A Randomized, Placebo-Controlled Trial of Citalopram for the Treatment of Major Depression in Children and Adolescents
Abstract
OBJECTIVE: Open-label trials with the selective serotonin reuptake inhibitor citalopram suggest that this agent is effective and safe for the treatment of depressive symptoms in children and adolescents. The current study investigated the efficacy and safety of citalopram compared with placebo in the treatment of pediatric patients with major depression. METHOD: An 8-week, randomized, double-blind, placebo-controlled study compared the safety and efficacy of citalopram with placebo in the treatment of children (ages 7–11) and adolescents (ages 12–17) with major depressive disorder. Diagnosis was established with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version. Patients (N=174) were treated initially with placebo or 20 mg/day of citalopram, with an option to increase the dose to 40 mg/day at week 4 if clinically indicated. The primary outcome measure was score on the Children’s Depression Rating Scale—Revised; the response criterion was defined as a score of ≤28. RESULTS: The overall mean citalopram dose was approximately 24 mg/day. Mean Children’s Depression Rating Scale—Revised scores decreased significantly more from baseline in the citalopram treatment group than in the placebo treatment group, beginning at week 1 and continuing at every observation point to the end of the study (effect size=2.9). The difference in response rate at week 8 between placebo (24%) and citalopram (36%) also was statistically significant. Citalopram treatment was well tolerated. Rates of discontinuation due to adverse events were comparable in the placebo and citalopram groups (5.9% versus 5.6%, respectively). Rhinitis, nausea, and abdominal pain were the only adverse events to occur with a frequency exceeding 10% in either treatment group. CONCLUSIONS: In this population of children and adolescents, treatment with citalopram reduced depressive symptoms to a significantly greater extent than placebo treatment and was well tolerated.
Up to 5% of children and 8% of adolescents meet diagnostic criteria for depression, with the incidence of depression increasing markedly after puberty (1–4). The mean duration of pediatric depressive episodes is 9 months, and there is substantial risk for relapse (4, 5). Like depression in adults, pediatric depression is associated with significant social and functional impairment. School performance, peer relationships, and family functioning all are affected negatively in children and adolescents with depression (6), and risk of suicide is increased (7). Furthermore, depression in children and adolescents frequently continues into adulthood, resulting in considerable morbidity and mortality (8).
Although childhood depression is increasingly being recognized, few randomized, placebo-controlled trials of antidepressant pharmacotherapy have been reported in this population, and those that have appeared in the literature have had mixed results. For example, trials with tricyclic antidepressants have not demonstrated superiority over placebo (9). However, several recently published studies have suggested that selective serotonin reuptake inhibitors (SSRIs) may be effective and well tolerated in children and adolescents. In double-blind, placebo-controlled trials, fluoxetine (10, 11) and sertraline (12) have shown efficacy in the treatment of children and adolescents with major depression, as has paroxetine for the treatment of depressed adolescents (13). The use of SSRIs in children and adolescents has increased dramatically as a result of these trials, case reports, and open-label studies. Indeed, approximately 70% of family physicians and pediatricians in one mail survey reported prescribing SSRIs to children and adolescents with psychiatric disorders, including depression (14).
Citalopram, an SSRI shown to be effective in the treatment of depression in the adult population (15–17), appears well suited for use in children and adolescents because it has a favorable side effect profile, a low potential for drug-drug interactions, and is relatively safe in overdose (18, 19), which is of concern in this population (7). Additionally, open-label trials and case reports suggest that citalopram is effective in younger patients (20, 21).
The objective of the current randomized, double-blind, placebo-controlled, flexible-dose study was to compare the efficacy and safety of citalopram with placebo in the treatment of children and adolescents with major depressive disorder.
Method
Study Population
This study was conducted at 21 hospital, academic, and research centers in the United States. Children (ages 7–11) and adolescents (ages 12–17) who met DSM-IV criteria for major depressive disorder and whose current episode of major depressive disorder was at least 4 weeks in duration at baseline were eligible for participation. The Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (22), a semistructured diagnostic interview, was used to establish that patients met DSM-IV criteria for major depressive disorder and to rule out other psychiatric diagnoses. A score of at least 40 on the Children’s Depression Rating Scale—Revised (23, 24) was required at the screening and baseline visits.
Additional inclusion criteria were a normal physical examination, laboratory tests, and ECG results. Female patients of childbearing potential were required to have negative serum human chorionic gonadotropin levels at screening and be willing to practice a reliable method of birth control. Patients were required to provide assent prior to participation, and the parent or legal guardian had to provide written consent. A parent or caregiver was required to accompany the patient at each visit. The study protocol was approved by institutional review boards at each study center.
Patients were excluded from the study for any of the following reasons: 1) primary psychiatric diagnoses other than major depressive disorder; 2) a DSM-IV diagnosis of attention deficit hyperactivity disorder (ADHD), posttraumatic stress disorder, bipolar disorder, pervasive development disorder, mental retardation, conduct disorder, or oppositional defiant disorder; 3) any psychotic features; 4) any personality disorder that would interfere with study participation; 5) a history of alcohol or substance abuse within the past year; or 6) anorexia or bulimia within the past year. Initiation of psychotherapy or behavioral therapy 3 months prior to the screening visit or during the study was not allowed. Patients who were considered a suicide risk, who had made an active suicide attempt within the past year, or had been hospitalized because of an attempt also were excluded.
Patients who had been treated with any antidepressant or anxiolytic medication within 2 weeks of baseline (4 weeks for fluoxetine), had been treated with a neuroleptic or stimulant within 6 months prior to screening, or received an investigational drug 30 days prior to study entry were excluded. Concomitant treatment with certain prescription or over-the-counter medications (e.g., antipsychotics, anticonvulsants, sedatives, hypnotics, and cardiovascular agents, among others) also was prohibited per protocol.
Study Design
Following an initial screening visit and a 1-week, single-blind placebo lead-in period, patients returned for a baseline visit to determine whether they remained eligible to participate. Eligible patients were then randomly assigned in double-blind fashion to 8 weeks of citalopram or placebo treatment. Citalopram was initiated at 20 mg/day, with the potential to increase the dose to 40 mg/day anytime after week 4 if deemed clinically necessary. Subsequent to week 4, the dose could be decreased to 20 mg/day for tolerability reasons. Evaluations were scheduled after 1, 2, 4, 6, and 8 weeks of double-blind treatment.
The primary outcome measure in this study was the change from baseline in score on the Children’s Depression Rating Scale—Revised at week 8 or upon termination. The Children’s Depression Rating Scale—Revised was administered at each study visit. Response was defined as a score of ≤28 (indicating minimal residual symptoms). Secondary measures included Clinical Global Impression (CGI) improvement and severity ratings (25). At the final visit (week 8), a physical examination and laboratory tests were performed, and ECG results were obtained. Adverse events were spontaneously reported by patients or observed by investigators.
Statistical Analyses
Treatment differences in the primary efficacy outcome, baseline-to-endpoint change in score on the Children’s Depression Rating Scale—Revised, in patients treated with citalopram versus patients treated with placebo were assessed using an analysis of covariance model, with treatment, study center, and age group as factors and the baseline score as covariate. A Cochran-Mantel-Haenszel test controlling for center and age group was applied for between-treatment comparison with respect to the response rate. These analyses were carried out using the last observation carried forward approach at week 8.
Results
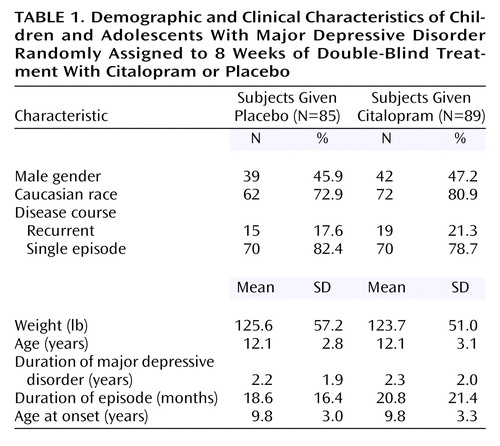
A total of 178 patients (93 in the citalopram group and 85 in the placebo group) were randomly assigned to double-blind treatment. Four patients (three children and one adolescent), all randomly assigned to the citalopram group, were lost to follow-up and did not receive study medication. These patients were not included in the intent-to-treat analyses. Thus, the intent-to-treat population consisted of 89 patients (45 children and 44 adolescents) assigned to citalopram and 85 patients (38 children and 47 adolescents) assigned to placebo. Of these, 18 patients from each group discontinued double-blind treatment prematurely. Demographic and clinical characteristics of the patient population at baseline are summarized in Table 1. There were no significant differences in age, gender, race, or weight between the placebo and citalopram groups. The mean age was 12.1 years in both treatment groups, and there were no differences between groups in the mean ages of children and adolescents. Additionally, responses to the K-SADS-PL (administered at the screening visit) indicated there were no clinically meaningful differences between the citalopram and placebo treatment groups in depression history. Ongoing secondary psychiatric disorders other than depression were reported in 23 patients, the most common diagnosis being dysthymia (citalopram group: 5.6%, placebo group: 1.2%) and enuresis (citalopram group: 4.5%, placebo group: 3.5%). A previous diagnosis of ADHD had been made in four citalopram-treated patients (4.5%) and one placebo-treated patient (1.2%); there were no reports of ongoing ADHD. Approximately 80% of the patients who participated in the study were experiencing a single episode of depression with a mean duration of 2 years. The mean duration of the current depressive episode was about 20 months. The age at onset was approximately 7 years for children and 12 years for the adolescent group. Twenty percent of the patients in the citalopram group and 18% of patients in the placebo group received previous antidepressant treatment, and approximately 15% of the patients in the citalopram group and 16% of the patients in the placebo group had a history of nonresponse to antidepressant treatment. Mean Children’s Depression Rating Scale—Revised scores at baseline were 58.8 (SD=10.9) and 57.8 (SD=11.1) in the citalopram and placebo groups, respectively, indicative of moderately severe illness.
As noted in the Method section, investigators had the option to increase the dose of study medication to 40 mg/day citalopram or placebo equivalent any time after the week 4 visit. By the end of the study, medication dose had been increased in 53 placebo-treated patients (62.4%) and in 48 citalopram-treated patients (53.9%). In the citalopram group, the mean daily dose over the 8-week treatment period was 23.3 mg/day (SD=5.0) for the children and 24.4 mg/day (SD=5.0) for the adolescents.
Citalopram treatment showed statistically significant improvement compared with placebo on the Children’s Depression Rating Scale—Revised as early as week 1 (F=6.58, df=1, 150, p<0.05), which persisted throughout the study (Figure 1). At week 8, the effect size on the primary outcome measure, Children’s Depression Rating Scale—Revised (last observation carried forward), was 2.9. Additionally, at endpoint more citalopram-treated patients (36%) met the prospectively defined criterion for response than did placebo-treated patients (24%), a difference that was statistically significant (χ2=4.178, df=1, p<0.05). The proportion of patients with a CGI improvement rating ≤2 at week 8 was 47% for the citalopram group and 45% for the placebo group (last observation carried forward values). For the CGI severity rating, baseline values were 4.4 for the citalopram group and 4.3 for the placebo group, and endpoint values (last observation carried forward) were 3.1 for the citalopram group and 3.3 for the placebo group.
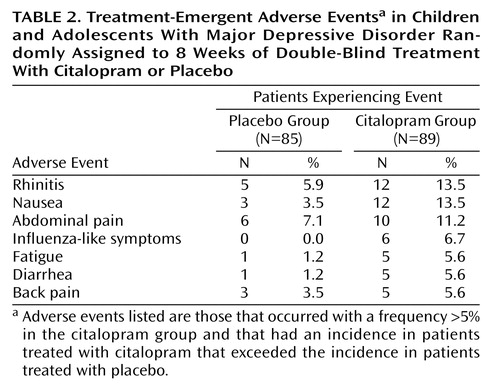
Citalopram treatment was well tolerated. All treatment-emergent adverse events that occurred with a frequency >5% in the citalopram group, and with an incidence in citalopram-treated patients that exceeded that in placebo-treated patients, are listed in Table 2. Of these, nausea, rhinitis, and abdominal pain were the only adverse events occurring in ≥10% of citalopram-treated patients. There were no reports of mania. The rate of discontinuation due to adverse events was comparable in the placebo and citalopram groups (5.9% versus 5.6%, respectively). The only adverse events that led to the discontinuation of more than one patient were aggravated depression (two placebo, no citalopram) and agitation (no placebo, two citalopram). No clinically significant ECG results, laboratory values, or weight changes were found. No serious adverse events were observed in any patient treated with citalopram in this study.
Discussion
This randomized, placebo-controlled, double-blind trial provides evidence that citalopram produces a statistically and clinically significant reduction in depressive symptoms in children and adolescents. Specifically, citalopram was superior to placebo on the primary efficacy measure, score on the Children’s Depression Rating Scale—Revised, as early as week 1 with efficacy continuing throughout the trial. Reported adverse events were mild, and the rate of discontinuation due to adverse events was similar in the placebo and citalopram groups.
The results of this trial are in agreement with previous open-label trials (20) and case reports (21), which suggest that citalopram is efficacious and safe in children and adolescents with depression. In the current trial, citalopram-treated patients demonstrated significant improvement in depressive symptoms compared with placebo at week 1, an earlier time point than has been reported in other published trials of SSRIs in children and adolescents (10–12), although one placebo-controlled trial demonstrated significance for fluoxetine at week 1 (11). This is a potentially important observation, since therapeutic treatment options in these young patients remain limited, and early amelioration of symptoms may serve to enhance adherence to an adequate course of therapy.
In our study, the prospectively defined criterion of response was an endpoint score of ≤28 on the Children’s Depression Rating Scale—Revised. In the Emslie et al. study of fluoxetine (11), a Children’s Depression Rating Scale—Revised endpoint total score ≤28 was defined as remission. It is noteworthy, therefore, that the rates of response for citalopram (36%) were similar to the remission rates for fluoxetine (41%).
It is tempting to speculate that similar clinical results would be achieved in children and adolescents treated with the recently developed single isomer compound escitalopram, since the serotonin reuptake activity of citalopram is attributable to its S-isomer (26). This hypothesis is being tested in a multicenter, randomized, double-blind study of escitalopram in pediatric patients that is currently under way.
The mean citalopram dose for children and adolescents over the 8-week trial was approximately 25 mg/day, which is similar to the minimum dose shown to be effective in adults (27). There were no serious adverse events observed in the citalopram group. In fact, the overall adverse event profile was similar to that reported in adult trials, with no new or unexpected adverse events reported. Notably absent in this population of children and adolescents were dizziness and somnolence, two events frequently reported by adult patients (18). In this study, psychiatric adverse events were reported infrequently by patients randomly assigned to citalopram. For example, adverse events associated with behavioral activation (such as insomnia or agitation) were not prevalent in this trial.
The consistent and significant reduction in Children’s Depression Rating Scale—Revised score in citalopram-treated patients compared with placebo-treated patients at weeks 1, 2, and 4 suggests that citalopram, 20 mg/day, is an effective dose in this population, since the citalopram dose was fixed throughout the first 4 weeks of the trial.
Of interest, early and sustained improvements in Children’s Depression Rating Scale—Revised scores were observed with citalopram treatment in this study despite a relatively high rate of placebo response (which is common in pediatric trials with antidepressants). The study was not powered sufficiently to detect treatment differences by age group. Other limitations include the low number of patients with comorbid conditions and concomitant medication use, both of which are common in younger patients. It is not known whether the presence of comorbid psychiatric disorders such as ADHD would have affected the observed outcomes of this trial; however, the exclusion criteria employed produced a study population comparable to those of Emslie et al. (11) and Wagner et al. (12).
In conclusion, citalopram treatment significantly improved depressive symptoms compared with placebo within 1 week in this population of children and adolescents. No serious adverse events were reported, and the rate of discontinuation due to adverse events among citalopram-treated patients was comparable to that of placebo. These findings further support the use of citalopram in children and adolescents suffering from major depression.
 |
 |
Presented in part at the 155th annual meeting of the American Psychiatric Association, Philadelphia, May 18–23, 2002. Received Dec. 4, 2002; revisions received Aug. 20 and Oct. 28, 2003; accepted Dec. 4, 2003. From the Department of Psychiatry and Behavioral Sciences, University of Texas Medical Branch, Galveston; Children’s National Medical Center, Washington, D.C.; University Hospitals of Cleveland; Forest Laboratories, Inc., New York; and Lexicon Pharmaceuticals, Princeton, N.J. Address reprint requests to Dr. Wagner, Department of Psychiatry and Behavioral Sciences, University of Texas Medical Branch, 301 University Blvd., Galveston, TX 77755-0188; [email protected] (e-mail). Supported by funding from Forest Pharmaceuticals.
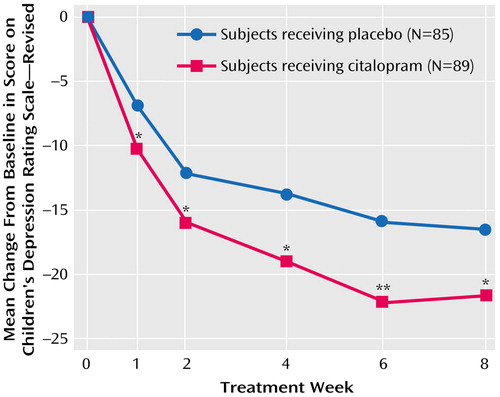
Figure 1. Baseline-to-Endpoint Changes in Score on the Children’s Depression Rating Scale—Revised in Children and Adolescents With Major Depressive Disorder Randomly Assigned to 8 Weeks of Double-Blind Treatment With Citalopram or Placebo
*p<0.05. **p<0.01.
1. Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B: Childhood and adolescent depression: a review of the past 10 years, part I. J Am Acad Child Adolesc Psychiatry 1996; 35:1427–1439Crossref, Medline, Google Scholar
2. Kroes M, Kalff AC, Kessels AG, Steyaert J, Feron FJ, van Someren AJ, Hurks PP, Hendriksen JG, van Zeben TM, Rozendaal N, Crolla IF, Troost J, Jolles J, Vles JS: Child psychiatric diagnoses in a population of Dutch schoolchildren aged 6 to 8 years. J Am Acad Child Adolesc Psychiatry 2001; 40:1401–1409Crossref, Medline, Google Scholar
3. Lewinsohn PM, Clarke GN, Seeley JR, Rohde P: Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry 1994; 33:809–818Crossref, Medline, Google Scholar
4. McCauley E, Myers K, Mitchell J, Calderon R, Schloredt K, Treder R: Depression in young people: initial presentation and clinical course. J Am Acad Child Adolesc Psychiatry 1993; 32:714–722Crossref, Medline, Google Scholar
5. Kovacs M, Obrosky DS, Gatsonis C, Richards C: First-episode major depressive and dysthymic disorder in childhood: clinical and sociodemographic factors in recovery. J Am Acad Child Adolesc Psychiatry 1997; 36:777–784Crossref, Medline, Google Scholar
6. Fleming JE, Offord DR: Epidemiology of childhood depressive disorders: a critical review. J Am Acad Child Adolesc Psychiatry 1990; 29:571–580Crossref, Medline, Google Scholar
7. Rao U, Weissman MM, Martin JA, Hammond RW: Childhood depression and risk of suicide: a preliminary report of a longitudinal study. J Am Acad Child Adolesc Psychiatry 1993; 32:21–27Crossref, Medline, Google Scholar
8. Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P: Depressed adolescents grown up. JAMA 1999; 281:1707–1713Crossref, Medline, Google Scholar
9. Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D: Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. BMJ 1995; 310:897–901Crossref, Medline, Google Scholar
10. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54:1031–1037Crossref, Medline, Google Scholar
11. Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2002; 41:1205–1215Crossref, Medline, Google Scholar
12. Wagner KD, Ambrosini P, Rynn M, Wohlberg C, Yang R, Greenbaum MS, Childress A, Donnelly C, Deas D (Sertraline Pediatric Depression Study Group): Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA 2003; 290:1033–1041Crossref, Medline, Google Scholar
13. Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, Hagino OR, Koplewicz H, Carlson GA, Clarke GN, Emslie GJ, Feinberg D, Geller B, Kusumakar V, Papatheodorou G, Sack WH, Sweeney M, Wagner KD, Weller EB, Winters NC, Oakes R, McCafferty JP: Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2001; 40:762–772Crossref, Medline, Google Scholar
14. Rushton JL, Clark SJ, Freed GL: Pediatrician and family physician prescription of selective serotonin reuptake inhibitors. Pediatrics 2000; 105:E82Google Scholar
15. Feighner JP, Overø K: Multicenter, placebo-controlled, fixed dose study of citalopram in moderate to severe depression. J Clin Psychiatry 1999; 60:824–830Crossref, Medline, Google Scholar
16. Mendels J, Kiev A, Fabre LF: Double-blind comparison of citalopram and placebo in depressed outpatients with melancholia. Depress Anxiety 1999; 9:54–60Crossref, Medline, Google Scholar
17. Willetts J, Lippa A, Beer B: Clinical development of citalopram. J Clin Psychopharmacol 1999; 19:36S-46SCrossref, Medline, Google Scholar
18. Noble S, Benfield P: Citalopram: a review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs 1997; 8:410–431Crossref, Google Scholar
19. Barbey JT, Roose SP: SSRI safety in overdose. J Clin Psychiatry 1998; 59(suppl 15):42–48Google Scholar
20. Bostic JQ, Prince J, Brown K, Place S: A retrospective study of citalopram in adolescents with depression. J Child Adolesc Psychopharmacol 2001; 11:159–166Crossref, Medline, Google Scholar
21. Thomsen PH: Child and adolescent obsessive-compulsive disorder treated with citalopram: findings from an open trial of 23 cases. J Child Adolesc Psychopharmacol 1997; 7:157–166Crossref, Medline, Google Scholar
22. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
23. Poznanski EO, Cook SC, Carroll BJ: A depression rating scale for children. Pediatrics 1979; 64:442–450Medline, Google Scholar
24. Poznanski EO, Mokros HB: Manual: Children’s Depression Rating Scale—Revised. Los Angeles, Western Psychological Services, 1996Google Scholar
25. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
26. Hyttel J, Bogeso KP, Perregaard J, Sanchez C: The pharmacological effect of citalopram resides in the (S)-(+)-enantiomer. J Neural Transm Gen Sect 1992; 88:157–160Crossref, Medline, Google Scholar
27. Montgomery SA, Pedersen V, Tanghøj P, Rasmussen C, Rioux P: The optimal dosing regimen for citalopram—a meta-analysis of nine placebo-controlled studies. Int Clin Psychopharmacol 1994; 1:35–40Crossref, Google Scholar



