Efficacy of the Branched-Chain Amino Acids in the Treatment of Tardive Dyskinesia in Men
Abstract
OBJECTIVE: The efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men with psychiatric disorders was tested. METHOD: Public-sector psychiatric patients with long histories of antipsychotic treatment and presumably long-standing tardive dyskinesia were randomly assigned to receive branched-chain amino acids or placebo. Treatment frequency was three times a day, 7 days a week for 3 weeks. The efficacy measure was a frequency count of videotaped tardive dyskinesia movements. RESULTS: A robust and highly significant difference was observed between patients who received high-dose branched-chain amino acids (222 mg/kg of body weight t.i.d.) (N=18) and those who received placebo (N=18) in the percent change in tardive dyskinesia symptoms from baseline to the end of the 3-week trial. Significant and marked differences were seen between the two groups at the ≥30% and ≥60% levels of decrease in tardive dyskinesia symptoms. No clinically significant differences were seen between the pre- and posttrial results of physical examinations and laboratory screening tests. Minimal gastrointestinal symptoms occurred during the trial. The reduction in tardive dyskinesia symptoms in the amino acids group was not related to changes in antipsychotic and glucose plasma levels. A mechanism of response related to decreased amine neurotransmitter synthesis was suggested by the significant positive correlations observed between decreases in tardive dyskinesia symptoms and decreases in aromatic amino acid plasma concentrations over the course of the trial. CONCLUSIONS: Branched-chain amino acids constitute a novel, safe treatment for tardive dyskinesia, with a strong potential for providing significant improvement in the diseased physiognomy of the afflicted person.
Findings from studies of the risk factors for tardive dyskinesia have been useful in setting treatment directions. One such study identified phenylketonuria as a risk factor for tardive dyskinesia in persons with mental retardation (1). Phenylketonuria impairs the kinetics of the large neutral amino acid phenylalanine and is characterized by high plasma and brain levels of phenylalanine. These findings led to the testing of phenylalanine kinetics in tardive dyskinesia in men with psychiatric disorders by means of a standardized oral challenge of pure phenylalanine (100 mg/kg of body weight) (2). The results showed significantly higher plasma levels of phenylalanine after the phenylalanine challenge in men with tardive dyskinesia than in those without tardive dyskinesia, suggesting greater availability of phenylalanine to the brain and supporting an association between tardive dyskinesia and impaired phenylalanine kinetics. In another study, complete tardive dyskinesia remission was seen 2 hours after ingestion of a protein meal in 21 of 46 patients with tardive dyskinesia (3, 4). The proportion of branched-chain amino acids (isoleucine, leucine, valine) in the protein meal was 19.6%, and the proportion of aromatic amino acids (phenylalanine, tyrosine, tryptophan) was 7.5%. Plasma analyses of the male subjects in the study (N=30) showed significantly higher plasma indices of branched-chain amino acids and significantly lower plasma indices of phenylalanine in patients whose tardive dyskinesia remitted. These cumulative findings led to the development of a medical food product for treatment of tardive dyskinesia (5). The food product has the same proportions of individual branched-chain amino acids and the same dose parameters as the protein meal used in the previous study. Use of this product, in a blindly rated open trial involving men with tardive dyskinesia, was associated with a significant decrease in tardive dyskinesia symptoms, as well as an increase in the plasma concentration and large neutral amino acid ratio of branched-chain amino acids and a decrease in the plasma concentration and large neutral amino acid ratio of the aromatic amino acid phenylalanine (6).
The branched-chain amino acids make up approximately 35% of the indispensable large neutral amino acids of muscle and 50% of the indispensable amino acids in food (7). Ingestion of branched-chain amino acids decreases the concentration of aromatic amino acids in plasma by the stimulation of protein synthesis (primarily in muscle) (8, 9) and insulin release (7, 10). These processes clear the plasma of the aromatic amino acids and suppress the flux of aromatic amino acids from muscle into plasma, allowing the use of branched-chain amino acids to protect or heal muscle (8, 11).
The branched-chain amino acids share a competitive blood-brain-barrier transport system with the aromatic amino acids. Thus the entry of branched-chain amino acids into the brain is enhanced by increasing their plasma concentration and/or by decreasing their competition for uptake, both of which occur after the ingestion of branched-chain amino acids (7, 12).
Since the aromatic amino acids are precursors of the amine neurotransmitters, ingestion of branched-chain amino acids can decrease the central synthesis of the neurotransmitters dopamine, noradrenaline, and serotonin. This treatment mechanism has been used successfully in disorders such as hepatic encephalopathy, in which a decrease in the central synthesis of these neurotransmitters is required for a therapeutic effect (13).
To our knowledge, the present study is the first placebo-controlled trial of branched-chain amino acids for the treatment of tardive dyskinesia. In addition to examining the efficacy of branched-chain amino acids, the study addresses possible mechanisms of action and confounding factors affecting treatment response through analysis of plasma levels of antipsychotics, glucose, branched-chain amino acids, and aromatic amino acids.
Method
Subjects
All male residents at a New York State psychiatric center and two community residential facilities were screened for study inclusion. After complete description of the study, written informed consent was obtained in accordance with the institutional review board guidelines and regulations of the Nathan S. Kline Institute and the patient’s treatment facility.
The patients’ lifetime DSM-IV diagnoses were determined on the basis of diagnostic interviews and clinical data from current and prior admissions. Medical reasons for exclusion from the study (FDA IND 40,382) included diabetes, hypoglycemia, clinically significant abnormal thyroid values, pancreatitis, amyloidosis, pernicious anemia, and history of malabsorption syndrome, acute or chronic renal disease, gout or family history compatible with gouty arthritis, a disorder of large neutral amino acid metabolism (other than phenylketonuria), and a proliferative disease such as multiple myeloma. Hepatitis was also an exclusion criterion; patients were excluded if they had a positive screen for hepatitis C antibody, hepatitis B antigen, hepatitis B antibody, or hepatitis A antibody and also had a serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, or gamma glutamyl transpeptidase value of 180 U/liter or higher or if they had a positive screen for any hepatitis antibody and had been experiencing unexplained flu-like symptoms for a period of 2 weeks. Had women been included in the study, pregnancy would have been an exclusion criterion and pregnancy tests would have been required.
Patients who were unable to sustain a stable antipsychotic dose for 2 weeks before the baseline study measurements were excluded from participation. Patients who began the trial with stable doses of medication but who had changes in antipsychotic, antiparkinson, antidepressant, or anticonvulsant drug doses during the trial were dropped from the study. Patients who had a record of a stable medication regimen but who showed highly variable antipsychotic plasma levels during the trial (suggestive of covert noncompliance) were not included in study analyses.
Health and Safety Monitoring
Pre- and posttrial physical examinations were conducted by the medical specialist in the patient’s unit. The psychiatrist in the patient’s unit authorized the inclusion of the patient in the protocol. The study physician cleared subjects for entry into the study after reviewing the results of the physical examination, CBC, SMA-20, and urinalysis screens. The results of posttrial physical examinations and laboratory tests were evaluated by the medical specialist in the patient’s unit and by the facility medical director. Before patients were administered the branched-chain amino acids formulation, their vital signs, general health, and psychiatric status were evaluated and their gastrointestinal condition was assessed by means of a symptom checklist. Patients’ fasting (morning) weight was measured at baseline, on the first day of treatment, and after 1, 2, and 3 weeks of treatment.
Dose and Dose Assignment
The branched-chain amino acids formulation (with a ratio of valine to isoleucine to leucine of 3:3:4) (5, 6) and the placebo formulation, both of which were manufactured by Scientific Hospital Supplies International Ltd. (Liverpool, U.K.) and supplied by Scientific Hospital Supplies, North America (Rockville, Md.), were in powder form and, for each dose, were dissolved in 148 ml of water (5 oz) to create a pineapple-flavored drink. The amino acid and placebo drinks had the same color and consistency. Doses were administered three times daily (after breakfast, 1 hour before lunch, and 1 hour before dinner) by the study nurses for the duration of the 3-week trial.
Patients were randomly assigned to receive placebo or low (56 mg/kg of body weight t.i.d), medium (167 mg/kg of body weight t.i.d.), or high (222 mg/kg of body weight t.i.d.) doses of the branched-chain amino acids formulation. After 24 subjects completed the 3-week trial, an interim analysis was conducted to test for ineffective dosing. A comparison of the numbers of subjects in each of the three dose groups with a decrease of 50% or more in tardive dyskinesia symptoms with the number of placebo group members having such a response indicated that both the low- and medium-dose groups did not meet the pretrial criteria for study continuation. Assignment to the low and medium treatment doses was discontinued, and for the remainder of the study subjects were randomly assigned to receive either placebo or the high (222 mg/kg) dose of the branched-chain amino acids formulation.
Efficacy Measure
The first and second authors made a consensual clinical diagnosis of tardive dyskinesia using a mild-to-severe global score based on a subscale of items from the Simpson Abbreviated Dyskinesia Scale (14). This criterion has been used by other investigators (15) and has been described in more detail elsewhere (16). The dependent variable for the study was a frequency count of videotaped tardive dyskinesia movements. The method for the frequency count was developed by the authors and has been used previously (6, 17, 18). Frequency counts have a long history of use in tardive dyskinesia treatment trials and have shown to have adequate validity, reliability, sensitivity, and specificity (19–22). The types of movement that were videotaped for counting had been preselected by the first and second authors after direct observation of the subjects during two 2.5-hour morning evaluation sessions. Each type of movement was videotaped for 4 minutes. Three to five types of movement were observed for each patient; thus a minimum of 12 minutes of videotaped movements per patient were evaluated. Movements were videotaped at baseline, after the first dose of the study formulation, and after 1, 2, and 3 weeks of treatment. Movement frequencies were counted from the videotapes by the first author, who was blind to the patients’ treatment status and the chronological order of the videotapes.
The use of a single rater can be seen as either a limitation or a benefit. The study may have been limited because the final ratings relied on the skill of one person, but it may have benefited from the continuity of having the same rater assess the subjects at every evaluation. The limitation of having a single rater was mollified by the following factors: 1) the first author had a long history of clinical and research experience in the differential diagnosis and rating of tardive dyskinesia, 2) the first and second authors had made a consensual diagnosis of tardive dyskinesia for patients’ entry into the study and had by consensus preselected the movements to be rated on the basis of two 2.5-hour observation periods, 3) the reliability of the first and second authors in rating tardive dyskinesia by evaluating videotapes of patients’ movements had been satisfactory established in a prior study (interclass correlation coefficient=0.89, 95% confidence interval=0.81–0.94 for 48 cases) (6), and 4) the reliability and validity of the first author’s differential diagnosis of tardive dyskinesia had been established in comparison with that of another tardive dyskinesia expert in prior studies (agreement=92.3%, kappa=0.84, z score=4.27, p<0.0001, N=26) (23, 24).
Control Plasma Variables
Glucose and antipsychotic drug plasma levels were determined to provide data on the safety of the study formulation and for analysis of their possible effects as confounding factors. Blood levels of large neutral amino acids were measured to monitor tolerance of the study formulation and to address treatment mechanisms. Blood samples were collected on the day of the first treatment and after 1, 2, and 3 weeks of treatment. Blood samples for the large neutral amino acid and glucose levels were collected both while the patient was fasting and at 2 hours after treatment. The antipsychotic levels were determined only from blood samples taken while the patient was fasting. Large neutral amino acid levels in plasma were measured as previously described (6). Antipsychotic plasma levels were assayed by using standard chromatographic methods (for risperidone, 9-hydroxy-risperidone, olanzapine, clozapine, norclozapine, haloperidol, reduced haloperidol, thioridazine, and mesoridazine) and radioimmunoassay methods (for fluphenazine). Glucose plasma concentrations were measured by using an automated BM/Hitachi 747-100 analyzer (Roche Diagnostics, Indianapolis).
Statistical Analyses
The primary efficacy variable was the percent change in tardive dyskinesia movements from baseline to either the last usable observation or the end of week 3. Percent change has been used as an efficacy variable by other investigators (25, 26). The percent change for each patient was calculated by determining the mean of the percent changes in the frequency counts for the movements observed for that patient. This method gives each discrete movement equal weight by removing the distortion caused in total movement counts by the higher frequencies of extremity movements (versus oral/facial movements) and thus maximizes clinical validity. In the placebo group, one patient who completed 3 weeks of treatment and had usable data for the entire period had a 220% increase in tardive dyskinesia movements (z score=3.5). This increase was recoded as a 55% increase (one unit above the next highest value in the placebo group) to preserve the deviancy of the data for this patient without allowing it to perturb the correlation analyses.
As a secondary efficacy measure, total movement counts at the last usable observation or at the end of week 3 were analyzed by using baseline measures as a covariate. For these analyses, assumptions of normality were satisfied by recoding the baseline and last usable observation of one outlier in the branched-chain amino acids group to one unit above the next highest value in the group (baseline value of 1,502 recoded to 589 and week-1 value of 959 recoded to 454).
Analysis of variance (ANOVA) was used to analyze the percent change in tardive dyskinesia movements, and analysis of covariance was used to analyze total movement counts with baseline counts as a covariate. The analyses were conducted 1) on an intent-to-treat basis with data from the last usable observation for all subjects who received at least 1 week of treatment and 2) with data from subjects in the final high-dose branched-chain amino acids group and the placebo group who remained in treatment for the entire 3-week trial (subjects in the efficacy analysis).
The demographic and baseline clinical characteristics of the patients in the amino acids and placebo groups were compared by using ANOVA and Fisher’s exact test. Repeated-measures ANOVA was used to test the effect of time and group-by-time differences for the continuous variables of percent change in tardive dyskinesia movements, weight, and plasma levels of branched-chain amino acids and antipsychotic medications. For analyses of plasma antipsychotic levels, an average percent change in levels for each drug received was calculated for each subject. Group differences in glucose plasma levels were analyzed by using ANOVA. The relationships between continuous variables within the high-dose branched-chain amino acids group were tested by using Spearman (rs) correlations.
Among all subjects randomly assigned to the low-, medium-, or high-dose branched-chain amino acids or placebo groups and who received at least one dose (N=68), the proportions of subjects with or without gastrointestinal side effects were compared by using a two-by-four Fisher’s exact test. Among the subjects in the efficacy analysis (N=36), the proportions with or without gastrointestinal side effects were compared by using a two-by-two Fisher’s exact test.
All subjects randomly assigned to the low-, medium-, or high-dose branched-chain amino acids groups who completed 3 weeks of treatment and who had usable data were included in analyses of possible mechanisms underlying tardive dyskinesia symptom change. These analyses were conducted by using Pearson (r) or Spearman (rs) correlations, as appropriate.
All statistical tests were two-tailed, with statistical significance set at 0.05, and were performed by using SAS (27).
Results
Subject Characteristics
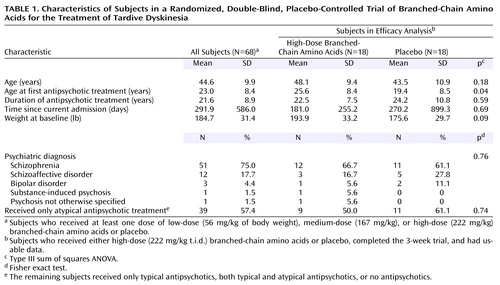
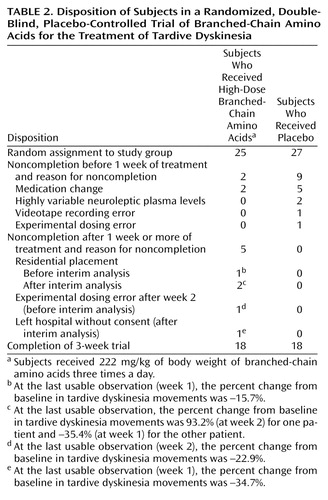
Sixty-eight patients (Table 1) were randomly assigned to treatment groups and received at least one treatment dose (of low-, medium-, or high-dose branched-chain amino acids or placebo). The subjects who did not complete 3 weeks of treatment (N=14) included eight with medication changes, five who left the hospital before study completion, and one whose unit physician requested that he be dropped from the study in week 1. Dropped from analyses at study completion (N=6) were three patients whose data were subject to experimental error (videotaping or dose calculation errors) and three patients with highly variable antipsychotic plasma levels. No patients left the protocol because of dissatisfaction with the branched-chain amino acids treatment or their participation in the trial. Table 2 reports information on the disposition of all subjects randomly assigned to receive high-dose branched-chain amino acids or placebo.
A comparison of the 48 subjects who remained in the study with those who either did not complete the study or whose data were not used (N=20) showed no significant group differences in age, age at first antipsychotic treatment, years of taking antipsychotic medications, days since the current admission, psychiatric diagnosis, or baseline weight (data not shown).
Intent-to-Treat Analysis
The intent-to-treat analysis was conducted by using data from the last usable observation for the 41 patients who were randomly assigned to receive high-dose branched-chain amino acids or placebo and who received at least 1 week of treatment. For the primary efficacy variable, percent change in tardive dyskinesia movements, a significant difference was found between the amino acids group (N=23) (mean=–29.3%, SD=40.7%) and the placebo group (N=18) (mean=3.4%, SD=31.0%) (F=7.98, df=1, 40, p=0.007). The secondary efficacy measure, total tardive dyskinesia movement count from the last usable observation (adjusted for baseline count), was also significantly different between the amino acids group and the placebo group (F=11.92, df=1, 40, p<0.002). The mean total tardive dyskinesia movement count decreased from 295.0 (SD=151.8) to 190.1 (SD=121.9) in the amino acids group (N=23) and from 303.7 (SD=148.2) to 283.0 (SD=120.4) in the placebo group (N=18).
Efficacy Analysis
A highly significant difference was observed between the high-dose branched-chain amino acids group (N=18) and the placebo group (N=18) in the final efficacy analysis of the primary efficacy variable of percent change in tardive dyskinesia movements from baseline to week 3 (F=13.38, df=1, 35, p=0.0009). The amino acids group had a mean decrease of 36.5% (SD=34.4%) and the placebo group a mean 3.4% increase (SD=31.0%) in tardive dyskinesia movement count from baseline to week 3. A robust significant difference was seen between the amino acids group and the placebo group in the number of responders with a reduction of 30% or more in tardive dyskinesia movements (p<0.005, Fisher’s exact test, N=36) and in the number of responders with a reduction of 60% or more (p<0.02, Fisher’s exact test, N=36). As Figure 1 shows, one-third of the subjects in the amino acids group had a reduction of 60% or more in tardive dyskinesia movements.
The mean baseline total tardive dyskinesia movement count for the high-dose branched-chain amino acids group was 295.1 (SD=138.1), compared to 303.7 (SD=148.2) for the placebo group (F=0.03, df=1, 35, p=0.86). At week 3, the mean total tardive dyskinesia movement count had decreased to 175.9 (SD=113.4) for the amino acids group, compared to 283.0 (SD=120.4) for the placebo group. The difference, with adjustment for baseline counts, was highly significant (F=13.66, df=1, 35, p=0.0008).
Possible Confounds to Efficacy
Among the 36 patients who completed 3 weeks of treatment with usable data, there were no significant differences between those who received high-dose branched-chain amino acids (N=18) and those who received placebo (N=18) in baseline demographic characteristics, diagnoses, and type of antipsychotic treatment, with the exception that the amino acids group was younger than the placebo group at the time of first antipsychotic treatment (Table 1).
The changes in antipsychotic plasma levels (from the first treatment morning to week 1, week 2, and week 3) were not different between the amino acids (N=18) and placebo (N=18) groups (F=2.33, df=1, 27, p=0.14), across time (F=0.90, df=2, 54, p=0.41), or by group-by-time interaction (F=0.54, df=2, 54, p=0.59). Further, in the amino acids group, the percent change in tardive dyskinesia movements was not predicted by the percent change in antipsychotic plasma level from the first treatment morning to week 3 (rs=–0.09, N=17, p=0.74).
There was no difference between the amino acids and placebo groups in the efficacy analysis in change in glucose plasma level from the fasting measure on the first treatment morning to the postdose measure at week 3 (F=2.60, df=1, 23, p=0.12). Further, within the amino acids group, the percent change in tardive dyskinesia movements was not predicted by change in overall glucose plasma levels (rs=0.30, N=13, p=0.32).
Time Course
Time in treatment was not significantly related to tardive dyskinesia symptom response (percent change from baseline to weeks 1, 2, and 3) in the high-dose branched-chain amino acids and placebo groups in the efficacy analysis (time-by-group interaction: F=2.38, df=2, 64, p=0.10). In addition, in the amino acids group, improvement in tardive dyskinesia symptoms at week 1 was significantly correlated with improvement at week 2 (rs=0.71, N=18, p=0.001) and week 3 (rs=0.74, N=18, p=0.0004).
In the high-dose branched-chain amino acids group in the efficacy analysis (N=18), plasma levels of branched-chain amino acids were substantially increased 2 hours after treatment administration (week 1: mean=201.0%, SD=52.7%; week 2: mean=199.2%, SD=41.4%; week 3: mean=200.6%, SD=45.9). Although group status (branched-chain amino acids or placebo) had a significant effect on the weekly changes in plasma level of branched-chain amino acids (F=321.17, df=1, 26, p<0.0001), there was no effect of time in trial (F=0.10, df=2, 52, p=0.91) and no time-by-dose interaction (F=0.01, df=2, 52, p=0.99), suggesting that tolerance in response to treatment with branched-chain amino acids had not developed over the 3-week trial.
Health and Safety
Laboratory tests and side effects
No clinically significant changes were found in a review of the results of the physical examination, CBC, SMA-20, and urinalysis screens conducted before and after the trial for the 68 patients who received at least one dose of branched-chain amino acids or placebo. Gastrointestinal side effects were monitored by the administration of a gastrointestinal symptom checklist three times daily. Single episodes of mild gastrointestinal side effects occurred variably across the trial in eight of 68 subjects and were not followed by further complaints. The proportion of subjects reporting symptoms did not differ significantly between the four groups of subjects who received at least one dose of branched-chain amino acids or placebo (p=0.38, Fisher’s exact test, N=68) or between the two groups in the efficacy analysis (p=0.23, two-by-two Fisher’s exact test, N=36).
Weight
Both the subjects receiving high-dose branched-chain amino acids and those receiving placebo in the efficacy analysis (N=36) gained weight during the trial (F=9.84, df=2, 68, p=0.001), but there was no significant difference between the two groups in the rate (F=2.44, df=2, 68, p=0.10) or amount (F=2.18, df=1, 34, p=0.15) of weight gained. In the amino acids group, there was no relationship between the amount of branched-chain amino acids ingested and the percent change in weight from baseline to the end of the 3-week trial (rs=0.08, N=18, p=0.74).
Mechanism of Action
For patients given branched-chain amino acids treatment (low, medium, or high dose) who completed 3 weeks of the trial with usable data (N=27), there was a strong and highly significant positive correlation between the amount of branched-chain amino acids ingested and the percent increase in plasma level of branched-chain amino acids across the trial (r=0.70, N=27, p<0.0001). Aromatic amino acids plasma levels in these subjects decreased over the trial (change in total aromatic amino acids plasma level: mean=–35.0%, SD=15.4%; change in phenylalanine plasma level: mean=–39.5%, SD=18.0%; change in tyrosine plasma level: mean=–34.8%, SD=16.9%; change in tryptophan plasma level: mean=–29.4%, SD=16.2%). The change in branched-chain amino acids plasma level was significantly and negatively correlated with the changes in phenylalanine (r=–0.49, N=27, p=0.01) and tryptophan (r=–0.41, N=27, p=0.03) plasma levels. The change in branched-chain amino acid plasma level was negatively related to the change in total aromatic amino acids plasma level, although the correlation only approached significance (r=–0.36, N=27, p=0.07), and was not related to change in tyrosine plasma level (r=–0.16, N=27, p=0.44). The decrease in tardive dyskinesia movements over the trial was significantly and positively correlated with the decrease in total aromatic amino acids (rs=0.39, N=27, p=0.04), phenylalanine (rs=0.39, N=27, p=0.05), and tryptophan (rs=0.42, N=27, p=0.03) plasma levels; the decrease in symptoms was positively related to the decrease in tyrosine plasma level, but the correlation only approached significance (rs=0.35, N=27, p=0.07).
Discussion
This randomized, double-blind, placebo-controlled trial demonstrates the clinical efficacy and tolerability of branched-chain amino acids for the treatment of tardive dyskinesia in a group of men with various psychiatric diagnoses, long histories of antipsychotic treatment, and most likely long-standing tardive dyskinesia.
The magnitude of the clinical response on a subject-by-subject basis (Figure 1) in the high-dose branched-chain amino acids group in the efficacy analysis (N=18) demonstrates the strong clinical efficacy of the amino acids treatment in a majority of patients. These findings also reflect the heterogeneity of tardive dyskinesia and serve as a reminder that no one treatment is appropriate for all patients (28, 29).
Symptom reductions occurred as soon as 1 week after treatment with branched-chain amino acids, and time in the trial was not significantly related to tardive dyskinesia symptom response. Treatment with branched-chain amino acids markedly increased plasma concentrations of branched-chain amino acids across the trial, without the appearance of tolerance. The demonstrated lack of time or tolerance effects suggests that tardive dyskinesia symptom response could extend beyond the 3 weeks of the trial. Longer-term studies would be clinically useful.
Careful monitoring of health and safety variables indicated that possible side effects were limited, with no clinically significant changes from pretrial to posttrial in laboratory screens. The branched-chain amino acids and placebo groups in the efficacy analysis (N=36) did not differ in the amount or pattern of weight gain, and no association was found between weight change and the amount of branched-chain amino acids (grams) ingested in the high-dose branched-chain amino acids group (N=18). However, patients’ weight should be carefully monitored when a branched-chain amino acids formulation such as the one used in this study is administered.
There was a lack of significant change in antipsychotic plasma level over the trial and no association between change in antipsychotic plasma level and either treatment group status (in the high-dose branched-chain amino acids and placebo groups in the efficacy analysis) or decrease in tardive dyskinesia symptoms. These findings demonstrate 1) that antipsychotic availability was not related to the demonstrated efficacy in treating tardive dyskinesia symptoms and 2) that treatment with branched-chain amino acids does not pose a safety issue in patients who require continued treatment with typical or atypical antipsychotics. Change in glucose plasma level was also not related to trial efficacy, and treatment with branched-chain amino acids did not challenge glycemic status in patients taking chronic antipsychotic treatment who did not have diabetes or hypoglycemia. It is unfortunate, given that diabetes has been shown to be a risk factor for tardive dyskinesia (30), that this disorder needs to be considered a contraindication for use of the current branched-chain amino acids formulation.
Whether branched-chain amino acids constitute an effective treatment for tardive dyskinesia in women has yet to be tested. We did not find an association between altered phenylalanine kinetics and tardive dyskinesia in a group of young women (N=103, mean age=38.7 years), as we had found for young men (N=209, mean age=33.0) (3). However, findings from a diverse literature suggest that this result may be age specific. These studies have shown that 1) tardive dyskinesia prevalence and severity increase with increasing age in women but not in men (31–33), 2) basal phenylalanine levels increase with increasing age in women but not in men (34), 3) sex differences in basal phenylalanine levels present in adolescence are not present in childhood (35), and 4) sex differences exist in gastric emptying (a critical process in phenylalanine kinetics), with premenopausal women having a slower rate than men and postmenopausal women having a rate similar to that of men (36). In addition, in a pilot study we found substantial tardive dyskinesia symptom reduction with high-dose branched-chain amino acids treatment in two 10-year-old girls and in one woman over age 60. These cumulative data suggest that the branched-chain amino acids may be an effective treatment for tardive dyskinesia in postmenopausal women and prepubertal girls and that controlled trials are warranted.
Studies using a branched-chain amino acids formulation similar to the one used in this study but at a dose that was 10% lower than the high dose we used demonstrated postingestion decrease in CSF phenylalanine and tyrosine (37, 38). In addition, in vivo magnetic resonance spectroscopy studies have shown that the ingestion of branched-chain amino acids prevents the increase in phenylalanine level in the brain that usually results from administration of phenylalanine (39, 40). These studies demonstrated that modulation of the plasma pool of large neutral amino acids by exogenous branched-chain amino acids or phenylalanine is reflected in the brain.
In prior studies we have shown that higher levels of phenylalanine in plasma and the brain are associated with tardive dyskinesia (1), that exogenous phenylalanine significantly elevates plasma phenylalanine in men with tardive dyskinesia (2, 3), and that exogenous branched-chain amino acids significantly decrease tardive dyskinesia symptoms and plasma aromatic amino acids in men (6).
In the present study we showed that the amount of exogenous branched-chain amino acids ingested has a robust and significant positive correlation with the postdose increase in plasma levels of branched-chain amino acids and significant negative correlations with the postdose decreases in plasma levels of phenylalanine and tryptophan (7–10). Exogenous branched-chain amino acids produce this effect by stimulating, primarily in muscle, 1) protein synthesis, which requires aromatic amino acids and thus depletes aromatic amino acids in plasma and suppresses aromatic amino acids flux from muscle back into plasma, preventing repletion (8, 9), and 2) insulin release, which clears aromatic amino acids from plasma and also stimulates protein synthesis (7, 10). Because of these changes in the periphery, exogenous branched-chain amino acids have a competitive advantage for brain transport over the aromatic amino acids, which are the nutrient precursors of the amine neurotransmitters dopamine, noradrenaline, and serotonin.
Novel to the present study, and a key to a possible mechanism of improvement in tardive dyskinesia symptoms, was the finding of a significant positive correlation between decrease in tardive dyskinesia symptoms and decrease in the total aromatic amino acids level. This finding suggests that decreased monoamine neurotransmitter synthesis affected by the decreased brain transport of the aromatic amino acids (7, 12) could relieve the effects of possibly long-lasting monoamine neurotransmitter supersensitivity by reducing the collective excesses of the catecholamines and indolamines, which may be the physiological engine of the hyperkinetic movements of tardive dyskinesia.
 |
 |
Presented at the 155th annual meeting of the American Psychiatric Association, Philadelphia, May 18–23, 2002. Received Dec. 11, 2001; revisions received Aug. 6 and Nov. 1, 2002; accepted Jan 2, 2003. From the Division of Movement Disorders and Molecular Psychiatry, Nathan S. Kline Institute for Psychiatric Research. Address reprint requests to Dr. Richardson, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Bldg. 35, Orangeburg, NY 10962; [email protected] (e-mail). Supported by NIMH grant MH-44153, institutional support from the New York State Office of Mental Health, and a grant and product support from Scientific Hospital Supplies International, Ltd. The authors thank Marianne Zarychta, the study nursing staff, and the medical, unit, and administrative staff of Rockland Psychiatric Center for their assistance and the study subjects for their participation.
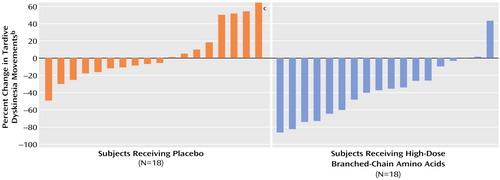
Figure 1. Percent Change in Tardive Dyskinesia Movements for 36 Subjects in a 3-Week Randomized, Double-Blind, Placebo-Controlled Trial of High-Dose Branched-Chain Amino Acids for the Treatment of Tardive Dyskinesiaa
aSubjects received 222 mg/kg of body weight of branched-chain amino acids or placebo three times a day.
bFrequency counts of videotaped tardive dyskinesia movements were made by means of previously used methods (6, 17, 18); negative values indicate improvement.
cOne patient with outlying data had a 220% increase in tardive dyskinesia movement count.
1. Richardson MA, Haugland G, Pass R, Craig TJ: The prevalence of tardive dyskinesia in a mentally retarded population. Psychopharmacol Bull 1986; 22:243-249Medline, Google Scholar
2. Richardson MA, Reilly MA, Read LL, Flynn CJ, Suckow RF, Maher TJ, Sziraki I: Phenylalanine kinetics are associated with tardive dyskinesia in men but not in women. Psychopharmacology (Berl) 1999; 143:347-357Crossref, Medline, Google Scholar
3. Richardson MA, Suckow R, Whittaker R, Boggiano W, Sziraki I, Kushner H, Perumal A: The plasma phenylalanine/large neutral amino acid ratio: a risk factor for tardive dyskinesia. Psychopharmacol Bull 1989; 25:47-51Medline, Google Scholar
4. Richardson MA, Bevans M, Weber J, Gonzalez J, Flynn C, Amira L: A dietary intervention decreases tardive dyskinesia symptoms, in 1996 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 1996, p 194Google Scholar
5. Richardson MA: Treatment of Tardive Dyskinesia With Leucine, Isoleucine, Valine, or Mixtures Thereof (patent number 5,393,784). Washington, DC, US Patent and Trademark Office, 1995Google Scholar
6. Richardson MA, Bevans ML, Weber JB, Gonzalez JJ, Flynn CJ, Amira L, Read LL, Suckow RF, Maher TJ: Branched chain amino acids decrease tardive dyskinesia symptoms. Psychopharmacology (Berl) 1999; 143:358-364Crossref, Medline, Google Scholar
7. Harper AE, Miller RH, Block KP: Branched-chain amino acid metabolism. Ann Rev Nutr 1984; 4:409-454Crossref, Medline, Google Scholar
8. Blomstrand E, Newsholme EA: Effect of branched-chain amino acid supplementation on the exercise-induced change in aromatic amino acid concentration in human muscle. Acta Physiol Scand 1992; 146:293-298Crossref, Medline, Google Scholar
9. Moldawer LL, Sakamoto A, Blackburn GL, Bistrian BR: Alterations in protein kinetics produced by branched chain amino acid administration during infection and inflammation, in Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids. Edited by Walser M, Williamson JR. New York, Elsevier-North Holland, 1981, pp 533-539Google Scholar
10. Malaisse WJ: Branched-chain amino and keto acids as regulators of insulin and glucagon release, in Branched-Chain Amino and Keto Acids in Health and Disease. Edited by Adibi SA, Fekl W, Langenbeck U, Schauder P. Basel, Karger, 1984, pp 119-133Google Scholar
11. Louard RJ, Barrett EJ, Gelfand RA: Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci 1990; 79:457-466Crossref, Medline, Google Scholar
12. Maher TJ: Plasma branched-chain amino acids as regulators of brain neurotransmitters, in Branched-Chain Amino and Keto Acids in Health and Disease. Edited by Adibi SA, Fekl W, Langenbeck U, Schauder P. Basel, Karger, 1984, pp 242-259Google Scholar
13. Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, Martines D, Abbiati R: Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy: a randomized double-blind casein-controlled trial. J Hepatol 1990; 11:92-101Crossref, Medline, Google Scholar
14. Simpson GM, Lee JH, Zoubok B, Gardos G: A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979; 64:171-179Crossref, Medline, Google Scholar
15. Lieberman J, Kane JM, Woerner M, Weinhold P, Basavaraju N, Kurucz J, Bergmann K: Prevalence of tardive dyskinesia in elderly samples. Psychopharmacol Bull 1984; 20:382-386Medline, Google Scholar
16. Richardson MA, Craig TJ: The coexistence of parkinsonism-like symptoms and tardive dyskinesia. Am J Psychiatry 1982; 139:341-343Link, Google Scholar
17. Branchey MH, Branchey LB, Bark NM, Richardson MA: Lecithin in the treatment of tardive dyskinesia. Commun Psychopharmacol 1979; 3:303-307Medline, Google Scholar
18. Richardson MA, Craig TJ, Branchey MH: Intra-patient variability in the measurement of tardive dyskinesia. Psychopharmacology (Berl) 1982; 76:269-272Crossref, Medline, Google Scholar
19. Chien C, Jung K, Ross-Townsend A: Methodological approach to the measurement of tardive dyskinesia: piezoelectric recording and concurrent validity test of five clinical rating scales, in Tardive Dyskinesia Research and Treatment. Edited by Fann WE, Smith RC, Davis JM, Domino EF. New York, SP Medical and Scientific Books, 1980, pp 233-241Google Scholar
20. Growdon JH, Hirsch MJ, Wurtman RJ, Wiener W: Oral choline administration to patients with tardive dyskinesia. N Engl J Med 1977; 297:524-527Crossref, Medline, Google Scholar
21. Gardos G, Cole JO, La Brie R: The assessment of tardive dyskinesia. Arch Gen Psychiatry 1977; 34:1206-1212Crossref, Medline, Google Scholar
22. Kazamatsuri H, Chien C, Cole JO: Treatment of tardive dyskinesia, I: clinical efficacy of a dopamine-depleting agent, tetrabenazine. Arch Gen Psychiatry 1972; 27:95-99Crossref, Medline, Google Scholar
23. Richardson MA, Casey DE, Lin SP: Tardive dyskinesia five-year outcome: a descriptive analysis, in Abstracts of the 1989 Meeting of the New Clinical Drug Evaluation Unit. Bethesda, Md, National Institute of Mental Health, NCDEU, 1989Google Scholar
24. Richardson MA, Casey DE: Tardive dyskinesia status: stability or change. Psychopharmacol Bull 1988; 24:471-475Medline, Google Scholar
25. Lerner V, Miodownik C, Kaptsan A, Cohen H, Matar M, Loewenthal U, Kotler M: Vitamin B6 in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry 2001; 158:1511-1514Link, Google Scholar
26. Sirota P, Mosheva T, Shabtay H, Giladi N, Korczyn AD: Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry 2000; 157:287-289Link, Google Scholar
27. SAS Version 8.1. Cary, NC, SAS Institute, 1999Google Scholar
28. Casey DE, Denney D: Pharmacological characterization of tardive dyskinesia. Psychopharmacology (Berl) 1977; 54:1-8Crossref, Medline, Google Scholar
29. Lieberman J, Lesser M, Johns C, Pollack S, Saltz B, Kane J: Pharmacologic studies of tardive dyskinesia. J Clin Psychopharmacol 1988; 8(suppl):57S-63SGoogle Scholar
30. Ganzini L, Heintz RT, Hoffman WF, Casey DE: The prevalence of tardive dyskinesia in neuroleptic-treated diabetics. Arch Gen Psychiatry 1991; 48:259-263Crossref, Medline, Google Scholar
31. Yassa R, Jeste DV: Gender differences in tardive dyskinesia: a critical review of the literature. Schizophr Bull 1992; 18:701-715Crossref, Medline, Google Scholar
32. Richardson MA, Pass R, Craig TJ, Vickers E: Factors contributing to the prevalence and severity of tardive dyskinesia. Psychopharmacol Bull 1984; 20:33-38Medline, Google Scholar
33. Smith JM, Oswald WT, Kucharski LT, Waterman LJ: Tardive dyskinesia: age and sex differences in hospitalized schizophrenics. Psychopharmacology (Berl) 1978; 58:207-211Crossref, Medline, Google Scholar
34. Caballero B, Gleason RE, Wurtman RJ: Plasma amino acid concentrations in healthy elderly men and women. Am J Clin Nutr 1991; 53:1249-1252Crossref, Medline, Google Scholar
35. Gregory DM, Sovetts D, Clow CL, Scriver CR: Plasma free amino acid values in normal children and adolescents. Metabolism 1986; 35:967-969Crossref, Medline, Google Scholar
36. Hutson WR, Roehrkasse RL, Wald A: Influence of gender and menopause on gastric emptying and motility. Gastroenterology 1989; 96:11-17Crossref, Medline, Google Scholar
37. Berry HK: Branched chain amino acids and inborn errors of metabolism, in Perspectives in Clinical Nutrition. Edited by Kinney JM, Borum PR. Baltimore, Urban & Schwarzenburg, 1989, pp 207-218Google Scholar
38. Berry HK, Bofinger MK, Hunt MM, Phillips PJ, Guilfaile MB: Reduction of cerebrospinal fluid phenylalanine after oral administration of valine, isoleucine and leucine. Pediatr Res 1982; 16:751-755Crossref, Medline, Google Scholar
39. Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, Bremer HJ: Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest 1999; 103:1169-1178Crossref, Medline, Google Scholar
40. Moller HE, Weglage J, Wiedermann D, Ullrich K: Blood-brain barrier phenylalanine transport and individual vulnerability in phenylketonuria. J Cereb Blood Flow Metab 1998; 18:1184-1191Crossref, Medline, Google Scholar



