Partial Recovery of Brain Metabolism in Methamphetamine Abusers After Protracted Abstinence
Abstract
OBJECTIVE: Methamphetamine is a highly addictive drug of abuse that is neurotoxic to dopamine terminals. The authors recently reported that decreases in dopamine transporters (used as markers of dopamine terminals) in the striatum of methamphetamine abusers recover with protracted abstinence and that relative to comparison subjects, recently detoxified methamphetamine abusers have lower metabolism in the striatum and thalamus. In this study, the authors assessed whether metabolism recovers with protracted abstinence. METHOD: Brain glucose metabolism was measured with positron emission tomography and [18F]fluorodeoxyglucose in five methamphetamine abusers who were evaluated after both a short (<6 months) and protracted (12–17 months) abstinence interval, eight methamphetamine abusers tested only after protracted abstinence, and 11 comparison subjects who were not drug users. RESULTS: Significantly greater thalamic, but not striatal, metabolism was seen following protracted abstinence relative to metabolism assessed after a short abstinence interval, and this increase was associated with improved performance in motor and verbal memory tests. Relative to the comparison subjects, the methamphetamine abusers tested after protracted abstinence had lower metabolism in the striatum (most accentuated in the caudate and nucleus accumbens) but not in the thalamus. CONCLUSIONS: The persistent decreases in striatal metabolism in methamphetamine abusers could reflect long-lasting changes in dopamine cell activity, and decreases in the nucleus accumbens could account for the persistence of amotivation and anhedonia in detoxified methamphetamine abusers. The recovery of thalamic metabolism could reflect adaptation responses to compensate for the dopamine deficits, and the associated improvement in neuropsychological performance further indicates its functional significance. These results suggest that while protracted abstinence may reverse some of the methamphetamine-induced alterations in brain function, other deficits persist.
The rapidly escalating abuse of methamphetamine places a sense of urgency on understanding its effects on the human brain and its medical consequences. Methamphetamine is a particularly problematic drug in that not only is it highly addictive but its administration to laboratory animals results in damage to dopamine terminals (1). Studies in the brains of methamphetamine abusers have also documented a significant loss of dopamine transporters, which have been used as markers of dopamine terminals (2–4). The dopamine transporter loss reported in methamphetamine abusers has been found to be associated with reduced motor speed and impaired verbal learning (3). Although early studies have proposed that methamphetamine-induced dopamine terminal damage is irreversible (5), subsequent studies in rodents (6, 7), nonhuman primates (8, 9), and humans (10) have revealed significant recovery with protracted abstinence. This suggests that methamphetamine-induced damage to the dopamine terminals may recover with protracted abstinence. However, while imaging studies have reported significant dopamine transporter increases with abstinence, this recovery has not been associated with the same degree of recovery in neuropsychological function. This could be explained if the dopamine transporter increases reflected 1) incomplete recovery of dopamine terminals, 2) enhanced arborization of viable dopamine synapses, or 3) methamphetamine’s effects on other neurotransmitter systems that have less recovery. Using positron emission tomography (PET), we have previously shown that methamphetamine abusers have significant reductions in striatal and thalamic metabolism, which likely reflected the effects of dopamine terminal damage on projection regions (11). In this study, we assessed whether the decreases in striatal and thalamic metabolism recover with protracted methamphetamine abstinence and the extent to which recovery is associated with improved neuropsychological function.
For this purpose, we used PET and [18F]fluorodeoxyglucose (FDG) to measure regional brain glucose metabolism, which serves as a marker of neuronal activity (12). The initial group (11), which comprised 12 methamphetamine abusers, was tested within 6 months of last methamphetamine use. They were followed and retested at least 9 months later if they remained drug free. Only five methamphetamine abusers were able to stay drug-free and were retested. Here we report the results from the repeated studies for these subjects and for eight additional methamphetamine abusers who were tested only once after having been abstinent for at least 9 months. The metabolic measures were compared with those of 11 age-matched comparison subjects who were not drug users and seven methamphetamine abusers whom we had previously tested within 6 months of abstinence (11). In parallel, we also measured selected neuropsychological functioning shown to correlate with dopamine transporter recovery (3).
Method
Subjects
Five methamphetamine users (three women and two men; mean age=29 years, SD=3) were evaluated twice, first after a short interval of abstinence (mean=3 months, SD=1.6) and then after at least 9 months of protracted abstinence (mean=14 months, SD=2). These five methamphetamine users were from an original group of 12 methamphetamine abusers (11) who were able to remain drug free for at least 9 months following the first PET scan evaluation. The seven other methamphetamine users of the original group of 12 who did not remain drug free constituted a short-term abstinence comparison group. In addition, we also evaluated eight additional methamphetamine users (six women and two men; mean age=36 years, SD=3) who were tested only once after having been detoxified for at least 9 months (mean=17 months, SD=10). The methamphetamine users fulfilled DSM-IV criteria for methamphetamine dependence (average methamphetamine use of at least 0.25 g/day at least 5 days per week for at least 2 years) and had been abstinent for at least 2 weeks. Subjects were excluded if seropositive for HIV or if comorbid neuropsychiatric disorder, medical illness, or current or past drug dependence other than methamphetamine and nicotine were found. Details for the screening procedures have been published (11). Methamphetamine users were enrolled in a California drug court monitoring rehabilitation program and were evaluated weekly or biweekly with drug screens, along with daily visits to the center, to ensure lack of regular drug use during the abstinence period.
The healthy comparison subjects were 11 volunteers (seven women and four men, mean age=31 years, SD=7) who were recruited by local advertisement. Exclusion criteria were the same as those for methamphetamine users except for dependence or abuse of methamphetamine. As for the methamphetamine users, a complete medical and psychiatric examination was performed in the subjects to ensure lack of medical, psychiatric, or neurological disease (G.-J.W., L.C.). The same screening laboratory tests as those given to the methamphetamine users (except for HIV serology) were obtained in these subjects. Prescan urine tests ensured absence of psychoactive drug use in all subjects. The protocol was approved by the Institutional Review Boards at Brookhaven National Laboratory, the State University of New York Stony Brook Medical Center, and the Harbor-UCLA Medical Center. All subjects gave written informed consent after the experimental procedure was explained and after they had read the consent form.
Scanning
Subjects were scanned by using FDG with a CTI-931 scanner. Details on procedures for positioning, arterial and venous catheterization, and quantification of radiotracer and transmission and emission scans have been published previously (13). Briefly, one emission scan (20 minutes) was taken 35 minutes after an intravenous injection of 4–6 mCi of FDG. During the study, subjects were positioned supine in the PET camera with their eyes open. The room was dimly lit, and noise was kept to a minimum. A nurse remained with the subjects throughout the procedure to ensure that they did not fall asleep during the study.
Neuropsychological Evaluation
For the methamphetamine users, we assessed performance on a selected set of neuropsychological tests that had previously been shown to be correlated with dopamine transporter levels (3). The tests were performed within 2 weeks of the PET studies and included 1) motor function tests (as quickly as possible, subjects walked in a straight line for a defined distance [timed gait test of gross motor function] and inserted pegs in small holes angled in different directions [grooved pegboard test of fine motor coordination]); 2) memory function tests (subjects had to learn and recall lists of unrelated words immediately, after a time delay, and after a distractor [Rey Auditory Verbal Learning Test]); and 3) attention tests (subjects had to identify the number associated with each one of the symbols arranged randomly in a row [Symbol Digit Modalities Test]).
Image Analysis and Modeling
Regions in the striatum (caudate, putamen) were obtained from three sequential planes, in the thalamus from two sequential planes, and in the occipital cortex from three sequential planes (as a control) using a template that we had previously published (13). We also computed a value for global metabolism by averaging the activity in the 15 planes scanned. To minimize the effects of overall changes in brain metabolism on the regional measures, we normalized them using the ratio of the region to the global metabolic measures (“relative” measures).
Statistical Analysis
Differences between metabolic measures obtained after short and protracted intervals of abstinence in the methamphetamine abusers were compared with paired t tests (two tailed). Pearson product correlations were used to assess the relationship between the changes in metabolism and the changes in neuropsychological performance between the first and second evaluation.
Differences in global and regional metabolism between the 11 comparison subjects, 10 methamphetamine users evaluated after protracted abstinence (the five methamphetamine abusers who were tested twice and five of the methamphetamine abusers evaluated only once during protracted abstinence), and the original sample of 12 methamphetamine users tested after a short interval of abstinence (seven women and five men; mean age=30 years, SD=6; mean days of abstinence=64, SD=40) were tested with unpaired t tests (two-tailed).
Differences between the two methamphetamine groups and the comparison subjects were also tested using the software package for statistical parametric mapping SPM 99 (14). Before the analysis, each subject’s PET image was mapped onto the Montreal Neurological Institute template closely resembling the Talairach brain and smoothed via a Gaussian kernel with full width half maximum at 16 mm. The relative (normalized) image is obtained by dividing the signal level of each voxel with the global mean, which is the average signal level of all voxels in the PET image. The analysis was performed using both the absolute and the relative metabolic images. Pixels that were significantly different (p<0.001) from those in the comparison subjects were identified with respect to the Talairach and Tournoux stereotactic coordinates and displayed on the axial MR images. The threshold for the cluster size was set at 200 voxels, and only the corrected p values <0.05 at the cluster level were considered significant.
Results
Brain Metabolism in Methamphetamine Users After Short and Protracted Abstinence Intervals
Among the methamphetamine users who were evaluated twice, there were no differences in global metabolism measured after short versus protracted intervals of abstinence (mean=42.6 μmol/100 g per minute [SD=4.0] and 40.8 μmol/100 g per minute [SD=3.4], respectively) or in the absolute metabolic measures in the striatum, thalamus, or occipital cortex. As seen in Figure 1, comparison of “relative” metabolism measured after short and protracted intervals of abstinence showed significant increases in thalamic activity after protracted abstinence (mean=12%, SD=9%) (t=2.3, df=9, p<0.05) but no significant changes in the striatum (mean=2.7%, SD=6.7%) (t=1.3, df=9, p<0.23) or occipital cortex (mean=0.4%, SD=9%) (t=1.2, df=9, p<0.28).
As seen in Figure 2, analysis of the correlation between thalamic activity changes and changes in performance on neuropsychological tests for which we hypothesized an association with metabolic changes showed a significant association for timed gait, Symbol Digit Modalities Test, and delayed recall but not with the changes in the grooved pegboard task (r=0.26, df=3, p=0.68) or the immediate or interference recall (r=0.32, df=3, p=0.60).
Comparison of Healthy Subjects With Methamphetamine Users Tested After Short and Protracted Abstinence Intervals
Absolute global brain metabolism was not significantly different in the methamphetamine abusers tested either after a short interval of abstinence (mean=38.5 μmol/100 g per minute, SD=8.5) or after protracted abstinence (mean=37.3 μmol/100 g per minute, SD=6.3) than in the comparison subjects (mean=33.8 μmol/100 g per minute, SD=7.4).
Regional changes were assessed by using the regional values normalized by the global measures. Compared with the relative metabolic measures seen in the striatum of the healthy subjects (caudate: mean=1.35, SD=0.06; putamen: mean=1.41, SD=0.09), the relative metabolic measures of the methamphetamine abusers were significantly lower after both a short abstinence interval (caudate: mean=1.2, SD=0.1 [t=–4.4, df=21, p<0.0002]; putamen: mean=1.31, SD=0.08 [t=–2.8, df=21, p<0.01]) and after protracted abstinence (caudate: mean=1.23, SD=0.09 [t=–4.4, df=22, p<0.0002; putamen: mean=1.29, SD=0.1 [t=–3.1, df=22, p<0.006]). Relative metabolism in the thalamus was significantly lower in the methamphetamine abusers after a short abstinence interval (mean=1.11, SD=0.08) relative to the comparison subjects (mean=1.31, SD=0.10) (t=–5.3, df=21, p<0.0001); however, thalamic metabolism in the methamphetamine abusers after protracted abstinence (mean=1.23, SD=0.09) did not differ from that of the comparison subjects.
The statistical parametric mapping analyses of the absolute metabolic values showed no differences between groups. The normalized metabolic values, with smaller intersubject variability, were also analyzed by using statistical parametric mapping, and the results were similar to those obtained with the region of interest method. The statistical parametric mapping analyses (Figure 3) yielded a large contiguous cluster (4,440 voxels) that encompassed three subclusters (left subcortical nuclei [including dorsal striatum, nucleus accumbens, and thalamus]: –8, 14, –6; left insula: –34, –6, –12; and right striatum: 14, 14, –6). The significance of this cluster is p<0.0001 (corrected at the cluster level).
For the region of interest method, metabolism in striatum was significantly lower in the methamphetamine abusers after both a short abstinence interval and protracted abstinence than in the comparison subjects. Similarly, the statistical parametric mapping analyses revealed significant decreases in the nucleus accumbens in the methamphetamine abusers whether they were tested after a short or protracted interval of abstinence. In contrast, thalamic metabolism, which was significantly lower in methamphetamine users after a short abstinence interval, was not different in the methamphetamine users after protracted abstinence relative to the comparison subjects. In addition, statistical parametric mapping revealed that relative to the comparison subjects, methamphetamine abusers had decreased metabolism in the left insular cortex. The decreases in insular metabolism were more accentuated in the methamphetamine abusers tested after a short than after a protracted interval of abstinence.
Comparison Between Methamphetamine Users Tested After Short Versus Protracted Intervals of Abstinence
Relative metabolism in thalamus was significantly lower (t=–3,8, df=23, p<0.001) in the 12 methamphetamine abusers tested after a short abstinence interval than in the 13 methamphetamine abusers tested after protracted abstinence (N=13). Metabolism in the striatum did not differ between methamphetamine abusers tested after a short abstinence interval and those tested after protracted abstinence.
Discussion
Recovery of Thalamic but Not Striatal Metabolism With Protracted Methamphetamine Abstinence
In this study, we did not document recovery in striatal metabolism with protracted methamphetamine abstinence. Because metabolism predominantly reflects activity from terminal regions (15), the decreased metabolism in the striatum of methamphetamine abusers is likely to reflect disrupted dopamine cell activity. Indeed, preclinical studies have shown that methamphetamine induces damage to dopamine cells (16, 17), and in certain instances it can even produce cell death (18). The fact that the striatal changes remained after protracted abstinence suggests that the effects of methamphetamine on dopamine cells in the human brain are long-lasting. These results differ from our previous findings showing dopamine transporter increases toward normal levels in the striatum of methamphetamine abusers with protracted abstinence (3). Since the dopamine transporter PET measures serve mostly as markers for the dopamine terminals, this suggests that the dopamine transporter increases in the striatum likely reflect adaptation responses in viable dopamine terminals rather than reversal of methamphetamine-induced damage to dopamine cells. However, the long-lasting decreases in striatal metabolism could also reflect decreased activity from cortical efferents to the striatum, since methamphetamine is also neurotoxic to cortical excitatory cells (19).
In contrast to the striatal findings, this study documented significant recovery of thalamic metabolism with protracted abstinence in methamphetamine abusers who were able to stay drug free for at least 9 months after the initial evaluation. The recovery in thalamic metabolism with protracted abstinence could reflect improvement of neurotransmission in striatothalamic circuits. Indeed, in Parkinson’s disease, where there is a significant loss of dopamine cells, improvement of dopamine neurotransmission with l-dopa treatment results in increases in thalamic metabolism (20). However, the increases in thalamic metabolism in the context of no recovery in striatal metabolism suggests that it may reflect adaptation responses in GABA cells, which are the main striatal efferents, in an attempt to compensate for dopamine deficits. The significant correlations observed between the increases in thalamic metabolism and the improvements on neuropsychological performance in some of the motor and verbal memory tasks further suggest that the thalamic changes with abstinence are functionally significant.
Comparison of Healthy Subjects With Methamphetamine Users Tested After Short and Protracted Intervals of Abstinence
Similar to the results obtained in the prospective studies assessing the effects of abstinence, the comparison in the cross-sectional studies revealed that relative to healthy subjects, striatal metabolism (including the nucleus accumbens) was significantly reduced in the methamphetamine users whether they were tested after a short or after a protracted interval of abstinence. Thalamic metabolism in the methamphetamine users, however, was lower only after a short abstinence interval, not after protracted abstinence. These results further corroborate that there is significant recovery of thalamic metabolism but persistence of decreased striatal metabolism with protracted methamphetamine abstinence.
The persistent reduction in metabolic activity in the striatum and nucleus accumbens could underlie some of the long-lasting deficits reported in abstinent methamphetamine abusers (21). In particular, the reduced activity in the nucleus accumbens could underlie the persistence of amotivation and anhedonia in detoxified methamphetamine abusers.
Changes in Insular Metabolism With Abstinence
The methamphetamine abusers also showed reduced metabolism in the insular cortex, which was much more accentuated in the subjects tested after a short abstinence interval than in those tested after protracted abstinence. The insular cortex is a region neuroanatomically connected with limbic structures and autonomic centers (22), and imaging studies have suggested its involvement in drug addiction (23–26). Structural imaging studies have reported reduced volumes in temporal insula in stimulant drug abusers and have suggested that this might be a brain region particularly sensitive to the neurotoxic effects of stimulant drugs (27).
Limitations
There are several limitations to this study. First, the small number of subjects who were able to remain abstinent for more than 9 months in our study may not be representative of subjects with protracted abstinence. Thus, further studies with larger sample sizes are required to compare the degree of regional brain metabolic recovery and neuropsychological improvements between methamphetamine abusers who are able to stay drug free and those who are not in order to assess the generalizability of the findings from this study and its prognostic implications. Second, since we previously found whole brain metabolism to be significantly increased in methamphetamine users who had been abstinent for 2 weeks to 35 months (11), the current finding of only a nonsignificant trend for increases in global brain metabolism in methamphetamine users tested during protracted detoxification may be due to insufficient sample size. If whole brain glucose metabolism is indeed higher in the methamphetamine users, this could contribute to the observed lower relative rates in striatum. Third, since the urine toxicology tests were performed randomly within a 2-week period, it is possible that some of the methamphetamine subjects were using the drug intermittently but were not detected. However, these subjects visited the rehabilitation center daily, received cognitive behavior therapy, and knew that if they relapsed they would be sent to jail, which minimized the chances that they took drugs during the detoxification period. Fourth, we chose only a small number of neuropsychological tests (those that were found to correlate with dopamine transporter recovery [3]) in order to avoid multiple comparisons and intercorrelations among tests. Future studies using a more comprehensive battery of neuropsychological tests should provide better assessment of improvements in other cognitive domains. Last, we excluded subjects who had relapsed during the follow-up studies; it might have been instructive to evaluate whether this group of subjects would have persistent brain metabolic abnormalities.
Summary
The results from this study provide evidence that there is some functional recovery (as assessed by regional brain glucose metabolism) in the thalamus and insula but not in the striatum with methamphetamine abstinence. The recovery in the thalamus was associated with some degree of recovery of neuropsychological performance, indicating that it is functionally significant. The findings from these studies have implications in the treatment of methamphetamine abusers, for they suggest that protracted abstinence and proper rehabilitation may reverse some methamphetamine-induced alterations in brain function.
Presented in part at the 48th annual meeting of the Society of Nuclear Medicine, Toronto, June 23–27, 2001; and the 41st annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 8–12, 2002. Received March 21, 2003; revision received Aug. 2, 2003; accepted Aug. 5, 2003. From the Medical and Chemistry Departments, Brookhaven National Laboratory; the Departments of Radiology, Psychiatry, and Applied Mathematics, State University of New York, Stony Brook; the Departments of Neurology and Psychiatry, University of California, Los Angeles; and the Department of Behavioral Neuroscience, Oregon Health Sciences University, Portland. Address reprint requests to Dr. Wang, Brookhaven National Laboratory, Upton, NY 11973; [email protected] (e-mail). Supported by a U.S. Department of Energy contract (DE-ACO2-76-CH-00016), grants from the National Institute on Drug Abuse (DA-7092-01, DA-00280) and the Office of National Drug Control Policy, and General Clinical Research Center grants for the Harbor-UCLA Medical Center (RR-00425) and University Hospital Stony Brook (RR-10710). The authors thank D. Schlyer and R. Carciello for Cyclotron operations; D. Warner for PET operations; C. Wong for data management; R. Ferrieri, C Shea, R. MacGregor, V. Garza, and P. King for radiotracer preparation and analysis; and D. Franceschi, M. Leonido-Yee, N. Pappas, N. Netusil, and Pauline Carter for patient care.
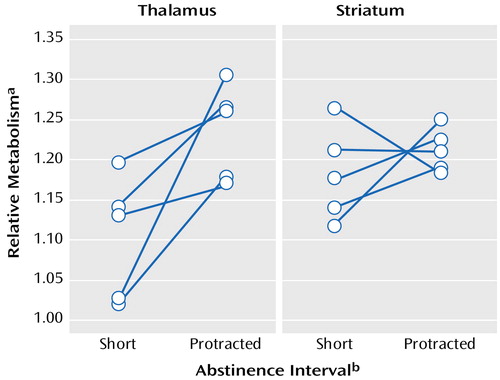
Figure 1. Thalamic and Striatal Activity After a Short Interval of Abstinence and Protracted Abstinence in Five Methamphetamine Abusers
aRatio of regional to global metabolism.
bIntervals of abstinence less than 6 months were classified as short; protracted abstinence referred to methamphetamine-free intervals of 12–17 months.
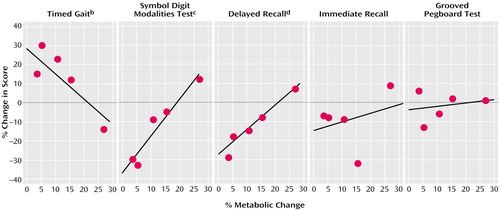
Figure 2. Correlation Between Changes in Thalamic Activity and Neuropsychological Test Performance in Five Methamphetamine Abusers Evaluated After Short and Protracted Intervals of Abstinencea
aIntervals of abstinence less than 6 months were classified as short; protracted abstinence referred to methamphetamine-free intervals of 12–17 months.
br=0.88, df=3, p<0.05.
cr=0.96, df=3, p<0.009.
dr=0.98, df=3, p<0.005.
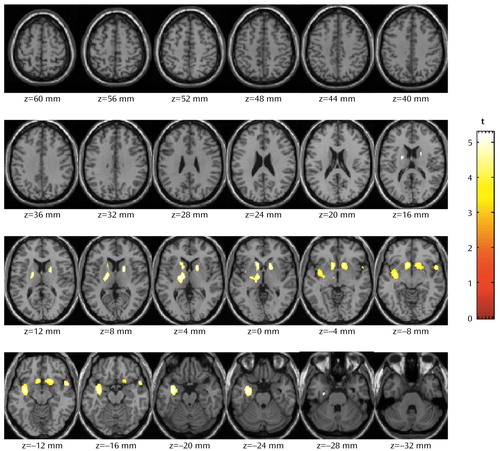
Figure 3. Brain Regions in Which Methamphetamine Abusers (N=12) Evaluated After a Short Abstinence Interval Had Significantly Lower Metabolism Than Comparison Subjects (N=11)a
aIntervals of abstinence less than 6 months were classified as short. The number under each slice represents the distance from the anterior and posterior commissures. The threshold for cluster size was set at 200 voxels, and the significance threshold was set at p<0.05 (corrected at the cluster level). At this threshold, three significant subclusters are seen: 1) left subcortical nuclei, which includes the dorsal striatum, nucleus accumbens, and thalamus (–8, 14, –6); 2) left insula (–34, –6, –12); and 3) right striatum (14, 14, –6). Significance for the overall cluster: p<0.0001.
1. Seiden LS, Sabol KE: Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr 1996; 163:251–276Medline, Google Scholar
2. McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA: Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998; 18:8417–8422Crossref, Medline, Google Scholar
3. Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 2001; 158:377–382Link, Google Scholar
4. Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ: Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996; 2:699–703Crossref, Medline, Google Scholar
5. Ricaurte GA, McCann UD: Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann NY Acad Sci 1992; 648:371–382Crossref, Medline, Google Scholar
6. Friedman SD, Castañeda E, Hodge GK: Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav 1998; 61:35–44Crossref, Medline, Google Scholar
7. Cass WA, Manning MW: Recovery of presynaptic dopaminergic functioning in rats treated with neurotoxic doses of methamphetamine. J Neurosci 1999; 19:7653–7660Crossref, Medline, Google Scholar
8. Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME: Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res 1997; 766:113–120Crossref, Medline, Google Scholar
9. Harvey DC, Lacan G, Tanious SP, Melega WP: Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 2000; 871:259–270Crossref, Medline, Google Scholar
10. Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J: Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 2001; 21:9414–9418Crossref, Medline, Google Scholar
11. Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Wong C, Logan J: Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry 2001; 158:383–389Link, Google Scholar
12. Schmidt KC, Lucignani G, Sokoloff L: Fluorine-18-fluorodeoxyglucose PET to determine regional cerebral glucose utilization: a re-examination. J Nucl Med 1996; 37:394–399Medline, Google Scholar
13. Wang G-J, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP: Functional significance of ventricular enlargement and cortical atrophy in normals and alcoholics as assessed by PET, MRI and neuropsychological testing. Radiology 1992; 186:59–65Crossref, Google Scholar
14. SPM 99 Software. London, MRC Cyclotron Unit, Hammersmith Hospital, 1999Google Scholar
15. Schwartz WJ, Smith CB, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink DJ, Gainer H: Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 1979; 205:723–725Crossref, Medline, Google Scholar
16. Burrows KB, Meshul CK: High-dose methamphetamine treatment alters presynaptic GABA and glutamate immunoreactivity. Neuroscience 1999; 90:833–850Crossref, Medline, Google Scholar
17. Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D: Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci 2002; 22:8951–8960Crossref, Medline, Google Scholar
18. Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL: Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology 2002; 42:837–845Crossref, Medline, Google Scholar
19. Pu C, Broening HW, Vorhees CV: Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse 1996; 23:328–334Crossref, Medline, Google Scholar
20. Hershey T, Black KJ, Stambuk MK, Carl JL, McGee-Minnich LA, Perlmutter JS: Altered thalamic response to levodopa in Parkinson’s patients with dopa-induced dyskinesias. Proc Natl Acad Sci USA 1998; 95:12016–12021Crossref, Medline, Google Scholar
21. Rawson RA, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W: Status of methamphetamine users 2–5 years after outpatient treatment. J Addict Dis 2002; 21:107–119Crossref, Medline, Google Scholar
22. Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley JS, Pappas NR, Wong CT, Felder C: Increased activity of the temporal insula in subjects with bradycardia. Life Sci 2000; 67:2213–2220Crossref, Medline, Google Scholar
23. Wang G-J, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775–784Crossref, Medline, Google Scholar
24. Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP: Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 2001; 58:334–341Crossref, Medline, Google Scholar
25. Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED: Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 2002; 26:376–386Crossref, Medline, Google Scholar
26. Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR: Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 2002; 51:134–142Crossref, Medline, Google Scholar
27. Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P: Age-related brain volume reductions in amphetamine and cocaine addicts and normal control subjects: implications for addiction research. Psychiatry Res 2000; 98:93–102Crossref, Medline, Google Scholar



