Repetitive Transcranial Magnetic Stimulation of the Right Dorsolateral Prefrontal Cortex in Posttraumatic Stress Disorder: A Double-Blind, Placebo-Controlled Study
Abstract
OBJECTIVE: The efficacy of repetitive transcranial magnetic stimulation (rTMS) of the right prefrontal cortex was studied in patients with posttraumatic stress disorder (PTSD) under double-blind, placebo-controlled conditions. METHOD: Twenty-four patients with PTSD were randomly assigned to receive rTMS at low frequency (1 Hz) or high frequency (10 Hz) or sham rTMS in a double-blind design. Treatment was administered in 10 daily sessions over 2 weeks. Severity of PTSD, depression, and anxiety were blindly assessed before, during, and after completion of the treatment protocol. RESULTS: The 10 daily treatments of 10-Hz rTMS at 80% motor threshold over the right dorsolateral prefrontal cortex had therapeutic effects on PTSD patients. PTSD core symptoms (reexperiencing, avoidance) markedly improved with this treatment. Moreover, high-frequency rTMS over the right dorsolateral prefrontal cortex alleviated anxiety symptoms in PTSD patients. CONCLUSIONS: This double-blind, controlled trial suggests that in PTSD patients, 10 daily sessions of right dorsolateral prefrontal rTMS at a frequency of 10 Hz have greater therapeutic effects than slow-frequency or sham stimulation.
Posttraumatic stress disorder (PTSD) is an incapacitating clinical syndrome characterized by intrusive recollections, emotional numbing and withdrawal, cue-related responses, and psychological and physiological hyperarousal (DSM-IV).
There is no definitive pharmacotherapy for PTSD. Agents studied that have resulted in various degrees of improvement in core PTSD symptoms and/or the associated anxiety and depressive symptoms (1–3) have included antidepressants (monoamine oxidase inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors [SSRIs]) (4–6), adrenergic agonists and antagonists (clonidine, propranolol) (7, 8), mood-stabilizing drugs (carbamazepine, lithium, valproic acid) (9–11), and benzodiazepines (alprazolam) (12). The Food and Drug Administration has to date approved the use of sertraline and paroxetine (which are SSRIs) for the indication of PTSD.
Transcranial magnetic stimulation (TMS) is a noninvasive technique for directly stimulating cortical neurons. Electrical energy crosses the brain almost painlessly, without causing convulsions or cognitive impairment but causing a depolarization of neurons (13) with cortical changes in monoamines (14). TMS has been shown to have some antidepressant-like effects (15–18).
In a prior open pilot study (19), one session of slow TMS with 30-m/sec pulses, 15 stimuli to each side of the vertex, was found to be effective in lowering the core PTSD symptom of avoidance, as well as anxiety and somatization, and in improving ratings on the Clinical Global Impression scale. These preliminary results were taken to show that TMS is a safe and tolerable intervention with possible therapeutic efficacy for PTSD patients (19).
Treatment with 1-Hz repetitive transcranial magnetic stimulation (rTMS) over the right frontal cortex has been reported (20) to demonstrate normalization of positron emission tomography (PET) findings, decreasing regional cerebral blood flow (rCBF) as well as improving the clinical condition of two patients with PTSD.
The prefrontal cortex has been implicated in the organization and control of behavior (21) by way of extensive dorsolateral prefrontal subcortical connections to limbic structures. The prefrontal cortex is involved in many complex cognitive and behavioral functions that are potentially relevant to PTSD, such as working memory (22–24), supervisory attentional control (25), reasoning and decision making (26), temporal organization of behavior (27), and inhibition of cognition.
Structural and functional neuroimaging studies have demonstrated abnormalities in the prefrontal cortex in PTSD patients. Low metabolism was found at baseline in temporal and prefrontal cortical areas (28) and in the parietal cortex of substance-dependent PTSD patients (29, 30), according to PET scans. PTSD patients showed increased rCBF in the amygdala and decreased blood flow in the medial prefrontal cortex in response to provocation of symptoms by script-driven imagery (31–34). These findings have been found in both subjects with combat-related PTSD and subjects whose PTSD was related to childhood abuse. Using a pharmacological challenge in Vietnam combat veterans with PTSD and healthy age-matched comparison subjects, Bremner et al. (28) found that the responses of brain metabolism to yohimbine were significantly smaller in the prefrontal, temporal, parietal, and orbitofrontal cortices of PTSD patients than those of healthy subjects.
Proton magnetic resonance spectroscopy (MRS) measurement of medial temporal lobe neural density in Vietnam combat veterans with PTSD and in healthy veterans (35) showed that the ratio of N-acetyl-l-aspartic acid to creatinine in the right hemisphere of the PTSD patients was significantly lower than that on the left and was also significantly lower than that of the comparison subjects. De Bellis et al. (36) reported lower levels of N-acetylaspartate in the anterior cingulate region in maltreated children with PTSD than in healthy matched subjects. The lower N-acetylaspartate/creatine ratio suggests neuronal loss in the anterior cingulate (36). Functional neuroimaging studies are beginning to reveal fairly consistent data indicating a hyperresponsive amygdala accompanied by hypoactivation of the prefrontal cortex in PTSD patients.
A specific pattern of prefrontal and limbic abnormalities is suggested by neuropsychological tests sensitive to frontal lobe damage, which demonstrate impaired performance on tests reflecting abnormalities of the dorsolateral prefrontal cortex, the orbitofrontal cortex (part of the ventral prefrontal system intimately connected with the limbic system), and the limbic system in general (37–39).
Taken together, these findings suggest that right limbic and paralimbic structures are intimately involved with PTSD abnormalities and could potentially be the target of neurobiological treatment strategies. Therefore, the stimulation in this study was focused on the right dorsolateral prefrontal cortex.
It is well documented that rTMS effects on cortical excitability may depend on the frequency of stimulation (15). rTMS to the motor cortex has been reported to increase the excitability of some cortical neurons when delivered at high frequencies (5–20 Hz) or to depress excitability at low frequencies (1–5 Hz) (40, 41). Therefore, we compared low-frequency (1 Hz), high-frequency (10 Hz), and sham stimulation.
The aim of this study was to evaluate the therapeutic effects of two different frequencies of active rTMS, as compared to sham stimulation, administered to the right dorsolateral prefrontal cortex of PTSD patients.
Method
Subjects
Twenty-nine consecutive patients fulfilling the DSM-IV diagnostic criteria for PTSD (as assessed by the Structured Clinical Interview) were recruited from the inpatient and outpatient treatment programs at the Beer Sheva Mental Health Center. Twenty-four patients completed a course of 10 daily rTMS sessions.
The exclusion criteria included substance use disorder, cardiac pacemaker implant, or a history of epilepsy, neurosurgery, or brain trauma. Patients suffering from chronic medical conditions of any sort were excluded from the study.
After receiving a full explanation of the procedures, all subjects signed a written informed consent statement approved by the Helsinki Ethics Committee of Ben-Gurion University. Subjects were randomly assigned (by N.G.) to either sham treatment or active treatment, with two different active treatment frequencies, faster (10 Hz) or slower (1 Hz).
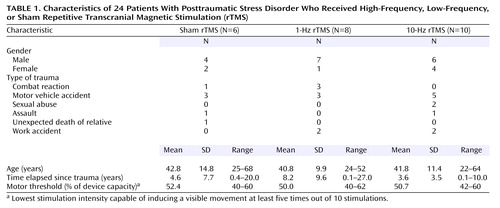
The subject group consisted of 17 men and seven women, with a mean age of 41.7 years (SD=11.4, range=22–68). The types of trauma were combat reaction (N=4), motor vehicle accident (N=11), sexual abuse (N=2), assault (N=2), work accident (N=4), and unexpected death of a relative (N=1). The mean time elapsed since the trauma was 5.4 years (SD=7.0, range=0.1–27.0) (Table 1). None of the patients suffered from any chronic medical disease.
The participants were asked to answer questions regarding their personal background, demographic characteristics, marital status, place of birth, education, and place of residence and the type of trauma that led them to seek help.
Four subjects were drug free. Polypharmacy was the rule for the majority. Two were receiving clomipramine, 15 were taking SSRIs or a serotonin and norepinephrine reuptake inhibitor, 19 were taking benzodiazepines (for five, the benzodiazepine was the only therapy), and four were receiving mood stabilizers. Three subjects with severe PTSD who were receiving long-term antipsychotic treatment enrolled in the study. Two of the three were taking olanzapine (10 mg/day), and one was taking clothiapine (40 mg/day); in addition, they were receiving mood stabilizers, SSRIs, and benzodiazepines. No significant differences in pharmacotherapy were found between groups. Drug treatment was neither stopped nor changed in the 3 weeks before the study or during the study. The patients continued to receive the same individual and group supportive psychotherapy as before the intervention.
Treatment Characteristics
In this double blind, placebo-controlled study, the patients were randomly assigned to one of three stimulation groups. None of the patients had any experience with rTMS before the study.
rTMS was performed with a Magstim stimulator (Magstim Company, Whitland, U.K.) having a circular coil with a 9-cm diameter.
The motor threshold was determined in each subject once, before treatment. This was defined as the lowest stimulation intensity capable of inducing a visible movement at least five times out of 10 stimulations (42).
The position of the right dorsolateral prefrontal cortex was defined as 5 cm anterior (in a parasagittal line) to the motor cortex. The stimulus intensity was 80% of the patient’s motor threshold intensity. The mean motor threshold for the group receiving sham treatment was 52.4%, with a range of 40%–60%; for the group receiving slow-frequency rTMS the mean was 50.0%, with a range of 40%–62%; and for the group receiving high-frequency treatment the mean was 50.7%, with a range of 42%–60%.
Treatments were given for 20 minutes per day over 10 working days. The patients were randomly assigned to the three treatment groups after assessment of their motor thresholds. Group 1 (sham rTMS) was treated in the same way as the group receiving high-frequency rTMS, but the coil was held at 90° vertical over the stimulated head area (no significant magnetic field was evoked, just the auditory artifact). Group 2 (slow-frequency rTMS) received 1 Hz for 5 seconds per train; the intertrain interval was 55 seconds. Group 3 (high-frequency rTMS) received 10 Hz for 2 seconds per train; the intertrain interval was 58 seconds.
For each participant the stimulus was administered over the right dorsolateral prefrontal cortex.
Rating Scales
The ratings of PTSD symptoms, anxiety, and depression were carried out by an expert investigator (R.M.) who was blind to the stimulation condition. The patients were assessed at four time points—before TMS (baseline), at day 5, at day 10, and at day 24 (14 days after the intervention). The instruments used were as follows.
The PTSD Checklist is a 17-item self-report checklist of PTSD symptoms based closely on the DSM-IV criteria (43). The respondents rate each item from 1 (“not at all”) to 5 (“extremely”) to indicate the degree to which they have been bothered by that particular symptom over the past month. Thus, the total scores can range from 17 to 85.
The Treatment Outcome PTSD Scale (44) is a clinician-rated instrument that measures the presence and severity of PTSD. This eight-item instrument measures symptoms that occur frequently within the PTSD population and is sensitive to the three major PTSD symptom dimensions (intrusive thoughts, avoidance behavior, and hyperarousal symptoms). Each symptom is rated on a defined step scale (0 to 4). Higher scores reflect greater severity on each measure.
The Hamilton Anxiety Rating Scale (45) is a clinician-rated instrument that measures the presence and severity of anxiety. This instrument covers 14 symptoms. Each symptom is rated on a defined scale (0 to 4). Here, too, a higher numeric rating reflects greater symptom severity.
The Hamilton Rating Scale for Depression (46) is a 23-item instrument that measures the presence and severity of depression. Each symptom is rated on a defined scale (0 to 4), whereby a higher numeric rating reflects greater symptom severity.
PTSD symptoms were assessed by using the Hebrew version of the Clinician-Administered PTSD Scale (47). This is a structured interview for assessing PTSD according to DSM-IV criteria. It quantifies symptom frequency and intensity for each of the criteria, yielding both a continuous measure of symptom severity and a dichotomous classification of PTSD status. A severity score for each symptom is calculated by summing the frequency and intensity scores. Thus, the total range of the instrument is 0–136. If a particular symptom was not present, the individual item was automatically scored as zero, as a default option. The Hebrew version of the scale has been extensively used and validated (49).
The questionnaires were filled out in the presence of an interviewer, and the subjects were assisted in answering the questions if necessary. The interviewer made sure that all subjects clearly understood the content of each item and the different aspects of the various component questions.
Statistical Methods
The ratings of psychopathology (PTSD Checklist, Treatment Outcome PTSD Scale, Hamilton anxiety scale, Hamilton depression scale) were entered into two-way repeated-measures analyses of variance with covariance for baseline scores (ANCOVAs), with treatment as the between-group factor (fast rTMS, slow rTMS, sham rTMS) and time as the within-subjects factor (day 5, day 10, and day 24). Repeated-measures analysis of variance (ANOVA) was used in order to compare the scores on the Clinician-Administered PTSD Scale of the three treatment groups (fast rTMS, slow rTMS, sham rTMS) and to estimate the effect of time (baseline and day 24, i.e., 14 days after the last treatment).
All scale scores were analyzed as change from baseline to the end of the treatment (day 10) or to follow-up 14 days later, by using ANOVA.
Dropouts
Five of the original 29 patients did not complete a course of rTMS. Two patients (receiving sham treatment) asked that the procedure stop immediately after motor threshold location, before stimulation began. One patient (receiving 1-Hz rTMS) refused to continue after one session, complaining that dizziness that existed before treatment had worsened. Another patient (1-Hz rTMS) asked that treatment stop after two sessions because of reasons unrelated to any side effect of the treatment. One patient (receiving 10-Hz rTMS) was excluded at the end of the treatment because of problems with device calibration.
Results
The demographic data are summarized in Table 1. The three groups did not differ significantly on age, gender, or duration of illness.
Scores on the PTSD Checklist, Treatment Outcome PTSD Scale, Hamilton anxiety scale, Hamilton depression scale, and Clinician-Administered PTSD Scale of the patients treated with active rTMS and sham rTMS are shown in Table 2.
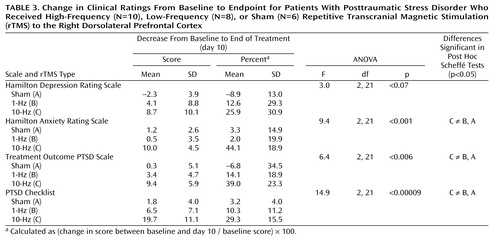
Two-way repeated-measures ANCOVA for the PTSD Checklist showed a significant effect of rTMS treatment group (Table 2). Post hoc Sheffé tests showed a significant effect of rTMS treatment group; active 10-Hz rTMS was significantly different from the sham (p<0.01) and 1-Hz (p<0.002) treatments. As shown in Table 3, during high-frequency active rTMS treatment, the mean PTSD Checklist scores decreased by 29.3% from baseline to the end of treatment (day 10).
Two-way repeated-measures ANCOVA for the Treatment Outcome PTSD Scale showed a significant effect of rTMS treatment type and a significant interaction of time and treatment (Table 2). Post hoc Sheffé tests showed a significant effect of rTMS treatment; active 10-Hz rTMS was significantly different from the sham (p<0.05) and 1-Hz (p<0.02) treatments. As shown in Table 3, during the active high-frequency rTMS treatment, the mean scores decreased by 39.0% from baseline to the end of treatment.
Two-way repeated-measures ANCOVA for the total Hamilton anxiety scale showed a significant effect of rTMS treatment and a significant interaction of time and rTMS treatment (Table 2). Post hoc Sheffé tests showed that active 10-Hz rTMS was significantly different from the sham (p<0.04) and 1-Hz (p<0.01) treatments. As shown in Table 3, during the active high-frequency rTMS treatment, the mean Hamilton anxiety scale scores decreased by 44.1% from baseline to the end of treatment.
Two-way repeated-measures ANCOVA for the Hamilton depression scale showed a significant interaction of time and rTMS treatment (Table 2). Post hoc tests revealed no significant difference between treatments or times.
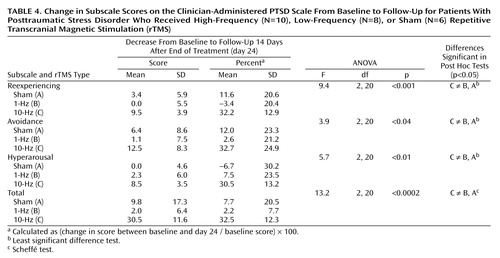
Two-way repeated-measures ANOVA for the reexperiencing subscale of the Clinician-Administered PTSD Scale showed a significant effect of time and a significant interaction of time and rTMS treatment groups (Table 2). Post hoc Sheffé tests showed a significant effect of time (p=0.0005). As shown in Table 4, for patients receiving the active high-frequency rTMS treatment, the mean score for reexperiencing decreased by 32.2% from baseline to follow-up 14 days after the end of treatment (day 24).
Two-way repeated-measures ANOVA for the avoidance subscale of the Clinician-Administered PTSD Scale showed a significant effect of time and a significant interaction of time and rTMS treatment group (Table 2). Post hoc Sheffé tests showed a significant effect of time (p=0.0007). As shown in Table 4, for patients receiving the active high-frequency rTMS treatment, the mean avoidance score decreased by 32.7% from baseline to follow-up.
Two-way repeated-measures ANOVA for the hyperarousal subscale of the Clinician-Administered PTSD Scale showed a significant effect of time and a significant interaction of time and rTMS treatment group (Table 2). Post hoc Sheffé tests showed a significant effect of time (p=0.002).
Two-way repeated-measures ANOVA for the total score on the Clinician-Administered PTSD Scale showed a significant effect of time and a significant interaction of time and rTMS treatment (Table 2). Post hoc Sheffé tests showed a significant effect of time (p=0.00001). As shown in Table 4, for patients receiving the active high-frequency rTMS treatment, the mean score decreased by 32.5% from baseline to follow-up.
Side Effects
Generally, the treatment was well tolerated, and no serious adverse effects were reported by any of the patients. Headache was the main side effect reported, regardless of stimulation group. It was reported by 14 patients: eight patients reported headache after one rTMS treatment, five patients reported it after two sessions, and one (receiving sham treatment) reported it after three sessions. In most cases, this side effect was reported several hours after the stimulation or on the following morning. Only four patients reported headache immediately after the stimulation. In three cases, headache was a symptom before the study. However, the total number of headaches after treatment was 21, out of approximately 250 treatment sessions, for an incidence of 8%.
Two patients receiving high-frequency rTMS reported neck pain and muscular contraction in the area. Another patient receiving high-frequency treatment reported an exacerbation of previously existing dizziness.
One patient in the group receiving slow-frequency rTMS and one patient from the high-frequency group developed a manic episode; in both cases this occurred after the third session of rTMS. One patient reported a mild rage attack, probably related to the stimulation. Although we did not use earplugs, only two patients reported ear discomfort, which lasted less than 1 minute.
In general, TMS was well tolerated and cooperation was excellent among all patients who completed the course of treatment. rTMS had no effect on any patient’s blood pressure or heart rate during the treatments. No serious side effects, such as seizures, neurological complications, or cognitive difficulties, occurred.
Additional Benefits
Eleven participants reported sleep improvement: six receiving 10-Hz rTMS, four receiving 1-Hz rTMS, and one receiving sham treatment. Six patients reported a sense of calmness or a deep sensation of comfort. One patient (receiving 1-Hz stimulation) reported a marked improvement and sharpening of taste and smell.
Discussion
In this double-blind study, high-frequency rTMS (10 Hz for 2 seconds) and slow-frequency rTMS (1 Hz for 5 seconds) were compared to sham rTMS. Our findings demonstrate that 10 daily sessions of 10-Hz rTMS at 80% motor threshold over the right dorsolateral prefrontal cortex has therapeutic effects on PTSD patients.
The PTSD symptoms markedly improved after 10 daily treatment sessions with 10-Hz rTMS, as reflected by the patients’ scores on the Treatment Outcome PTSD Scale and other PTSD scales. At follow-up 14 days after the end of the treatments, the total score on the Clinician-Administered PTSD Scale and the scores on the subscales reflecting the PTSD core symptoms of reexperiencing and avoidance were significantly reduced. These findings therefore widen the scope and possibilities of treatment applications of TMS.
Active 10-Hz rTMS, relative to 1-Hz treatment and sham, significantly reduced Hamilton anxiety scale scores but not Hamilton depression scale scores. Improvement in anxiety symptoms during high-frequency rTMS treatment has been reported previously. Greenberg et al. (49) administered single sessions of high-frequency rTMS stimulation to the left and right dorsolateral prefrontal cortex and to a parieto-occipital control site, in a randomized design, to patients with obsessive-compulsive disorder (OCD). They reported that rTMS treatment to the right dorsolateral prefrontal cortex, but not to the left or to the parieto-occipital area, significantly reduced the compulsive urges. The patients also reported significant mood elevation after right prefrontal stimulation. Conversely, Alonso et al. (50) reported that low-frequency rTMS of the right prefrontal cortex failed to produce significant improvement of OCD and was not significantly different from sham treatment. These results suggest that right prefrontal high-frequency rTMS might affect mechanisms involved in anxiety disorders.
The effect of 10-Hz rTMS was significant and stable for at least 14 days after the last treatment. It is suggested that in further studies the stimulation could be repeated as maintenance therapy, as in ECT procedures.
In an attempt to explain our results, several variables such as frequency and location of stimulation must be discussed. First is the potential lateralization of effects. Several findings suggest that the right hemisphere, especially the right paralimbic and limbic structures, is involved in the emotional and cognitive symptoms associated with traumatic memories and PTSD symptoms (51–53). Therefore, we stimulated the right dorsolateral prefrontal cortex, as did McCann et al. (20), who reported normalization of metabolic activity, as shown by PET imaging, and symptom improvement after 17 treatments with low-frequency rTMS. Second, our results, comparing high- and slow-frequency rTMS over the dorsolateral prefrontal cortex, show that only high-frequency rTMS (10 Hz) achieved clinically significant efficiency, as opposed to the findings of the study by McCann et al. The open design in that study did not exclude placebo effects or changes in severity due to the natural course of the illness as explanations for the observed changes in clinical state. The differences in design and the small patient group may explain that study’s conflicting findings.
The medial prefrontal cortex has an important role in mediating responses to stressful situations. It does so by modulating the hypothalamic-pituitary-adrenal (HPA) axis (54–56), acting as a site for glucocorticoids to exert negative-feedback modulation of HPA activity (57) and regulating a variety of autonomic functions (58, 59).
In a series of studies conducted in rats, Sullivan and Gratton (60) showed that unilateral lesions of the right medial prefrontal cortex abolished stress-induced secretion of glucocorticoids, whereas lesions of the left medial prefrontal cortex were without effect. Animals with lesions failed to interpret sensory input related to stress and failed to integrate this with neuroendocrine responses. The laterality of control of HPA axis activity by the prefrontal cortex is highly relevant to PTSD patients, because these patients suffer from symptoms associated with both dysfunction in HPA axis regulation (61) and prefrontal cortical functional abnormalities (28–34, 36, 62–65). Although still controversial, there is a large body of evidence pointing to low baseline cortisol levels and greater sensitivity of negative-feedback inhibition in the HPA axis as characteristics of PTSD patients (66). Moreover, two studies have shown a deficiency of HPA activity in the aftermath of a traumatic event among individuals who developed PTSD (67, 68). It is not clear whether hypoactivity of the HPA axis in PTSD patients is a consequence of down-regulation of the endocrine stress system, resulting from chronic anxiety/stress, or is a preexisting condition, which may contribute to vulnerability to PTSD.
On the basis of the preceding animal experiments, the findings of low basal cortisol together with several findings revealing low cerebral blood flow in the prefrontal cortex in PTSD patients suggest a predictive association between hypoactivity of the HPA axis and right hypofrontality. High-frequency rTMS to the right dorsolateral prefrontal cortex of PTSD patients may cause activation in this area, resulting in improvement in PTSD symptoms through activation of the HPA axis. This hypothesis may be applicable to the findings of several studies of depressive patients. An antidepressant effect of TMS was found in depressive patients when high-frequency impulses were applied over the left prefrontal cortex or when low-frequency stimuli were applied to the right prefrontal cortex (69–71). Both of these treatments lead to inhibition of HPA axis activity, if the preceding hypothesis is correct.
There is a growing body of evidence implicating the medial prefrontal cortex as a modulator not only of the HPA axis but also of the autonomic nervous system (72). This area contains neurons (principally in the prelimbic cortex and infralimbic cortex) that, when stimulated, result in an inhibitory influence on sympathetic function. Although the precise mechanisms of how the medial prefrontal cortex affects autonomic regulation are not completely understood, these findings suggest the hypothesis that hypoactivation of the medial prefrontal cortex (as reported in PTSD patients) elicits a sympathoexcitatory response, resulting in an excessive autonomic function.
Lesions of the medial prefrontal cortex have been shown to alter behavioral measures of anxiety in rats (ultrasonic vocalization, immobility duration, and respiratory rate) (73, 74). Morgan and LeDoux (73) examined the emotional reactivity of rats with lesions of the dorsal portion of the medial prefrontal cortex, using a classical fear-conditioning paradigm. Conditioned fear behavior (freezing responses) was measured during both the acquisition and extinction phases of the task. Lesions of the dorsal portion of the medial prefrontal cortex were associated with enhanced fear reactivity to both the conditioned stimulus and contextual stimuli during both phases.
Another area involved in regulating the cardiovascular component of the conditioned fear response is the amygdala (75). Neurons in layers II, III, and V of the prelimbic and infralimbic cortex project to the amygdaloid complex, and a proportion of these projections issue collaterals to the contralateral medial prefrontal cortex. The medial prefrontal cortex modulates emotional responses through inhibition of amygdalar responsiveness to fearful cues. Thus, hypofrontality may abolish this suppression response, resulting in an elevated fearful response as shown in PTSD patients.
Overall, one may speculate that the improvement seen in PTSD symptoms is the result of activation of the right dorsolateral prefrontal cortex by the high-frequency rTMS. This improvement may also be associated with activation of the HPA axis, enhanced activity of the depressor area of autonomic responsiveness, or increased suppression of the amygdala. Although these hypotheses are supported by empirical evidence at several points, more studies are needed to directly assess our hypotheses.
Grosso modo, the brain appears to adhere to two fundamental principles: functional organization/specialization and functional integration (where the integration within and among specialized areas is mediated by effective connectivity). Therefore, in order to achieve complete characterization of brain responses in imaging experiments, it is necessary to assess both specific regional changes and regional interaction (connectivity). Shaw and colleagues (76) found direct evidence of differences in functional connectivity between PTSD patients and comparison subjects. They reported that the functional connectivity pattern of the PTSD patients was characterized by more activation in the bilateral inferior parietal lobes and the left precentral gyrus than in the comparison group and less activation in the inferior medial frontal lobe, bilateral middle frontal gyri, and right inferior temporal gyrus. Thus, we may speculate that high-frequency rTMS over the dorsolateral prefrontal cortex improves functional connectivity in these PTSD patients.
In conclusion, this double-blind, controlled trial suggests that in PTSD patients, 10 days of daily right prefrontal high-frequency rTMS has therapeutic effects greater than those of low-frequency or sham stimulation. However, given the small number of patients, our study must be considered preliminary. Our findings suggest that trials using high frequencies to the right prefrontal cortex may be a promising avenue for future research with PTSD patients.
 |
 |
 |
 |
Received Oct. 1, 2002; revisions received March 19 and July 28, 2003; accepted Aug. 2, 2003. From the Ministry of Health Mental Health Center, Faculty of Health Sciences, Ben-Gurion University of the Negev. Address reprint requests to Dr. Cohen, Anxiety and Stress Research Unit, Ministry of Health Mental Health Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, P.O. Box 4600, Beer Sheva 84170, Israel; [email protected] (e-mail). Supported by a grant from the Israel Defense Force—Medical Corps Research and Development Branch.
1. Davidson JR, Malik ML, Sutherland SN: Response characteristics to antidepressants and placebo in post-traumatic stress disorder. Int Clin Psychopharmacol 1997; 12:291–296Crossref, Medline, Google Scholar
2. Davidson J: Drug therapy of post-traumatic stress disorder. Br J Psychiatry 1992; 160:309–314Crossref, Medline, Google Scholar
3. Solomon SD, Gerrity ET, Muff AM: Efficacy of treatments for posttraumatic stress disorder: an empirical review. JAMA 1992; 268:633–638Crossref, Medline, Google Scholar
4. Smajkic A, Weine S, Djuric-Bijedic Z, Boskailo E, Lewis J, Pavkovic I: Sertraline, paroxetine, and venlafaxine in refugee posttraumatic stress disorder with depression symptoms. J Trauma Stress 2001; 14:445–452Crossref, Medline, Google Scholar
5. Marshall RD, Beebe KL, Oldham M, Zaninelli R: Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 2001; 158:1982–1988Link, Google Scholar
6. Davidson J, Pearlstein T, Londborg P, Brady KT, Rothbaum B, Bell J, Maddock R, Hegel MT, Farfel G: Efficacy of sertraline in preventing relapse of posttraumatic stress disorder: results of a 28-week double-blind, placebo-controlled study. Am J Psychiatry 2001; 158:1974–1981Link, Google Scholar
7. Harmon RJ, Riggs PD: Clonidine for posttraumatic stress disorder in preschool children. J Am Acad Child Adolesc Psychiatry 1996; 35:1247–1249Crossref, Medline, Google Scholar
8. Southwick SM, Bremner JD, Rasmusson A, Morgan CA III, Arnsten A, Charney DS: Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 1999; 46:1192–1204Crossref, Medline, Google Scholar
9. Fesler FA: Valproate in combat-related posttraumatic stress disorder. J Clin Psychiatry 1991; 52:361–364Medline, Google Scholar
10. Forster PL, Schoenfeld FB, Marmar CR, Lang AJ: Lithium for irritability in post-traumatic stress disorder. J Trauma Stress 1995; 8:143–149Crossref, Medline, Google Scholar
11. Looff D, Grimley P, Kuller F, Martin A, Shonfield L: Carbamazepine for PTSD. J Am Acad Child Adolesc Psychiatry 1995; 34:703–704Crossref, Medline, Google Scholar
12. Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY: Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996; 57:390–394Medline, Google Scholar
13. Belmaker RH, Fleischmann A: Transcranial magnetic stimulation: a potential new frontier in psychiatry. Biol Psychiatry 1995; 38:419–421Crossref, Medline, Google Scholar
14. Ben-Shachar D, Belmaker RH, Grisaru N, Klein E: Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm 1997; 104:191–197Crossref, Medline, Google Scholar
15. George MS, Lisanby SH, Sackeim HA: Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999; 56:300–311Crossref, Medline, Google Scholar
16. Post A, Keck ME: Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001; 35:193–215Crossref, Medline, Google Scholar
17. Manes F, Crespo-Facorro B: [Transcranial magnetic stimulation in psychiatry]. Actas Esp Psiquiatr 1999; 27:51–55 (Spanish)Medline, Google Scholar
18. Levkovitz Y, Grisaru N, Segal M: Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology 2001; 24:608–616Crossref, Medline, Google Scholar
19. Grisaru N, Amir M, Cohen H, Kaplan Z: Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry 1998; 44:52–55Crossref, Medline, Google Scholar
20. McCann UD, Kimbrell TA, Morgan CM, Anderson T, Geraci M, Benson BE, Wassermann EM, Willis MW, Post RM: Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry 1998; 55:276–279Crossref, Medline, Google Scholar
21. Bor D, Duncan J, Wiseman RJ, Owen AM: Encoding strategies dissociate prefrontal activity from working memory demand. Neuron 2003; 37:361–367Crossref, Medline, Google Scholar
22. Goldman-Rakic PS: Circuitry of primate prefrontal cortex and regulation of behavior by representational memory, in Handbook of Physiology, The Nervous System, Higher Functions of the Brain, I. Edited by Plum F. Bethesda, Md, American Physiological Society, 1987, pp 373–417Google Scholar
23. Goldman-Rakic PS: The cortical dopamine system: role in memory and cognition. Adv Pharmacol 1998; 42:707–711Crossref, Medline, Google Scholar
24. Postle BR, Berger JS, Taich AM, D’Esposito M: Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci 2000; 12(suppl 2):2–14Google Scholar
25. Shallice T, Burgess P: The domain of supervisory processes and the temporal organisation of behaviour, in The Prefrontal Cortex: Executive and Cognitive Functions. Edited by Roberts AC, Robbins T, Weiskrantz L. New York, Oxford University Press, 1998, pp 22–35Google Scholar
26. Damasio AR: On some functions of the human prefrontal cortex. Ann NY Acad Sci 1995; 769:241–251Crossref, Medline, Google Scholar
27. Fuster JM: Executive frontal functions. Exp Brain Res 2000; 133:66–70Crossref, Medline, Google Scholar
28. Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS: Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry 1997; 54:246–254Crossref, Medline, Google Scholar
29. Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF Jr, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC: Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry 2000; 63:65–74Crossref, Medline, Google Scholar
30. Semple WE, Goyer PF, McCormick R, Compton-Toth B, Morris E, Donovan B, Muswick G, Nelson D, Garnett ML, Sharkoff J, Leisure G, Miraldi F, Schulz SC: Attention and regional cerebral blood flow in posttraumatic stress disorder patients with substance abuse histories. Psychiatry Res 1996; 67:17–28Crossref, Medline, Google Scholar
31. Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK: A positron emission tomographic study of symptom provocation in PTSD. Ann NY Acad Sci 1997; 821:521–523Crossref, Medline, Google Scholar
32. Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK: A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996; 53:380–387Crossref, Medline, Google Scholar
33. Bremner JD: Alterations in brain structure and function associated with post-traumatic stress disorder. Semin Clin Neuropsychiatry 1999; 4:249–255Medline, Google Scholar
34. Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS: Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999; 45:806–816Crossref, Medline, Google Scholar
35. Freeman TW, Cardwell D, Karson CN, Komoroski RA: In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med 1998; 40:66–71Crossref, Medline, Google Scholar
36. De Bellis MD, Keshavan MS, Spencer S, Hall J: N–Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry 2000; 157:1175–1177Link, Google Scholar
37. Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152:973–981Link, Google Scholar
38. Vasterling JJ, Brailey K, Constans JI, Sutker PB: Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 1998; 12:125–133Crossref, Medline, Google Scholar
39. Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsom S, Huang MT, Schlesinger L: Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn 2001; 45:64–78Crossref, Medline, Google Scholar
40. Wassermann EM, Grafman J, Berry C, Hollnagel C, Wild K, Clark K, Hallett M: Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr Clin Neurophysiol 1996; 101:412–417Crossref, Medline, Google Scholar
41. Wassermann EM, Wang B, Zeffiro TA, Sadato N, Pascual-Leone A, Toro C, Hallett M: Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET. Neuroimage 1996; 3:1–9Crossref, Medline, Google Scholar
42. Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS: Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT 1998; 14:25–27Crossref, Medline, Google Scholar
43. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA: Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 1996; 34:669–673Crossref, Medline, Google Scholar
44. Davidson JR, Colket JT: The eight-item treatment-outcome post-traumatic stress disorder scale: a brief measure to assess treatment outcome in post-traumatic stress disorder. Int Clin Psychopharmacol 1997; 12:41–45Crossref, Medline, Google Scholar
45. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
46. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
47. Blake DD, Weathers FW, Nagy LN, Kaloupek DG, Klauminzer G, Charney DS, Keane TM: A clinician rating scale for assessing current and lifetime PTSD: the CAPS:1. Behavior Therapist 1990; 18:187–188Google Scholar
48. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T: Predicting PTSD in trauma survivors: prospective evaluation of self-report and clinician-administered instruments. Br J Psychiatry 1997; 170:558–564Crossref, Medline, Google Scholar
49. Greenberg BD, Ziemann U, Harmon A, Murphy DL, Wassermann EM: Decreased neuronal inhibition in cerebral cortex in obsessive-compulsive disorder on transcranial magnetic stimulation. Lancet 1998; 352:881–882Crossref, Medline, Google Scholar
50. Alonso P, Pujol J, Cardoner N, Benlloch L, Deus J, Menchón JM, Capdevila A, Vallejo J: Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Am J Psychiatry 2001; 158:1143–1145Link, Google Scholar
51. Galletly C, Clark CR, McFarlane AC, Weber DL: Working memory in posttraumatic stress disorder—an event-related potential study. J Trauma Stress 2001; 14:295–309Crossref, Medline, Google Scholar
52. Schiffer F, Teicher MH, Papanicolaou AC: Evoked potential evidence for right brain activity during the recall of traumatic memories. J Neuropsychiatry Clin Neurosci 1995; 7:169–175Crossref, Medline, Google Scholar
53. Lucey JV, Costa DC, Adshead G, Deahl M, Busatto G, Gacinovic S, Travis M, Pilowsky L, Ell PJ, Marks IM, Kerwin RW: Brain blood flow in anxiety disorders: OCD, panic disorder with agoraphobia, and post-traumatic stress disorder on 99mTcHMPAO single photon emission tomography (SPET). Br J Psychiatry 1997; 171:346–350Crossref, Medline, Google Scholar
54. Meaney MJ, Aitken DH: [3H]Dexamethasone binding in rat frontal cortex. Brain Res 1985; 328:176–180Crossref, Medline, Google Scholar
55. McEwen BS, De Kloet ER, Rostene W: Adrenal steroid receptors and actions in the nervous system. Physiol Rev 1986; 66:1121–1188Crossref, Medline, Google Scholar
56. Herman JP, Cullinan WE: Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 1997; 20:78–84Crossref, Medline, Google Scholar
57. Diorio D, Viau V, Meaney MJ: The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 1993; 13:3839–3847Crossref, Medline, Google Scholar
58. Sullivan RM, Henke PG: The anterior midline cortex and adaptation to stress ulcers in rats. Brain Res Bull 1986; 17:493–496Crossref, Medline, Google Scholar
59. Neafsey EJ: Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res 1990; 85:147–165Crossref, Medline, Google Scholar
60. Sullivan RM, Gratton A: Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 1999; 19:2834–2840Crossref, Medline, Google Scholar
61. Yehuda R: Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am 2002; 25:341–368, viiCrossref, Medline, Google Scholar
62. Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575–584Abstract, Google Scholar
63. Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL: An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50:932–942Crossref, Medline, Google Scholar
64. Rauch SL, Shin LM: Functional neuroimaging studies in posttraumatic stress disorder. Ann NY Acad Sci 1997; 821:83–98Crossref, Medline, Google Scholar
65. Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47:769–776Crossref, Medline, Google Scholar
66. Yehuda R: Are glucocortoids responsible for putative hippocampal damage in PTSD? how and when to decide. Hippocampus 2001; 11:85–89Crossref, Medline, Google Scholar
67. Resnick HS, Yehuda R, Pitman RK, Foy DW: Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry 1995; 152:1675–1677Link, Google Scholar
68. McFarlane AC, Atchison M, Yehuda R: The acute stress response following motor vehicle accidents and its relation to PTSD. Ann NY Acad Sci 1997; 821:437–441Crossref, Medline, Google Scholar
69. Pascual-Leone A, Rubio B, Pallardo F, Catala MD: Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233–237Crossref, Medline, Google Scholar
70. Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Ben-Shachar D, Feinsod M: Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry 1999; 56:315–320Crossref, Medline, Google Scholar
71. Evers S, Hengst K, Pecuch PW: The impact of repetitive transcranial magnetic stimulation on pituitary hormone levels and cortisol in healthy subjects. J Affect Disord 2001; 66:83–88Crossref, Medline, Google Scholar
72. Verberne AJ, Owens NC: Cortical modulation of the cardiovascular system. Prog Neurobiol 1998; 54:149–168Crossref, Medline, Google Scholar
73. Morgan MA, LeDoux JE: Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 1995; 109:681–688Crossref, Medline, Google Scholar
74. Frysztak RJ, Neafsey EJ: The effect of medial frontal cortex lesions on respiration, “freezing,” and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex 1991; 1:418–425Crossref, Medline, Google Scholar
75. Reis DJ, LeDoux JE: Some central neural mechanisms governing resting and behaviorally coupled control of blood pressure. Circulation 1987; 76(1, part 2):I2-I9Google Scholar
76. Shaw ME, Strother SC, McFarlane AC, Morris P, Anderson J, Clark CR, Egan GF: Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage 2002; 15:661–674Crossref, Medline, Google Scholar



