realigned thereafter to optimize coregistration. The MRI scan was used to confirm the absence of structural brain abnormalities and to aid in localization of drawn regions of interest.
PET Image Analysis
Regions were drawn on transaxial summed PET images from both the pre- and postbreak series of frames (0 to 50 minutes and 60 to 90 minutes) with reference to coregistered MRI scans. Consistency between data obtained with PET and MRI region-of-interest drawing has previously been reported for [11C]raclopride PET (38). Two regions of interest were drawn bilaterally—the ventral caudate/nucleus accumbens and putamen (Figure 1), consisting of three slices each. Two additional regions of interest (dorsal caudate and dorsal putamen, consisting of three slices each) were drawn bilaterally as control regions. For this study, the ventral caudate/nucleus accumbens region of interest corresponded to the region reported by others as being associated with the rewarding properties of other addictive drugs (15–17). A single region-of-interest slice of cerebellum was drawn as a reference region. Interrater reliability performed for this study by using mean counts in three complete sets of regions of interest (including the cerebellar region of interest) was 0.99 (range for the three subjects was 0.99 to 1.00).
Binding potential for regions of interest was calculated by using the simplified reference tissue model (39), with binding potential=Bmax/Kd = CROI/Ccerebellum – 1, where C is radioligand concentration and ROI is the region of interest. The PET scanning time periods of interest were the 10 minutes before and the 10 minutes after the break, so that mean count densities used for data analysis (for the regions of interest, including the cerebellar region of interest) were the averages from the two 5-minute frames before and after the break. This time frame is similar to one that has been recommended as optimal for signal-to-noise ratio (40).
Statistical Analysis
Means for the two study groups were determined for demographic characteristics, exhaled carbon monoxide levels, and rating scale scores. These variables were compared between groups by using Student’s t tests. In addition to these baseline comparisons, differences in mean changes pre- to postbreak in craving (Urge to Smoke Scale) and state anxiety (State-Trait Anxiety Inventory) ratings were assessed by using Student’s t tests. Mean Urge to Smoke Scale and State-Trait Anxiety Inventory state anxiety scores were reported as the mean score per item on scales ranging from 0 to 6 and from 1 to 4, respectively.
To examine pre- to postbreak group differences in regional [11C]raclopride binding, a repeated-measures multivariate analysis of covariance (MANCOVA) was performed by using [11C]raclopride binding potential for the two regions of interest bilaterally pre- and postbreak as the repeated measures and group (no smoking versus smoking) as the between-subject factor. Age and sex were used as nuisance covariates in this (and all) analyses, given reports of differences in D2 receptor density associated with these variables (41, 42). On the basis of the results of this overall analysis, repeated-measures analyses of covariance (ANCOVAs) were performed for individual experimental regions of interest to determine which ones accounted for the between-group difference. Repeated-measures ANCOVAs (using the variables described earlier) were also performed for control regions of interest. For descriptive purposes, percentage change in binding potential was calculated as 100 times the mean region-of-interest change in binding potential divided by the pretreatment mean binding potential.
For regions of interest found to have significant effects in the preceding analyses, Pearson product-moment correlation coefficients were determined for the relationship between change in region-of-interest binding potential and change in mean Urge to Smoke Scale and State-Trait Anxiety Inventory scores. The correlations were done to determine if change in extracellular dopamine concentration was associated with change in craving and anxiety states, respectively, with the recognition that the modest number of study subjects did not permit a meaningful evaluation of the effect of group on these correlations.
Results
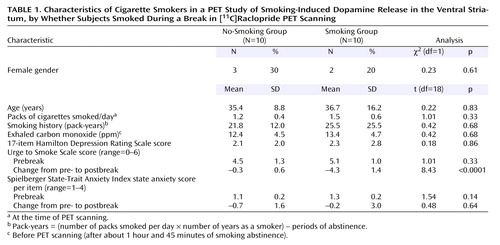
There were no significant differences between the groups at baseline in demographic characteristics, CO levels, or rating scale scores (Table 1). There were also no significant prebreak between-group differences in binding potential for any of the regions of interest (Student’s t tests, two-tailed, range of p values=0.14 to 0.89). As expected, from pre- to postbreak, the group that smoked had a significantly greater reduction in craving (mean Urge to Smoke Scale score) than the group that did not smoke (Student’s t test, two-tailed, p<0.0001). No difference was found between groups in pre- to postbreak change in state anxiety (as measured by the State-Trait Anxiety Inventory) (Student’s t test, two-tailed, p=0.64).
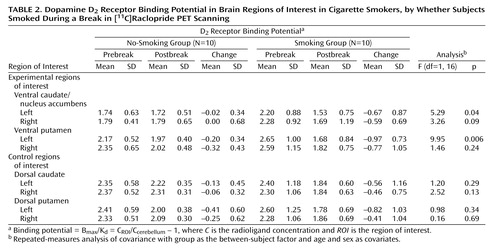
In the overall repeated-measures MANCOVA, a significant between-group effect was found (F=7.6, df=1, 16, p<0.02), indicating an overall group difference in change in [11C]raclopride binding potential in ventral striatum regions between those who did and did not smoke (Figure 2). Follow-up repeated-measures ANCOVAs for specific regions demonstrated that the group that smoked had significantly greater reductions in binding potential in the left ventral caudate/nucleus accumbens (F=5.3, df=1, 16, p=0.04) and left ventral putamen (F=9.9, df=1, 16, p=0.006) than the group that did not smoke (Table 2). The difference in the reduction in binding potential in the right ventral caudate/nucleus accumbens did not reach significance (F=3.3, df=1, 16, p=0.09). The right ventral putamen and control regions of interest did not have significant between-group differences in binding potential change. Percent changes in the experimental regions of interest for those who did not smoke versus those who did smoke were –1.1% versus –30.5% for the left ventral caudate/nucleus accumbens, –9.2% versus –36.6% for the left ventral putamen, 0.0% versus –25.9% for the right ventral caudate/nucleus accumbens, and –13.6% versus –29.7% for the right ventral putamen. All regions of interest had greater mean reductions in binding potential from pre- to postbreak in the group that smoked than in the group that did not smoke (Table 2).
Among the regions in which differences reached significance in the preceding analysis, the change in binding potential in both the left ventral caudate/nucleus accumbens (r=0.49, df=18, p=0.04) and putamen (r=0.65, df=18, p=0.004) was significantly and positively associated with change in the Urge to Smoke Scale score, possibly indicating that dopamine release in these regions is associated with decreased craving.
Discussion
Nicotine-dependent subjects who smoked a cigarette during PET scanning had greater reductions in [11C]raclopride binding potential (an indirect measure of dopamine release) than nicotine-dependent subjects who underwent the same procedure but did not smoke. These group differences were significant in the ventral striatum (left ventral caudate/nucleus accumbens and putamen; the difference for the right ventral caudate/nucleus accumbens did not reach significance) and were numerically present in all basal ganglia regions studied (including both ventral and dorsal regions). These results provide evidence for smoking-induced dopamine release in humans. Furthermore, reductions in binding potential in the left ventral caudate/nucleus accumbens and putamen were significantly associated with reductions in self-reports of craving, possibly indicating an association between dopamine release and craving reduction.
The magnitudes of smoking-induced decreases in ventral striatal [11C]raclopride binding potential (–25.9% to –36.6%) here were similar to those found in human (and nonhuman primate) studies that used the [11C]raclopride PET method paired with administration of amphetamine (–15% to –42%) (15–17, 22, 23), cocaine (–29%) (20), or methylphenidate (–32%) (20). Changes here were also greater than those observed in studies that used similar methods paired with nonpharmacological stimuli (–7.9% to –21.2%) (24–26). Microdialysis studies in animals have demonstrated that doses of nicotine that emulate human smoking result in roughly a doubling (on average) of extracellular dopamine concentration (range=26% to 600%, depending on dose) in the nucleus accumbens (3–6, 8). Furthermore, one study indicated that dopamine concentrations increase more with cocaine (330%) (3) or amphetamine (550%) (3) than with nicotine, while another (4) indicated similarities between nicotine and other addictive drugs.
There are several potential explanations for the similarity between the magnitude of the current findings and those of studies of other addictive drugs. In [11C]raclopride PET studies of other drugs, nonaddicted subjects have most often been studied. In contrast, our study included nicotine-dependent smokers and was designed to elicit the greatest possible change in ventral striatum binding potential by examining change in this measure in nicotine-dependent subjects at a time point (after 3 hours of abstinence) when craving for cigarettes has been reported to be at its peak (34) and then having them smoke. Indeed, subjects in this study had a mean craving score of about 80% of the maximum score on the Urge to Smoke Scale (Table 1) at the 3-hour time point before the break in scanning. This design feature may have maximized the changes in binding potential so that they were comparable to those seen in prior studies with potent dopamine-releasing drugs (e.g., amphetamine, cocaine, or methylphenidate). An alternative explanation is that there is an upper limit to possible displacement of [11C]raclopride binding potential detectable with PET and that all dopamine-releasing drugs reach this limit of displacement. A third possibility is that in humans smoking produces changes in dopamine concentration that are comparable to those seen with other drugs of dependence, regardless of the subjects being studied.
The correlation found here between craving and left ventral caudate/nucleus accumbens (and putamen) binding potential is consistent with the theory that increases in ventral striatum dopamine concentration are associated with craving reduction and satiety. This relationship is supported by research with animal models of nicotine dependence (43), other drug dependencies (44, 45), food (46, 47), and sex (48, 49). The current findings are also in agreement with the prior report of euphoria ratings correlating inversely with [11C]raclopride binding potential in the ventral striatum (17) (assuming that craving and euphoria are opposing states).
Study findings also support the use of medications that modulate the dopamine system for the treatment of nicotine dependence. Bupropion hydrochloride (HCl) is widely used in the treatment of nicotine dependence and is known to block presynaptic reuptake of dopamine (50, 51), although this effect has been reported to be somewhat weak (52). Bupropion HCl has also been shown to increase synaptic ventral striatum dopamine concentrations with both acute (53) and chronic (54) administration, although at least one study found greater effects on nondopaminergic systems with bupropion HCl (55). In addition, other medications that enhance dopamine neurotransmission, such as the dopamine agonist bromocriptine (56) and the monoamine oxidase-B inhibitor l-deprenyl (57) decrease the number of cigarettes smoked in nicotine-dependent subjects and reduce associated withdrawal symptoms. Dopamine receptor antagonists (including haloperidol [58] and others [43]) have also been shown to acutely diminish smoking behavior, although one study reported the opposite effect with haloperidol (59). Thus, change in dopaminergic tone (either through enhancement of dopamine neurotransmission, acute stimulation, or acute blockade) appears to be effective in reducing smoking behavior in nicotine-dependent subjects.
Results of the study should be interpreted in the context of several limitations. First, subjects were removed from the scanner and repositioned after smoking, which could potentially lead to differences in region placement on pre- and postbreak scans because of imperfect repositioning. Although efforts were made to reposition the subjects precisely (by using a laser light from the scanner and markings on the subject’s head) and to draw regions by using the same criteria on pre- and postbreak scans, the possibility remains that region placement was different for the two scans. For the present study, removing subjects from the scanner to smoke was done in a deliberate attempt to emulate naturalistic smoking (based on the hypothesis that this method would lead to the greatest change in dopamine concentration). A second potential confound is that smoking might alter [11C]raclopride binding potential by effects on blood flow (60, 61) rather than dopamine release. If global blood flow increased uniformly and radioligand delivery to the striatum and cerebellum increased equally, the [11C]raclopride binding potential ratio would be expected to decrease in those who smoked. However, in the current group of subjects, cerebellar radioactivity did not increase significantly for either study group (–4% for the smoking group and –2% for the comparison group). Furthermore, [15O]H2O PET studies have not demonstrated changes in striatal blood flow in response to nicotine (19, 62–65). Taken together, these findings indicate that it is unlikely that smoking-related blood flow changes confounded our study results. Third, although subjects were monitored continuously during the break while they were standing in the outdoor area, subtle movements of hands or feet were not systematically examined. Rapid repetitive movements, such as finger tapping (66) and foot extension/flexion (67) (but not vigorous exercise [68]), have been shown to decrease [11C]raclopride binding potential, although the decreases in binding potential in the past studies were generally smaller than those seen here. A fourth limitation was the modest number of subjects.
To our knowledge, this study is the first to demonstrate dopamine release (indirectly) in response to human cigarette smoking and craving alleviation. The present study supports continued attempts to develop medications that alter dopaminergic tone for the treatment of nicotine dependence.
 |
 |
Received July 24, 2003; revision received Oct. 28, 2003; accepted Oct. 31, 2003. From the Veterans Affairs Greater Los Angeles Healthcare System, Los Angeles; the Department of Psychiatry and Biobehavioral Sciences and the Department of Molecular and Medical Pharmacology, University of California, Los Angeles; the Centre for Addiction and Mental Health, University of Toronto, Toronto, Ont.; and the Department of Physics, University of California, Irvine. Address reprint requests to Dr. Brody, 300 UCLA Medical Plaza, Suite 2200, Los Angeles, CA 90095; [email protected] (e-mail). Supported by a Department of Veterans Affairs Type I Merit Review Award to Dr. Brody, grants 7KT-0098 and 11RT-0024 to Dr. Brody and 10RT-0091 to Dr. London from the Tobacco-Related Disease Research Program, and grants R01 DA-15059 to Dr. Brody and RO1 DA-14093 to Dr. London from the National Institute on Drug Abuse. The authors thank Josephine Ribe and Michael Clark for technical assistance in performing positron emission tomography and magnetic resonance imaging scans, respectively.
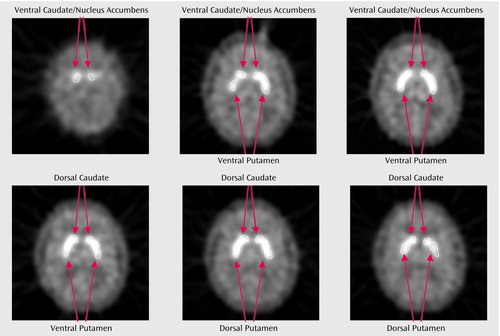
Figure 1. Regions of Interest Drawn on a Summed PET Scan of a Subject in a Study of Smoking-Induced Dopamine Release in the Ventral Striatuma
aExperimental regions of interest were the left and right ventral caudate/nucleus accumbens and ventral putamen. Control regions were the left and right dorsal caudate and putamen.
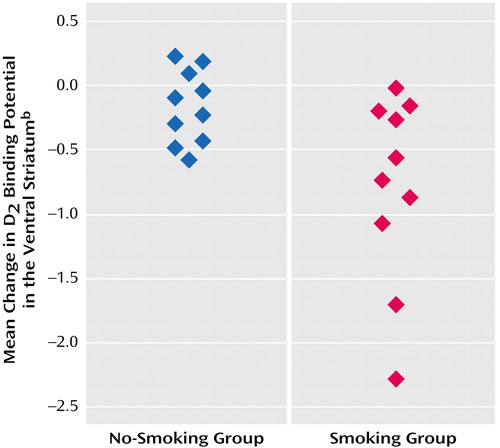
Figure 2. Change in Dopamine D2 Receptor Binding Potential in the Ventral Striatum of Cigarette Smokers, by Whether Subjects Smoked During a Break in PET Scanninga
aFive subjects who smoked had greater reductions in ventral striatum D2 binding potential than all 10 subjects who did not smoke. All 10 subjects who smoked had overall reductions in ventral striatum D2 binding potential.
bD2 binding potential was measured with [11C]raclopride, the radioligand used in the bolus-plus-continuous-infusion PET study. Volume-weighted mean changes for the ventral striatum include all four experimental regions of interest (left and right ventral caudate and left and right ventral putamen).
1. Leshner AI, Koob GF: Drugs of abuse and the brain. Proc Assoc Am Physicians 1999; 111:99–108Crossref, Medline, Google Scholar
2. Koob GF: Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 1992; 13:177–184Crossref, Medline, Google Scholar
3. Di Chiara G, Imperato A: Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988; 85:5274–5278Crossref, Medline, Google Scholar
4. Pontieri FE, Tanda G, Orzi F, Di Chiara G: Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382:255–257Crossref, Medline, Google Scholar
5. Sziraki I, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A: Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res 2001; 26:609–617Crossref, Medline, Google Scholar
6. Damsma G, Day J, Fibiger HC: Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol 1989; 168:363–368Crossref, Medline, Google Scholar
7. Corrigall WA, Franklin KB, Coen KM, Clarke PB: The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992; 107:285–289Crossref, Medline, Google Scholar
8. Rowell PP, Carr LA, Garner AC: Stimulation of [3H]dopamine release by nicotine in rat nucleus accumbens. J Neurochem 1987; 49:1449–1454Crossref, Medline, Google Scholar
9. Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CRJ: A pharmacologic strategy for the treatment of nicotine addiction. Synapse 1999; 31:76–86Crossref, Medline, Google Scholar
10. Salokangas RKR, Vilkman H, Ilonen T, Taiminen T, Bergman J, Haaparanta M, Solin O, Alanen A, Syvälahti E, Hietala J: High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry 2000; 157:632–634Link, Google Scholar
11. Laruelle M: Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20:423–451Crossref, Medline, Google Scholar
12. Vallone D, Picetti R, Borrelli E: Structure and function of dopamine receptors. Neurosci Biobehav Rev 2000; 24:125–132Crossref, Medline, Google Scholar
13. Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, Eckelman WC, Carson RE: Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab 1997; 17:932–942Crossref, Medline, Google Scholar
14. Schlaepfer TE, Pearlson GD, Wong DF, Marenco S, Dannals RF: PET study of competition between intravenous cocaine and [11C]raclopride at dopamine receptors in human subjects. Am J Psychiatry 1997; 154:1209–1213Link, Google Scholar
15. Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, Mathis C: PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology 1999; 21:694–709Crossref, Medline, Google Scholar
16. Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M: Imaging human mesolimbic dopamine transmission with positron emission tomography, part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23:285–300Crossref, Medline, Google Scholar
17. Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA: Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 2001; 49:81–96Crossref, Medline, Google Scholar
18. Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, et al: Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 1994; 16:255–262Crossref, Medline, Google Scholar
19. Cumming P, Rosa-Neto P, Watanabe H, Smith D, Bender D, Clarke PBS, Gjedde A: Effects of acute nicotine on hemodynamics and binding of [11-C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage 2003; 19:1127–1136Crossref, Medline, Google Scholar
20. Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A: Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse 1999; 31:59–66Crossref, Medline, Google Scholar
21. Park MH, Park EH: Synaptic concentration of dopamine in rat striatal slices in relationship to [3H]raclopride binding to the dopamine D2 receptor. Arch Pharm Res 2000; 23:360–366Crossref, Medline, Google Scholar
22. Hartvig P, Torstenson R, Tedroff J, Watanabe Y, Fasth KJ, Bjurling P, Langstrom B: Amphetamine effects on dopamine release and synthesis rate studied in the Rhesus monkey brain by positron emission tomography. J Neural Transm 1997; 104:329–339Crossref, Medline, Google Scholar
23. Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC: Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab 1997; 17:437–447Crossref, Medline, Google Scholar
24. de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ: Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 2001; 293:1164–1166Crossref, Medline, Google Scholar
25. Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC: Increased dopamine tone during meditation-induced change of consciousness. Brain Res Cogn Brain Res 2002; 13:255–259Crossref, Medline, Google Scholar
26. Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM: Evidence for striatal dopamine release during a video game. Nature 1998; 393:266–268Crossref, Medline, Google Scholar
27. Wang GJ, Volkow ND, Fowler JS, Logan J, Pappas NR, Wong CT, Hitzemann RJ, Netusil N: Reproducibility of repeated measures of endogenous dopamine competition with [11C]raclopride in the human brain in response to methylphenidate. J Nucl Med 1999; 40:1285–1291Medline, Google Scholar
28. Hietala J, Nagren K, Lehikoinen P, Ruotsalainen U, Syvalahti E: Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab 1999; 19:210–217Crossref, Medline, Google Scholar
29. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
30. Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL: Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 2000; 66:553–558Crossref, Medline, Google Scholar
31. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
32. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
33. Ito H, Hietala J, Blomqvist G, Halldin C, Farde L: Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab 1998; 18:941–950Crossref, Medline, Google Scholar
34. Schuh KJ, Stitzer ML: Desire to smoke during spaced smoking intervals. Psychopharmacology (Berl) 1995; 120:289–295Crossref, Medline, Google Scholar
35. Farde L, Hall H, Ehrin E, Sedvall G: Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231:258–261Crossref, Medline, Google Scholar
36. Ehrin E, Gawell L, Hogberg T, Depaulis T, Strom P: Synthesis of [methoxy-H-3]- and [methoxy-C-11]-labeled raclopride—specific dopamine-D2 receptor ligands. J Labelled Comp Radiopharm 1987; 24:931–940Crossref, Google Scholar
37. Woods RP, Mazziotta JC, Cherry SR: MRI-PET registration with automated algorithm. J Comp Assist Tomogr 1993; 17:536–546Crossref, Medline, Google Scholar
38. Wang GJ, Volkow ND, Levy AV, Fowler JS, Logan J, Alexoff D, Hitzemann RJ, Schyler DJ: MR-PET image coregistration for quantitation of striatal dopamine D2 receptors. J Comp Assist Tomogr 1996; 20:423–428Crossref, Medline, Google Scholar
39. Lammertsma AA, Hume SP: Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4:153–158Crossref, Medline, Google Scholar
40. Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE: Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med 2000; 41:522–530Medline, Google Scholar
41. Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J: Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry 1998; 155:768–773Abstract, Google Scholar
42. Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, Farde L, Rinne J: Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 2000; 21:683–688Crossref, Medline, Google Scholar
43. Le Foll B, Schwartz JC, Sokoloff P: Disruption of nicotine conditioning by dopamine D-3 receptor ligands. Mol Psychiatry 2003; 8:225–230Crossref, Medline, Google Scholar
44. Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben Shahar O: Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology 2001; 25:361–372Crossref, Medline, Google Scholar
45. Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P: Selective inhibition of cocaine-seeking behaviour by a partial dopamine D-3 receptor agonist. Nature 1999; 400:371–375Crossref, Medline, Google Scholar
46. Duong A, Weingarten HP: Dopamine antagonists act on central, but not peripheral, receptors to inhibit sham and real feeding. Physiol Behav 1993; 54:449–454Crossref, Medline, Google Scholar
47. Chance WT, Foley-Nelson T, Nelson JL, Fischer JE: Neurotransmitter alterations associated with feeding and satiety. Brain Res 1987; 416:228–234Crossref, Medline, Google Scholar
48. Rodriguez-Manzo G: Yohimbine interacts with the dopaminergic system to reverse sexual satiation: further evidence for a role of sexual motivation in sexual exhaustion. Eur J Pharmacol 1999; 372:1–8Crossref, Medline, Google Scholar
49. Fiorino DF, Coury A, Phillips AG: Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. J Neurosci 1997; 17:4849–4855Crossref, Medline, Google Scholar
50. Holm KJ, Spencer CM: Bupropion: a review of its use in the management of smoking cessation. Drugs 2000; 59:1007–1024Crossref, Medline, Google Scholar
51. Horst WD, Preskorn SH: Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Disord 1998; 51:237–254Crossref, Medline, Google Scholar
52. Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S: Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002; 163:102–105Crossref, Medline, Google Scholar
53. Nomikos GG, Damsma G, Wenkstern D, Fibiger HC: Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology 1989; 2:273–279Crossref, Medline, Google Scholar
54. Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al: Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995; 56:395–401Medline, Google Scholar
55. Dong J, Blier P: Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 2001; 155:52–57Crossref, Medline, Google Scholar
56. Jarvik ME, Caskey NH, Wirshing WC, Madsen DC, Iwamoto-Schaap PN, Elins JL, Eisenberger NI, Olmstead RE: Bromocriptine reduces cigarette smoking. Addiction 2000; 95:1173–1183Crossref, Medline, Google Scholar
57. Houtsmuller EJ, Thornton JA, Stitzer ML: Effects of selegiline (l-deprenyl) during smoking and short-term abstinence. Psychopharmacology (Berl) 2002; 163:213–220Crossref, Medline, Google Scholar
58. Brauer LH, Cramblett MJ, Paxton DA, Rose JE: Haloperidol reduces smoking of both nicotine-containing and denicotinized cigarettes. Psychopharmacology (Berl) 2001; 159:31–37Crossref, Medline, Google Scholar
59. Caskey NH, Jarvik ME, Wirshing WC: The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol 1999; 7:72–78Crossref, Medline, Google Scholar
60. Mathew RJ, Wilson WH: Substance abuse and cerebral blood flow. Am J Psychiatry 1991; 148:292–305Link, Google Scholar
61. Boyajian RA, Otis SM: Acute effects of smoking on human cerebral blood flow: a transcranial Doppler ultrasonography study. J Neuroimaging 2000; 10:204–208Crossref, Medline, Google Scholar
62. Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE: PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry 2003; 160:323–333; correction, 160:810Link, Google Scholar
63. Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF: Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry 2001; 49:906–913Crossref, Medline, Google Scholar
64. Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK: Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 2000; 38:313–321Crossref, Medline, Google Scholar
65. Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J: Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 1998; 136:179–189Crossref, Medline, Google Scholar
66. Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, Brooks DJ: Dopamine release during sequential finger movements in health and Parkinson’s disease: a PET study. Brain 2003; 126:312–325Crossref, Medline, Google Scholar
67. Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Sakamoto M: Effect of simple motor performance on regional dopamine release in the striatum in Parkinson disease patients and healthy subjects: a positron emission tomography study. J Cereb Blood Flow Metab 2002; 22:746–752Crossref, Medline, Google Scholar
68. Wang GJ, Volkow ND, Fowler JS, Franceschi D, Logan J, Pappas NR, Wong CT, Netusil N: PET studies of the effects of aerobic exercise on human striatal dopamine release. J Nucl Med 2000; 41:1352–1356Medline, Google Scholar



