Are Gender Differences Important for the Clinical Effects of Antidepressants?
Abstract
OBJECTIVE: Gender differences in antidepressant treatment response, side effects, dropout rates, and plasma concentrations were examined in patients with major and predominantly melancholic depression. METHOD: The study included a subgroup of 292 inpatients (96 men, 196 women) from three Danish double-blind, randomized, controlled trials. All patients completed a 5-week treatment period and fulfilled the DSM-III or DSM-III-R criteria for major depression. Clomipramine (150 mg/day) was the reference treatment, and comparable treatments were citalopram (40 mg/day), paroxetine (30 mg/day), and moclobemide (400 mg/day). Assessments were performed by using the 17-item Hamilton Depression Rating Scale and the Udvalg for Kliniske Undersøgelser Side Effect Rating Scale. In a subgroup of 110 patients, weekly measurements of clomipramine plasma concentrations were obtained. Nonparametric statistical tests and multiple linear and logistic regression models were used for statistical evaluations. RESULTS: Both genders had similar remission rates (Hamilton depression scale score <8) when treated with clomipramine and had significantly higher remission rates with clomipramine than with the comparable treatments. The plasma concentrations of clomipramine were significantly higher for female than for male patients. No gender differences were found in posttreatment Hamilton depression scale scores, nor did the therapeutic effects of treatment depend on gender. Rates of dropout and side effects were similar for men and women. No relationship between plasma concentrations, gender, and therapeutic outcome was found. CONCLUSIONS: In a group of patients with major and predominantly melancholic depression, differentiation according to gender was not important in treatment with common antdepressants. Women appeared to have higher plasma concentrations of tricyclic antidepressants than men. The consequences of this difference for clinical effects are unclear. Gender-specific recommendations for dosing of tricyclic antidepressants may be considered.
The role of gender differences in depressive disorders has been extensively studied in the psychiatric literature. The gender ratio (male:female) in the prevalence of major depression is roughly constant at 1:2 (1–3).
Very little attention has thus far been paid to potential gender differences regarding the clinical effects of antidepressant treatment. The authors of a recent review of pharmacological treatment of depression concluded, “There are data supporting sex differences in the activity of various antidepressant-metabolizing enzymes. However, there is a paucity of investigation regarding how these differences might translate into differences in clinical efficacy” (4). An earlier commentary noted the lack of attention to gender differences in the clinical evaluation of drugs: “There is little work using existing databases to perform the subgroup analyses recommended by the U.S. Food and Drug Administration (FDA)” (5).
To our knowledge, only three studies (6–8) have explicitly analyzed the role of gender differences in the response to antidepressant treatment. Two of the studies reported on subgroups of depressive patients, i.e., patients with major chronic or double depression (7) and patients with atypical depression (6). The two studies compared tricyclic antidepressants with either selective serotonin reuptake inhibitors (SSRIs) (7) or monoamine oxidase inhibitors (MAOIs) (6). In both studies tricyclic antidepressants were found to be superior to comparable treatments in men. In contrast, the comparable treatments were found to be superior to tricyclic antidepressants in women. In the third study (8), a total of 1,746 subjects from a depression outpatient clinic were examined with respect to treatment effect of tricyclic antidepressants, MAOIs, SSRIs, or placebo. The authors found that women and men had similar response rates to tricyclic antidepressants and SSRIs and that women had a statistically superior response to MAOIs, which, however, may not have been clinically relevant. Regarding gender differences in the metabolism of tricyclic antidepressants, other studies (9, 10) have reported higher plasma concentrations of tricyclic antidepressants in women than in men.
The goal of the present study was to analyze potential gender differences in clinical effects of antidepressant treatment in a homogeneous group of inpatients with major and predominantly melancholic depression. We examined gender differences in the clinical effects of clomipramine (a tricyclic antidepressant), citalopram (an SSRI), paroxetine (an SSRI), and moclobemide (a reversible inhibitor of monoamine oxidase). Furthermore, we analyzed the relationship between plasma concentration of clomipramine, gender, and therapeutic outcome.
Method
The present study was based on data from three double-blind, randomized, controlled trials (11–13) carried out by the Danish University Antidepressant Group in six clinical centers in Denmark (Table 1). Data were collected between 1980 and 1990. In each of the studies, depressed inpatients were selected by using similar inclusion and exclusion criteria.
Inclusion Criteria
Inclusion criteria were a DSM-III or DSM-III-R diagnosis of depression requiring antidepressant treatment and a total score of ≥18 on the Hamilton Depression Rating Scale (14, 15) and/or a total score of ≥9 on a subscale of the Hamilton depression scale (16).
Exclusion Criteria
Exclusion criteria were age <19 years or >65 years (11), ≥68 years (12), or ≥70 years (13); duration of present depressive episode >12 months; a diagnosis of schizophrenia, paranoid psychosis, oligophrenia, or organic brain syndrome; chronic drug or alcohol abuse; serious somatic disease; pregnancy or puerperium (≤2 months postpartum); and severe suicidality or mental retardation and the consequent requirement to use of ECT to treat the depression. Patients were selected on the basis of the presence of major depression without consideration of the presence or lack of previous manic episodes, which were not systematically recorded. The development of mania during the study period resulted in the patient being excluded from the study.
Study Design
Eligible patients underwent a 7-day patient-blind placebo washout period. Diagnostic and quantitative depression ratings were performed at the end of the placebo week. Patients still fulfilling the inclusion criteria were stratified according to diagnostic rating (melancholic/nonmelancholic) and clinical center before being randomly allocated to treatment for 5 (11) or 6 (12, 13) weeks, respectively. A 5-week treatment period was examined in the present analysis.
Patients
A total of 351 inpatients (115 men, 236 women) were started on active treatment. Of those, 292 patients (96 men, 196 women) completed 5 weeks of active treatment. The number of patients varied slightly by analysis because fewer patients completed the diagnostic rating (N=273) and the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale (17) rating (N=286). Clomipramine plasma concentration data from two of the studies (12, 13) were available for the present analysis (N=110).
Study Medications
Patients were randomly assigned to receive either standard treatment or comparable treatment. The standard fixed-dose treatment was 150 mg/day of clomipramine. The comparable treatments were 40 mg/day of citalopram (11), 30 mg/day of paroxetine (12), and 400 mg/day of moclobemide (13). Additional medication was restricted to the occasional use of oxazepam as sedative/hypnotic. Chronically used medications such as oral contraceptives were allowed, provided the medication was not started during the study periods. Antipsychotics and barbiturates were not allowed.
Clinical Assessments
Time intervals for clinical assessments are given in Table 2. Qualitative ratings were carried out by using the Newcastle diagnostic depression scales (18, 19). The patients’ scores on the Newcastle 1 Scale were used to classify the patients into two groups: endogenous/melancholic, for patients with a score ≥5.5, and nonendogenous/nonmelancholic, for patients with a score <5.5. Quantitative ratings were carried out by using the 17-item Hamilton depression scale, and side effect ratings were made with the UKU Side Effect Rating Scale. The side effects considered were tremor, visual accommodation disturbances, reduced salivation, orthostatic dizziness, palpitations/tachycardia, and increased tendency to sweat.
Reliability
Joint rating sessions were held during the study periods. The intraclass correlation coefficients (20) were between 0.35 and 0.76 for the Newcastle 1 Scale and between 0.75 and 0.86 for the Hamilton depression scale.
Plasma Drug Concentrations
Plasma drug concentrations were measured in blood collected at weekly intervals during the study period (N=110). Clomipramine and desmethylclomipramine were assayed by high-performance liquid chromatography (21).
Ethics and Consent
Written informed consent was obtained from all subjects after complete description of the study. The regional Ethics Committees approved all three protocols.
Effect Measures
Rate of remission (an endpoint Hamilton depression scale total score <8), rate of response (a 50% reduction in Hamilton depression scale score from baseline to endpoint), and difference in Hamilton depression scale score from baseline to endpoint (endpoint minus baseline Hamilton depression scale total score) were used as the primary effect measures. Dropout rates, the prevalence of six relevant side effects, and a factor analysis of Hamilton depression scale scores were used as secondary effect measures. In addition, gender differences in clomipramine plasma concentration and the relation between plasma concentration, gender, and therapeutic outcome were analyzed.
Statistical Analysis
Gender differences in effect measures were analyzed by using data from study completers. The principle of last observation carried forward was used for analyses of data on clomipramine concentrations. Nonparametric statistical tests were used for simple statistical evaluations. The Mann Whitney U test or Kruskal-Wallis test was used for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. All tests were two-tailed.
Logistic regression models were used when testing for gender differences in rates of dropout, response, and remission. Multiple linear regression models, were used when testing for gender differences in Hamilton depression scale difference scores, the interaction of gender and type of antidepressant treatment, and gender differences in clomipramine plasma concentrations. In all regression models the robust variance estimation was used, as provided by Stata’s robust and cluster option (Stata Corp., College Station, Tex.), thus relaxing the assumptions of constant variance of observations and of Gaussian distribution of residuals and allowing dependence among the error terms for a subject at different time points. Treatment (four groups: clomipramine, citalopram, paroxetine, and moclobemide), age, and presence of melancholic depression were included in all models when testing for gender differences in effect measures. In the interaction models, the effects of the two SSRIs (citalopram and paroxetine) were analyzed together for additional power. When testing for gender differences in clomipramine plasma concentrations, age and weight were included in the analysis.
Baseline Hamilton depression scale scores were analyzed by factor analysis (varimax analysis). The number of factors to be retained was decided from a scree plot. A cutoff value of >0.30 or <–0.30 defined the significance of the factor loadings. Factor scores were calculated at baseline and endpoint.
Results
Baseline Characteristics and Endpoint Hamilton Depression Scale Total Score
The baseline characteristics and the mean endpoint Hamilton depression scale total scores for the treatment and gender groups are shown in Table 3. Higher proportions of male (81%) than female (70%) patients fulfilled the criteria for melancholic depression (χ2=3.7, df=1, p=0.05). There were no gender differences in Hamilton depression scale baseline (z=–0.7, p=0.50) or endpoint scores (z=–0.1, p=0.88). Previous depressive episodes were more prevalent among female (13.1%) than among male (5.5%) patients, but the difference was not statistically significant (χ2=4.6, df=2, p=0.10).
Primary Effect Measures
Remission rates
Both genders had significantly higher rates of remission (Hamilton depression scale score <8) when treated with clomipramine than with the comparable treatments (χ2=5.4, df=1, p=0.02). The remission rates were similar for male (31.3%) and female (30.1%) patients. Logistic regression analysis showed no significant gender differences in remission rates (odds ratio=1.08, 95% confidence interval [CI]=0.61–1.91).
Response rates
The rate of response (50% reduction in Hamilton depression scale score from baseline to endpoint) was 56.3% for male and 53.3% for female patients. Gender differences in response rates were nonsignificant when tested in a logistic regression model (odds ratio=0.98, 95% CI=0.57–1.69).
Hamilton depression scale difference scores
The weekly differences in Hamilton depression scale total scores for male and female patients are shown in Figure 1. There was an approximately linear decrease in Hamilton depression scale total scores from baseline to endpoint for both men and women. At endpoint the mean Hamilton depression scale difference score was –11.6 (SD=7.7) for female and –12.1 (SD=6.9) for male patients. Gender effects were nonsignificant when tested in a multiple linear regression model (difference=0.56, 95% CI=–0.55 to 1.66).
Interaction between gender and type of treatment
The mean Hamilton depression scale difference scores (from baseline to endpoint) are listed by gender and treatment group in Table 4. The interaction of gender and treatment was nonsignificant when tested in a multiple linear regression model (p>0.10). When the remission rates or response rates were used as effect measures, again no interaction between gender and treatment was found.
Secondary Effect Measures
Dropouts
Dropout rates by gender and treatment are shown in Table 5. The total number of dropouts was 59 (19 men, 40 women). Overall dropout rates were similar for male and female patients (χ2=0.01, df=1, p=0.92), as they were for the particular treatment groups. In the clomipramine group the main reason for dropout was severe side effects, particularly orthostatic hypotension or palpitations. In the three other treatment groups, the main reason for dropout was “no effect of treatment” or “worsening of depression” (11–13).
Side effects
Gender differences in the prevalence of side effects at baseline and after 4 weeks of treatment are shown in Figure 2. Of the six UKU side effects analyzed, tremor was more prevalent in female (42%) than in male (33%) patients, whereas orthostatic dizziness was more prevalent in male (33%) than in female (24%) patients after 4 weeks of treatment. However, in the pooled sample and in each treatment group, all gender differences were statistically nonsignificant (p>0.09, chi-square test).
Factor analysis
The factor analysis of Hamilton depression scale scores produced four significant factors explaining 42% of the variance. An anxiety factor (agitation, psychic and somatic anxiety), a sleep factor (initial, middle, and delayed insomnia), a somatic factor (gastrointestinal and general somatic symptoms, hypochondriasis, loss of insight, and loss of weight), and a depression factor (depression, guilt, suicidal ideation or behavior, effects on work/interests, and retardation) were produced. Male and female patients had similar scores on all factors at baseline and endpoint. The difference scores (endpoint minus baseline scores) were similar for male and female patients. All gender differences were nonsignificant (p>0.05, Mann-Whitney U test).
Additional Analyses
Clomipramine plasma concentration
Figure 3 shows the weekly measures of clomipramine plasma concentration for male (N=45) and female (N=65) patients. Mean plasma concentrations varied from 613 to 1022 nmol/liter for female patients and from 446 to 693 nmol/liter for male patients. When tested in a multiple linear regression model, the difference in plasma concentration between men and women was statistically significant (p<0.0001). Plasma concentrations were a mean of 309 nmol/liter (95% CI=156–464) higher for female than for male patients.
Relationship between plasma concentration level, gender, and outcome
There was no correlation between plasma concentrations and Hamilton depression scale difference scores (rs=–0.10, N=58, p=0.5). In stratified groups of male (rs=–0.25, N=24, p=0.3) and female patients (rs=0.14, N=34, p=0.5), the correlations were also nonsignificant.
Discussion
Gender Differences in Clinical Effects of Antidepressant Treatment
Our analyses showed similar clinical effects of antidepressant treatment for male and female patients with major and predominantly melancholic depression. There were no gender differences with respect to the primary effect measures (Hamilton depression scale difference scores, response and remission rates as measured with the Hamilton depression scale) or secondary effect measures (dropout rates, side effects, and factor analysis).
Other studies have shown a more favorable effect of tricyclic antidepressants for men (6, 7, 9) and a more favorable effect of SSRIs (7) and MAOIs (6) for women.
Kornstein et al. (7) examined gender differences in treatment response to the SSRI, sertraline (mean dose of 140 mg/day), versus the tricyclic antidepressant, imipramine (mean dose of 200 mg/day). They reported on 635 patients with chronic depression or major depression superimposed on dysthymia. Men had a higher response rate (57%) than women (46%) while taking imipramine, and women had a higher response rate (62%) than men (45%) while taking sertraline. In addition to the gender differences in treatment response, women who were taking imipramine and men who were taking sertraline were more likely to withdraw from the study. One explanation for their findings regarded depressive subtypes. Kornstein et al. (7) argued that women were more likely to have atypical depressive symptoms (22), which have been shown to respond preferentially to SSRIs or MAOIs (23, 24), and that men often have more classic “neurovegetative features” of depression, which are responsive to tricyclic antidepressants (25, 26).
Davidson and Pelton (6) reported on 151 patients with atypical depression. They pooled treatment data for phenelzine and isocarboxazid as MAOI treatment and data for imipramine and amitriptyline as tricyclic antidepressant treatment. In a subsample (N=48) of patients with associated panic attacks, they found that MAOIs were superior to tricyclic antidepressants in women and that tricyclic antidepressants were superior to MAOIs in men. The authors (6) pointed out that the number of subjects in their study was small and that their findings needed further confirmation.
Hamilton et al. (9) analyzed gender differences in treatment response to imipramine (a tricyclic antidepressant) in a meta-analysis of 180 studies published between 1957 and 1991. Thirty-five studies reported imipramine treatment response rates separately by gender. In 53% of the 35 studies, men showed more benefit from imipramine than women. In 19% of the studies, women received more benefit than men, and in 28% of the studies, no gender difference was observed. They argued that although the meta-analysis of gender and imipramine was comprehensive, it combined studies that used different diagnostic criteria and methods. They suggested that the apparent gender difference in treatment response to imipramine resulted from the inclusion of relatively heterogeneous populations in early clinical trials. They concluded that gender differences in imipramine outcome might have been an artifact of the inclusion of more women with atypical, dysthymic, or anxious depression and of more men with melancholic or endogenous depression (9).
Quitkin et al. (8) reported on a large sample of 1,746 outpatients and found no clinically relevant gender differences in treatment response to tricyclic antidepressants, MAOIs, SSRIs, or placebo. Based on the results of studies previously described, they concluded that men might have a slightly better outcome with imipramine, but that it is unclear whether this small advantage is a result of pharmacokinetics, pharmacodynamics, or diagnostic differences.
In summary, the studies discussed in this section indicated that the finding of men’s better treatment response to tricyclic antidepressants may be confounded by gender differences in distribution of depressive subtypes in the study samples. There may have been relatively more male patients with the neurovegetative or melancholic subtype of depression and relatively more female patients with the atypical or anxious subtype of depression. These uneven gender distributions of depressive subtypes may have given rise to the findings of gender differences in response to different types of antidepressants.
In the present study, we examined a more homogeneous subgroup of hospitalized patients with major and predominantly melancholic depression. Patients were stratified according to melancholic or nonmelancholic depression before being randomly assigned to treatment. A larger proportion of male patients (81%) had melancholic depression, compared with female patients (70%). To avoid the confounding effects of this difference, we adjusted for melancholia in the analyses.
However, this restriction of the subjects to patients with major and predominantly melancholic depression may have been a limitation of this study. Previously we showed that this group of hospitalized patients, who were participating in controlled clinical trials, differed significantly from outpatient subjects in the distribution of melancholic depression (27). Furthermore, previous findings suggested that in this group with major and predominantly melancholic depression, tricyclic antidepressants seem to be the most effective treatment, more effective than SSRIs (27, 28).
In the studies used for the present analysis, clomipramine was chosen as the comparison pro-drug because it has the most potent action on 5-hydroxytryptamine (5-HT) of any classic tricyclic antidepressant. Furthermore, it is similar to other types of tricyclic antidepressants (28).
Gender Differences in Plasma Concentrations of Tricyclic Antidepressant
Our analyses showed that female patients had significantly higher clomipramine plasma concentrations than male patients (Figure 3). However, no relationship between plasma concentration, gender, and therapeutic outcome was found. In concordance with our findings, other studies have reported higher plasma concentrations of tricyclic antidepressants in women than in men (9, 10).
Hamilton et al. (9) included in their meta-analysis 12 studies reporting imipramine steady-state plasma concentrations. They found that women had significantly higher dose-adjusted plasma concentrations than men. Since men on average have a higher body weight and thereby a higher volume of distribution, an analysis that controls the effects of weight might find a smaller gender difference in dose-adjusted plasma concentrations. In three of the studies included in the meta-analysis by Hamilton et al. (9), dose and weight were controlled in determining differences in imipramine plasma concentrations. It appeared that the dose-by-weight adjustment removed the effect of gender in the three studies. However, the authors concluded that there was some support for the hypothesis that clearance of tricyclic antidepressants is slower in women. They suggested that, although data are scant, the use of oral contraceptives and hormonal replacement therapy may further increase the absolute bioavailability of tricyclic antidepressants. Finally, they concluded that little is known about the effects of the menstrual cycle and menopause on the pharmacokinetics of tricyclic antidepressants.
The review article by Frackiewicz et al. (10) discussed gender differences in depression and in antidepressant pharmacokinetics and adverse events. Based on nine studies reporting higher plasma concentrations of tricyclic antidepressants in women, they concluded that dose adjustment might be necessary for women to ensure a favorable treatment response, compliance, and a low incidence of adverse events. In the present analysis we also found higher plasma concentrations of tricyclic antidepressants in women than in men. However, our results do not entirely suggest that a dose adjustment should be required to ensure the clinical outcomes of treatment with tricyclic antidepressants. Additional studies examining the pharmacokinetics and clinical effects of antidepressants are needed before any conclusions on this topic can be made.
To our knowledge, only one study (29) has analyzed the pharmacokinetics of clomipramine in relation to gender. In this drug monitoring analysis, the researchers found that women had a significantly lower hydroxylation clearance of clomipramine than men. Potential reasons for our finding in the present study of women’s higher plasma concentrations could be related to time-of-day variance or gender differences in pharmacokinetics influenced by the varying hormonal milieu in women. Medication was given at exact hours, and likewise plasma concentrations were measured at exact hours, so time-of-day variance is quite unlikely. Gender differences in pharmacokinetics influenced by the varying hormonal milieu in women are a considerably more reasonable explanation.
Pharmacokinetics can be divided into the subprocesses of absorption, bioavailability, distribution, and metabolism. There are several sites for potential gender differences within these subprocesses. Absorption of a drug is dependent on its acid-base and lipophilic properties and on the physiology of the gastrointestinal tract. It has been suggested that women may secrete less gastric acid than men, leading to potential gender differences in absorption (30). A decrease in gastric acid would lead to an increase in absorption of weak bases such as the tricyclic antidepressants. Other sites for potential gender differences in absorption and bioavailability are gastric emptying and gastrointestinal transit time. The gastrointestinal transit time may be prolonged in women during the premenstrual phase (31). The distribution of a drug is dependent on the drug’s acid-base properties, water and lipid solubility, and affinity for binding proteins. Potential gender differences in blood volume, cardiac output, and percentage of lean body mass could affect the volume of distribution of a drug. The larger blood volume and cardiac output of male subjects and the lower ratio of lean body mass to adipose tissue for female subjects would generally increase the volume of distribution of lipophilic drugs such as the tricyclic antidepressants. The main gender-related metabolic factors that can affect the metabolism of tricyclic antidepressants are first-pass metabolism and the activity of some antidepressant metabolizing enzymes of the cytochrome P450 group. Significant differences according to gender are found for the hydroxylation and elimination of hydroxy metabolites of clomipramine; a lower hydroxylation clearance has been found for female than for male subjects (29).
The varying hormonal milieu in women may lead to possible menstrual-phase-specific changes that can affect the pharmacokinetics of psychotropic drugs. In addition, the use of oral contraceptives and hormonal replacement therapy may exert an influence on the pharmacokinetics of psychotropic drugs (9, 32). Unfortunately, data on menstrual cycle, menopausal status, and the use of oral contraceptives and hormonal replacement therapy were not available in the present study. The varying hormonal milieu in women may also lead to gender differences in pharmacodynamics of psychotropic drugs. However, it is unlikely that such differences could account for the higher plasma concentrations of tricyclic antidepressants in women. Female sex hormones have been found to modulate the function of some major neurotransmitters (e.g., γ-aminobutyric acidA, dopamine, serotonin) (9), and it is reasonable to expect that the varying hormonal milieu might influence the clinical profile in women. Despite this likelihood, to our knowledge, no publications have addressed gender differences in the pharmacodynamics of psychotropic medication in relation to clinical effects.
In conclusion, to verify that the gender-related pharmacokinetic effects are clinically relevant, it will be necessary to show that they are associated with gender-related differences in therapeutic outcome. In the present study, we found no such relationship with therapeutic outcome. Additional larger studies are needed to examine the pharmacokinetics of antidepressants with dose-by-weight adjustment and to explore the concentration-effect relationship with controls for the effects of hormonal factors in women. In addition, studies examining the pharmacodynamics of antidepressants, with controls for the effects of hormonal factors in women, must produce findings of gender-related differences in clinical profile before conclusions on this topic can be drawn.
 |
 |
 |
 |
 |
Received Nov. 2, 2001; revisions received Aug. 21, 2002, and Feb. 27, 2003; accepted March 19, 2003. From the Department of Psychiatry, Center for Depression Research, Odense University Hospital; and the Departments of Public Health and Medical Psychology and Psychotherapy, Erasmus Medical Center, Rotterdam, the Netherlands. Address reprint requests to Dr. Hildebrandt, Department of Psychiatry, Center for Depression Research, Odense University Hospital, Sdr. Boulevard 29, DK-5000 Odense C, Denmark; [email protected] (e-mail). Supported by the Danish Research Councils and the Faculty of Health Sciences, University of Southern Denmark, Odense. This research was performed as part of the Netherlands Institute for Health Sciences Master of Science program. The authors thank Professor Werner Vach for statistical support.
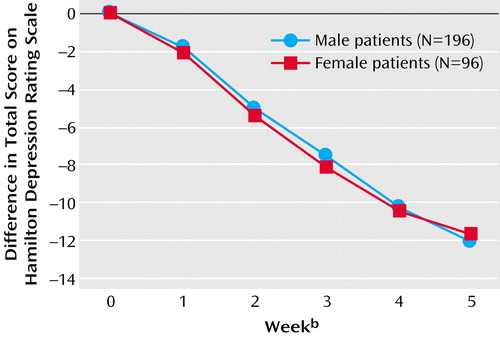
Figure 1. Baseline-to-Endpoint Changes in Hamilton Depression Rating Scale Scores Over a 5-Week Study Period in Male and Female Patients With Predominantly Melancholic Depression (N=292) in Three Double-Blind, Randomized, Controlled Trials Comparing Antidepressantsa
aNo significant difference between male and female patients (p=0.32, multiple linear regression model).
bWeek 0=baseline; week 5=after 5 weeks of active treatment.
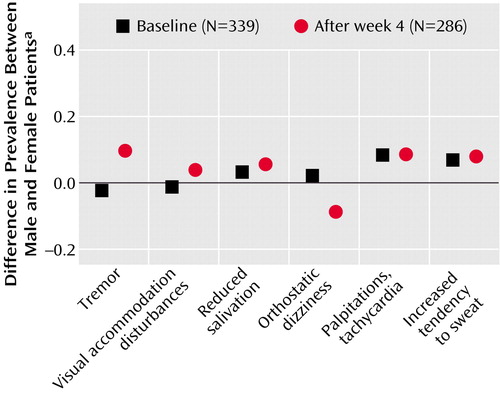
Figure 2. Gender Difference in Prevalence of Side Effects at Baseline (N=339) and After 4 Weeks of Treatment (N=286) in Three Double-Blind, Randomized, Controlled Trials Comparing Antidepressants
aPercentage of women minus percentage of men.
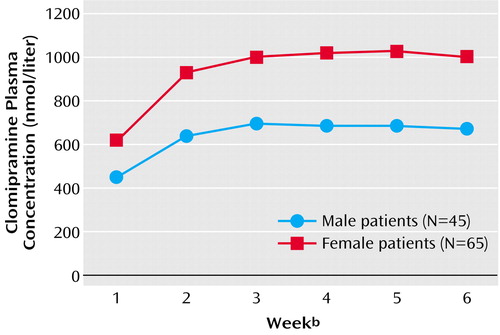
Figure 3. Clomipramine Plasma Concentration Over a 6-Week Study Period in Male and Female Patients in Three Double-Blind, Randomized, Controlled Trials Comparing Antidepressantsa
aSignificant difference between male and female patients (p<0.0001, multiple linear regression model).
bWeek 1=after 1 week of active treatment; week 6=after 6 weeks of active treatment.
1. Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO: Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry 1998; 55:405–413Crossref, Medline, Google Scholar
2. Weissman MM, Klerman GL: Sex differences and the epidemiology of depression. Arch Gen Psychiatry 1977; 34:98–111Crossref, Medline, Google Scholar
3. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK: Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996; 276:293–299Crossref, Medline, Google Scholar
4. Yonkers KA, Brawman-Mintzer O: The pharmacologic treatment of depression: is gender a critical factor? J Clin Psychiatry 2002; 63:610–615Crossref, Medline, Google Scholar
5. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs: notice. Federal Register 1993; 58:39406–39416Medline, Google Scholar
6. Davidson J, Pelton S: Forms of atypical depression and their response to antidepressant drugs. Psychiatry Res 1986; 17:87–95Crossref, Medline, Google Scholar
7. Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison W, Keller MB: Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 2000; 157:1445–1452Link, Google Scholar
8. Quitkin FM, Stewart JW, McGrath PJ, Taylor BP, Tisminetzky MS, Petkova E, Ma YG, Klein DF: Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry 2002; 157:1848–1854Link, Google Scholar
9. Hamilton JA, Grant M, Jensvold MF: Sex and treatment of depressions: when does it matter? in Psychopharmacology and Women: Sex, Gender and Hormones. Edited by Jensvold MF, Halbreich U, Hamilton JA. Washington, DC, American Psychiatric Press, 1996, pp 241–260Google Scholar
10. Frackiewicz EJ, Sramek JJ, Cutler NR: Gender differences in depression and antidepressant pharmacokinetics and adverse events. Ann Pharmacother 2000; 34:80–88Crossref, Medline, Google Scholar
11. Danish University Antidepressant Group: Citalopram: clinical effect profile in comparison with clomipramine: a controlled multicenter study. Psychopharmacology (Berl) 1986; 90:131–138Medline, Google Scholar
12. Danish University Antidepressant Group: Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance but weaker antidepressant effect than clomipramine in a controlled multicenter study. J Affect Disord 1990; 18:289–299Crossref, Medline, Google Scholar
13. Danish University Antidepressant Group: Moclobemide: a reversible MAO-A-inhibitor showing weaker antidepressant effect than clomipramine in a controlled multicenter study. J Affect Disord 1993; 28:105–116Crossref, Medline, Google Scholar
14. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
15. Bech P, Kastrup M, Rafaelsen OJ: Mini-compendium of rating scales for states of anxiety, depression, mania, schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl 1986; 326:1–37Medline, Google Scholar
16. Bech P, Allerup P, Reisby N, Gram LF: Assessment of symptom change from improvement curves on the Hamilton depression scale in trials with antidepressants. Psychopharmacology (Berl) 1984; 84:276–281Crossref, Medline, Google Scholar
17. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K: The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 334:1–100Crossref, Medline, Google Scholar
18. Bech P, Gjerris A, Andersen J, Bojholm S, Kramp P, Bolwig TG, Kastrup M, Clemmesen L, Rafaelsen OJ: The Melancholia Scale and the Newcastle Scales: item-combinations and inter-observer reliability. Br J Psychiatry 1983; 143:58–63Crossref, Medline, Google Scholar
19. Mullaney JA: The validity of two Newcastle Diagnostic Scales in the affective disorders: bimodality and other correlates. J Affect Disord 1985; 9:239–247Crossref, Medline, Google Scholar
20. Bartko JJ, Carpenter WT Jr: On the methods and theory of reliability. J Nerv Ment Dis 1976; 163:307–317Crossref, Medline, Google Scholar
21. Nielsen KK, Brosen K: High-performance liquid chromatography of clomipramine and metabolites in human plasma and urine. Ther Drug Monit 1993; 15:122–128Crossref, Medline, Google Scholar
22. Kornstein SG: Gender differences in depression: implications for treatment. J Clin Psychiatry 1997; 58(suppl 15):12–18Google Scholar
23. Pande AC, Birkett M, Fechner-Bates S, Haskett RF, Greden JF: Fluoxetine versus phenelzine in atypical depression. Biol Psychiatry 1996; 40:1017–1020Crossref, Medline, Google Scholar
24. Quitkin FM, Stewart JW, McGrath PJ, Tricamo E, Rabkin JG, Ocepek-Welikson K, Nunes E, Harrison W, Klein DF: Columbia atypical depression: a subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry Suppl 1993; 21:30–34Medline, Google Scholar
25. Roose SP, Glassman AH, Attia E, Woodring S: Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry 1994; 151:1735–1739Link, Google Scholar
26. Parker G: “New” and “old” antidepressants: all equal in the eyes of the lore? Br J Psychiatry 2001; 179:95–96Crossref, Medline, Google Scholar
27. Stage KB, Bech P, Gram LF, Kragh-Sorensen P, Rosenberg C, Ohrberg S (Danish University Antidepressant Group): Are in-patient depressives more often of the melancholic subtype? Acta Psychiatr Scand 1998; 98:432–436Crossref, Medline, Google Scholar
28. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder (Revision). Am J Psychiatry 2000; 157(April suppl)Google Scholar
29. Gex-Fabry M, Balant-Gorgia AE, Balant LP, Garrone G: Clomipramine metabolism: model-based analysis of variability factors from drug monitoring data. Clin Pharmacokinet 1990; 19:241–255Crossref, Medline, Google Scholar
30. Grossman MI, Kirsner JB, Gillespie IE: Basal and histolog-stimulated gastric secretion in control subjects and in patients with peptic ulcer or gastric cancer. Gastroenterology 1963; 45:14–26Crossref, Medline, Google Scholar
31. Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R: Gastrointestinal transit: the effect of the menstrual cycle. Gastroenterology 1981; 80:1497–1500Crossref, Medline, Google Scholar
32. Yonkers KA, Kando JC, Cole JO, Blumenthal S: Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. Am J Psychiatry 1992; 149:587–595Link, Google Scholar



