Relation of Shyness in Grade School Children to the Genotype for the Long Form of the Serotonin Transporter Promoter Region Polymorphism
Abstract
OBJECTIVE: Studies have shown that genetic factors are significant in predisposing individuals to shyness and social phobia. Toward further elucidating the genetic structure of shyness, the authors examined four functional polymorphisms that make biological sense for contributing to the development of this phenotype: serotonin transporter promoter region 44 base pair insertion/deletion (5-HTTLPR), dopamine D4 receptor exon III repeat (DRD4), catechol O-methyltransferase (COMT), and monoamine oxidase A promoter region repeat (MAOA). METHOD: The authors assessed shyness after recruitment of a nonclinical sample (N=118, unscreened second-grade children) using a composite scale derived from questionnaires administered to the children, parents, and teachers. DNA from buccal smears successfully obtained from 98 children was genotyped by polymerase chain reaction methods for the 5-HTTLPR, DRD4, COMT, and MAOA polymorphisms. RESULTS: Significant correlations were observed for parents’, teachers’, and children’s ratings of shyness, and Cronbach’s alpha reliability was high for all three scales. A significant association was observed between the long 5-HTTLPR polymorphism and shyness, both by the functional classification of Lesch as well as by consideration of all three genotypes. No significant association was observed for the DRD4, COMT, or MAOA polymorphisms. CONCLUSIONS: This study provisionally identifies a common genetic polymorphism, 5-HTTLPR, that modestly (effect size=7%) contributed to greater shyness scores in a nonclinical group of second-grade students. These first findings may be relevant to previous reports that have shown an association between the 5-HTTLPR long form and obsessive-compulsive disorder and autism.
“Shyness,” as used in developmental psychology, refers to a pattern of behaviors and emotional states that includes inhibition of approach behaviors and discomfort on exposure to unfamiliar people and situations, a constricted social life, and physiological markers such as high heart rate and cortisol levels (1). The term may encompass a broad range of severity, from normal traits such as mild social awkwardness to clinical disorders such as totally inhibiting social phobia. Shyness is often pervasive, in that it is manifest at school, at home (with strangers), and in other social situations. Various authors have suggested that its temperamental antecedents can be seen as early as the first 6 months of life (2) and that it is an important risk factor for clinical anxiety disorders in childhood and later (3).
Numerous studies have examined genetic factors involved in shyness, and socially related fears and heritability have been shown to be significant (4). However, to our knowledge, association of a specific gene to shyness has been shown in only one study of a phenotype—behavioral inhibition (5). In that study, four candidate genes first flagged in mouse models of emotionality were examined in a selected group of behaviorally inhibited children, and a modest association with glutamic acid decarboxylase was observed.
We assessed shyness after recruitment of unscreened second-grade children using a composite questionnaire administered to the children, parents, and teachers. Children were initially genotyped for the serotonin transporter promoter region 44 base pair deletion/insertion polymorphism (5-HTTLPR), which has been demonstrated to moderate transcriptional efficiency and was originally associated (reviewed in reference 6) with anxiety-related personality traits (7). Other studies have shown an association between the 5-HTTLPR polymorphism and obsessive-compulsive disorder (OCD) (8–13) and autism (14–16).
Three additional polymorphisms of considerable interest in human behavioral genetics—the dopamine D4 receptor (DRD4) (17), catechol O-methyltransferase (COMT) (18), and the MAOA promoter region (MAOA) (19)—were also examined for association with shyness in our study sample.
Method
Subjects
Children (age=7.29 years, SD=0.45, range=7–8) were recruited from second-grade classes in schools in the Beer Sheva municipal area and represent a population-based sample. The interviews were carried out by a psychiatrist (M.G.). Parents and teachers completed a short questionnaire for each child participating in the study. Sixty-seven percent of the children were boys, and 33% were girls. Altogether, 118 children were recruited; DNA, genotyping, and questionnaires were completed for 98 children. After complete description of the study to the parents of the children, written informed consent was obtained. The study was approved by Ben Gurion University’s institutional review board and the Israel Ministry of Health.
Assessment
We assessed shyness in second-grade children with three converging approaches: self-reports with questions from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (20), parental reports with questions from the Martin Temperament Assessment Battery (21) and the Achenbach Behavior Checklist (22), and teacher reports with questions from the teacher versions of the latter instruments. Relevant questions relating to shyness were taken from each of these questionnaires. The teachers’ questionnaire consisted of eight questions, the parents’ questionnaire eight questions, and the children’s questionnaire six questions. The questionnaires used a Likert-type format.
Questions for Teachers
The teachers’ questions were the following:
| 1. | The child is bashful with adults (s)he doesn’t know. | ||||
| 2. | The child avoids new games or activities and prefers to watch others from the side. | ||||
| 3. | The child “gets his/her act together” quickly when learning conditions change. | ||||
| 4. | The child adapts to new activities and situations without reservations. | ||||
| 5. | The child stands and performs in front of the class (sings, recites) without reservations, even for the first time. | ||||
| 6. | When presented with a new learning task, the child reacts immediately and with interest. | ||||
| 7. | The child prefers familiar games to new games. | ||||
| 8. | The child is bashful when (s)he meets a child (s)he doesn’t know. | ||||
Questions for Parents
The parents’ questions were the following:
| 1. | My child is shy with adults (s)he doesn’t know. | ||||
| 2. | My child does not feel at ease appearing or performing at home in front of unfamiliar guests. | ||||
| 3. | When my child meets new children, (s)he is bashful. | ||||
| 4. | In the playground, at a party, or during a visit, my child approaches children (s)he does not know and joins in their games. | ||||
| 5. | When the family goes on a trip, my child quickly feels at ease in unfamiliar places. | ||||
| 6. | My child makes friends easily and connects with unfamiliar adults visiting our home. | ||||
| 7. | The first time (s)he stayed alone (without his/her parents) in a new place, my child felt very unsettled. | ||||
| 8. | My child feels free to laugh and smile in the presence of people (s)he has just met for the first time. | ||||
Questions for Children
The children’s questions were the following:
| 1. | Many children are shy. Some children do not feel at ease with people outside their families. Have you ever been like those children? | ||||
| 2. | Have you felt uneasy or tense with your teacher? | ||||
| 3. | Have you felt uneasy or tense with other children at school? | ||||
| 4. | Some children feel very shy with people they don’t know and feel unable to say anything. Has this happened to you? | ||||
| 5. | Is it hard for you to speak to children you don’t know? | ||||
| 6. | Some children do not like to answer questions in class or speak in front of the class even if they know the answer. Are you like those children? | ||||
Genotyping
DNA was obtained from a buccal smear (brush) from each child. The DNA was extracted by using a MasterPure kit (Epicentre, Madison, Wis.). The 5-HTTLPR (23), DRD4 (24), COMT (25), and MAOA(26) polymorphisms were genotyped in our laboratory, as previously described.
Statistics
All statistical analyses were carried out by using SPSS for Windows (SPSS, Chicago). Associations with shyness scores were examined by using analysis of variance (ANOVA). In the univariate ANOVA analyses, the effect size of the genotype for shyness is given as eta squared. Sample size and power were estimated by using the program PS (W.D. Dupont and W.D. Plummer, Jr.), which is downloadable from Vanderbilt Medical Center (http://www.mc. vanderbilt.edu/prevmed/ps/index.htm).
Results
The children were rated on shyness by themselves, their parents, and their teachers. Significant correlations were observed between parents’ and teachers’ ratings and by self-ratings of shyness (child and teacher: r=0.38, N=116, p<0.001; child and parent: r=0.72, N=118, p<0.001; parent and teacher: r=0.35, N=116, p<0.001). Cronbach’s alpha reliability, which measures internal consistency of test items, was also high for all three rating scales as well as for the combined scale (eight parent items: alpha=0.89, N=118; eight teacher items: alpha=0.88, N=116; six child self-rating items: alpha=0.70, N=118; 22 total items: alpha=0.90, N=116). For the genetic analysis, we used the combined rating scale, arrived at by summing the parent, teacher, and child questionnaire ratings.
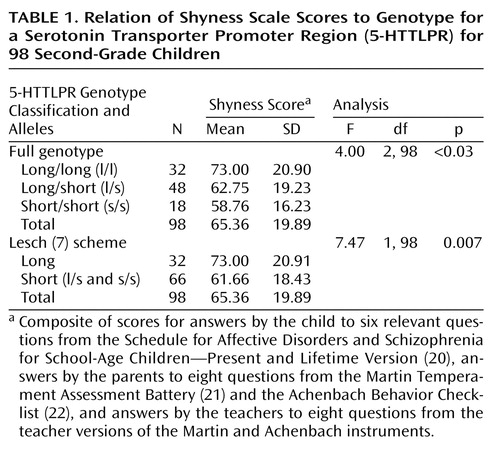
Shyness scores were grouped by all three (long/long homozygotes, short/long heterozygotes, and short/short homozygotes) genotypes and also by a genotype classification (long/long versus short/short and short/long) first suggested by Lesch et al. (7), which reflects the effect of the polymorphism on transcription (Table 1). A significant association was observed between the long 5-HTTLPR polymorphism and shyness, both by the functional classification of Lesch et al. (ANOVA: F=7.47, df=1, 98, p=0.007, N=98; eta squared [a measure of effect size]=0.072, observed power=0.77) as well as by consideration of all three genotypes (ANOVA: F=4.00, df=2, 98, p<0.03, N=98; eta squared=0.078; observed power=0.70). Note that after Bonferroni correction a significant difference at the p<0.05 level was observed between the long/long versus the short/short genotypes. The Bonferroni correction provided by ANOVA performed by SPSS is applicable only with more than two independent variables (in this instance, the long/long, long/short, and short/short genotypes). Of interest, the boys were the main contributors to the association (Lesch scheme [ANOVA], only boys: F=12.53, df=1, 64, p=0.001, N=65; only girls: F=0.04, df=1, 32, p=0.85, N=33). Genotype frequencies were distributed according to the Hardy-Weinberg equilibrium.
Another way of estimating the effect size of the 5-HTTLPR genotype on shyness scores is from the correlation coefficient derived from a linear regression analysis of our data. For example, in our data set, Pearson’s correlation coefficient was 0.269 (p=0.004, one-tailed) for this analysis, which was obtained by regressing composite shyness scores on the Lesch genotype classification scheme. Such a correlation is similar to an odds ratio of ∼1.2–1.9, which, by Cohen’s definition (27), is a small effect.
Since population admixture and stratification may confound interpretation of association studies, we examined the distribution of the 5-HTTLPR genotype in a large sample that represents the two principal ethnic groups examined in the present study. These subjects were previously recruited in our studies of normal personality (6). No significant difference in genotype frequency (long/long, long/short, or short/short) for the 5-HTTLPR genotype was observed between the Ashkenazi (N=575) and non-Ashkenazi (N=383) Jewish populations (χ2=0.01, df=2, p=1.00). Genotype frequencies were in Hardy-Weinberg equilibrium.
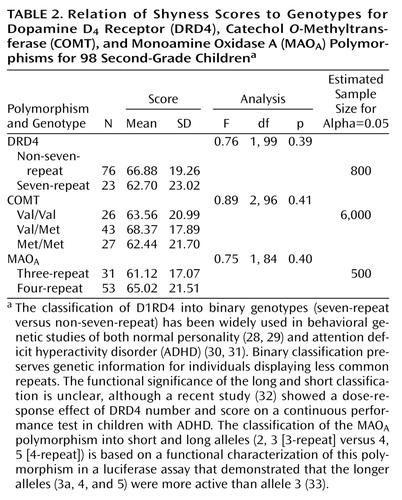
We also examined three additional polymorphisms for possible association with composite shyness scores: the DRD4, COMT, and MAOA (Table 2). No association was observed for any of these genes with shyness (DRD4: F=0.76, df=1, 99, p=0.39, observed power=0.14; COMT: F=0.89, df=2, 96, p=0.41, observed power=0.20; MAOA: F=0.75, df=1, 84, p=0.40, observed power=0.14). We included in Table 2 the estimated sample size for a significant effect for each of these genes at alpha=0.05, based on observed differences in mean values and standard deviations in the current sample.
A Bonferroni correction for multiple testing (four genes tested) showed that the association with the 5-HTTLPR polymorphism was still significant (4×0.007=0.028).
Discussion
The current study shows an association between shyness measured in a group of second-grade schoolchildren by using a composite rating scale (of self-reports, parent reports, and teacher reports) and the long allele of the 5-HTTLPR polymorphism. Shyness, a phenotype akin to behavioral inhibition (34)—a well-described childhood temperament—has been suggested to be an antecedent of later anxiety disorders in adolescents and adults, especially social phobia (35). Although there is considerable evidence that genetic factors significantly contribute to the etiology of social phobia (4), the current study is one of a very few investigations (5, 36) that has examined the role of a specific and common gene variant in conferring risk for this temperament construct. Stein et al. (36) examined 17 multiplex families comprising 76 probands (from ages 18 to 65) who were diagnosed with social phobia and failed to observe linkage to 5-HTTLPR. However, as the authors noted, their study had power to observe only a major gene effect. In contrast to these two previous studies, we examined a group of unscreened schoolchildren in the second grade. Although using subjects who exhibit extremes of a phenotype in genetic studies is generally considered the most efficient strategy, a recent genome-wide linkage study on human stature (height) (37) suggested otherwise. Indeed, the authors reached the conclusion that selective ascertainment of families may provide only modest gains in efficiency.
A longitudinal study from Australia that measured a broad range of temperament and behavioral problem variables found an association between the long 5-HTTLPR allele and higher anxiety at 13–14 and 15–16 years (38), which is consistent with the current findings of an association between the same allele and shyness. At earlier developmental stages, no association was observed between temperament traits and the transporter gene. In previous studies (39–42), we examined the role of the 5-HTTLPR polymorphism in human temperament from 2 weeks to 1 year. However, in contrast to the present findings and the Australian longitudinal study (38), it was the short allele (by an interaction with the DRD4 repeat variant) that enhanced avoidant-like behaviors during this early developmental period. It should be considered, however, that despite the apparent face validity of neonatal and infant temperament traits (distress to limitations, negative emotionality) to measures of adult behavioral traits (social and general anxiety), infant temperament is likely to be only weakly correlated with adult traits. Extrapolating gene effects on behavior from infancy to adult personality constructs, therefore, may lead to ambiguous conclusions. It is also worth noting that the original Lesch et al. finding (7) showed an association between the short 5-HTTLPR alleles that was shown to reduce gene transcription (and presumably make more serotonin available in the synapse) and that adult neuroticism is opposite from what might be expected from the action of selective serotonin reuptake inhibitors (SSRIs).
The currently reported association of the long 5-HTTLPR allele with shyness may be relevant to recent findings in adult anxiety disorder, OCD, and this gene (8–13). Both a family-based (12) and an association (11) study showed associations between the long 5-HTTLPR polymorphism and OCD. OCD is often comorbid (43) with social phobia and other anxiety disorders, suggesting the possibility that such disorders share some, but not all, common genetic determinants—for example, the 5-HTTLPR polymorphism. Such a notion is strengthened by the effectiveness of SSRIs across the range of anxiety disorders (44). Of interest, clinical response to fluvoxamine in OCD patients is related to the 5-HTTLPR genotype (45, 46), and carriers of the long allele are more responsive to treatment.
Some studies have also shown an association between the long 5-HTTLPR allele and autism (14–16). It is worth noting that Cook et al. (47) found an association between autism and the short HTTLPR allele. Tordjman et al. (16) suggested that this promoter region polymorphism by itself does not convey risk for autism but instead influences the behavioral phenotypic expression of autism. A core phenotype in autism is the repetitive behaviors that also characterize OCD. Most intriguing is the epidemiological study of Smalley et al. (48), showing that the frequency of social phobia—20.2%—in autistic families is approximately 10 times more common than that found among the relatives of comparison probands (2.4%).
Although we observed no differences between 5-HTTLPR genotype frequencies when grouped by the two main ethnic groups examined in this study, population stratification cannot be excluded as a contributing cause to the observed association. Future studies should include the use of more robust family-based designs, such as the transmission disequilibrium test (49, 50). Additionally, more sophisticated case-control designs (51) and the use of so-called genomic control subjects (52) offer alternative strategies for handling population admixture in association studies.
We also examined three additional polymorphisms (DRD4, COMT, and MAOA) that have been studied in many other human behavioral genetic investigations and were therefore likely candidates for contributing to shyness. No association was observed between any of these polymorphisms and shyness in the current study. However, our failure to detect an association between these genes and shyness may reflect the difficulty in observing genes of small effect size in a sample of 98 children.
A word of caution is called for regarding association studies in general and the present report in particular. Ioannidis et al. (53) noted that “validation of statistical hypotheses in genetic epidemiology is a task of unprecedented scale” because of the increasing number of candidate genes generated by the mapping of the human genome that are being intensively examined in association studies of human disease. Meta-analysis offers a robust solution to making sense of numerous investigations.
Similarly, the provisional association reported here between shyness and the long 5-HTTLPR allele needs to be examined using both case-control and family-based designs in numerous independent study groups before the validity of this observation is either strengthened or weakened. Nevertheless, the role of shyness as a risk factor for subsequent psychopathology and the importance of elucidating its genetic architecture suggest the importance of the current study as a catalyst that will undoubtedly generate additional investigations of this and other genes in contributing to this intriguing phenotype.
Shyness in the children we examined may be considered an intermediate phenotype (a so-called endophenotype [54]) that is more proximally related to the genetic substrate than is the higher-order construct of a disorder (e.g., social phobia) (5). To the extent that shyness is an intermediate phenotype, genetic influence on it may be stronger and less complex, making shyness in children an excellent target for genetic dissection that may shed light on adult anxiety disorders.
 |
 |
Received Feb. 11, 2002; revision received June 26, 2002; accepted July 8, 2002. From the Unit for Child Psychiatry, Soroka Hospital, Beer Sheva, Israel; the Faculty of Health Sciences, Ben Gurion University of the Negev, Beer Sheva, Israel; the Department of Psychiatry, Emek Hospital, Afula, Israel; and S. Herzog Memorial Hospital. Address reprint requests to Dr. Ebstein, Research Laboratory, S. Herzog Memorial Hospital, P.O. Box 35300, Jerusalem 91351, Israel; [email protected] (e-mail). Supported in part by a grant from the Israel Ministry of Health (to Dr. Benjamin) and the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities (to Dr. Ebstein).
1. Kagan J, Reznick JS, Snidman N: Biological bases of childhood shyness. Science 1988; 240:167-171Crossref, Medline, Google Scholar
2. Chess S, Thomas A: Temperament: Theory and Practice. New York, Brunner/Mazel, 1996Google Scholar
3. Schmidt LA, Fox NA, Schulkin J, Gold PW: Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Dev Psychobiol 1999; 35:119-135Crossref, Medline, Google Scholar
4. Hudson JL, Rapee RM: The origins of social phobia. Behav Modif 2000; 24:102-129Crossref, Medline, Google Scholar
5. Smoller JW, Rosenbaum JF, Biederman J, Susswein LS, Kennedy J, Kagan J, Snidman N, Laird N, Tsuang MT, Faraone SV, Schwarz A, Slaugenhaupt SA: Genetic association analysis of behavioral inhibition using candidate loci from mouse models. Am J Med Genet 2001; 105:226-235Crossref, Medline, Google Scholar
6. Ebstein RP, Benjamin J, Belmaker RH: Personality and polymorphisms of genes involved in aminergic neurotransmission. Eur J Pharmacol 2000; 410:205-214Crossref, Medline, Google Scholar
7. Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527-1531Crossref, Medline, Google Scholar
8. Kinnear CJ, Niehaus DJ, Moolman-Smook JC, du Toit PL, van Kradenberg J, Weyers JB, Potgieter A, Marais V, Emsley RA, Knowles JA, Corfield VA, Brink PA, Stein DJ: Obsessive-compulsive disorder and the promoter region polymorphism (5-HTTLPR) in the serotonin transporter gene (SLC6A4): a negative association study in the Afrikaner population. Int J Neuropsychopharmacol 2000; 3:327-331Crossref, Medline, Google Scholar
9. Camarena B, Rinetti G, Cruz C, Hernandez S, de La Fuente JR, Nicolini H: Association study of the serotonin transporter gene polymorphism in obsessive-compulsive disorder. Int J Neuropsychopharmacol 2001; 4:269-272Crossref, Medline, Google Scholar
10. Frisch A, Michaelovsky E, Rockah R, Amir I, Hermesh H, Laor N, Fuchs C, Zohar J, Lerer B, Buniak SF, Landa S, Poyurovsky M, Shapira B, Weizman R: Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharmacol 2000; 10:205-209Crossref, Medline, Google Scholar
11. Bengel D, Greenberg B, Cora-Locatelli G, Altemus M, Heils A, Li Q, Murphy DL: Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry 1999; 4:463-466Crossref, Medline, Google Scholar
12. McDougle CJ, Epperson CN, Price LH, Gelernter J: Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol Psychiatry 1998; 3:270-273Crossref, Medline, Google Scholar
13. Billett EA, Richter MA, King N, Heils A, Lesch KP, Kennedy JL: Obsessive compulsive disorder, response to serotonin reuptake inhibitors and the serotonin transporter gene. Mol Psychiatry 1997; 2:403-406Crossref, Medline, Google Scholar
14. Yirmiya N, Pilowsky T, Nemanov L, Arbelle S, Feinsilver T, Fried I, Ebstein RP: Evidence for an association with the serotonin transporter promoter region polymorphism and autism. Am J Med Genet 2001; 105:381-386Crossref, Medline, Google Scholar
15. Klauck SM, Poustka F, Benner A, Lesch KP, Poustka A: Serotonin transporter (5-HTT) gene variants associated with autism? Hum Mol Genet 1997; 6:2233-2238Crossref, Medline, Google Scholar
16. Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Carsalade V, Cohen DJ, Ferrari P, Roubertoux PL, Anderson GM: Role of the serotonin transporter gene in the behavioral expression of autism. Mol Psychiatry 2001; 6:434-439Crossref, Medline, Google Scholar
17. Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V: Multiple dopamine D4 receptor variants in the human population. Nature 1992; 358:149-152Crossref, Medline, Google Scholar
18. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM: Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996; 6:243-250Crossref, Medline, Google Scholar
19. Sabol SZ, Hu S, Hamer D: A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998; 103:273-279Crossref, Medline, Google Scholar
20. Orvaschel H, Thompson WD, Belanger A, Prusoff BA, Kidd KK: Comparison of the family history method to direct interview: factors affecting the diagnosis of depression. J Affect Disord 1982; 4:49-59Crossref, Medline, Google Scholar
21. Martin RP: Assessment of Personality and Behavior Problems: Infancy Through Adolescence. New York, Guilford, 1988Google Scholar
22. Achenbach TM, Edelbrock CS: Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev 1981; 46:1-82Crossref, Medline, Google Scholar
23. Manor I, Eisenberg J, Tyano S, Sever Y, Cohen H, Ebstein RP, Kotler M: Family-based association study of the serotonin transporter promoter region polymorphism (5-HTTLPR) in attention deficit hyperactivity disorder. Am J Med Genet 2001; 105:91-95Crossref, Medline, Google Scholar
24. Kotler M, Manor I, Sever Y, Eisenberg J, Cohen H, Ebstein RP, Tyano S: Failure to replicate an excess of the long dopamine D4 exon III repeat polymorphism in ADHD in a family-based study. Am J Med Genet 2000; 96:278-281Crossref, Medline, Google Scholar
25. Eisenberg J, Mei-Tal G, Steinberg A, Tartakovsky E, Zohar A, Gritsenko I, Nemanov L, Ebstein RP: Haplotype relative risk study of catechol-O-methyltransferase (COMT) and attention deficit hyperactivity disorder (ADHD): association of the high-enzyme activity Val allele with ADHD impulsive-hyperactive phenotype. Am J Med Genet 1999; 88:497-502Crossref, Medline, Google Scholar
26. Manor I, Tyano S, Mel E, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP: Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA). Mol Psychiatry 2002; 7:626-632Crossref, Medline, Google Scholar
27. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
28. Kluger AN, Siegfried Z, Ebstein RP: A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Mol Psychiatry 2002; 7:712-717Crossref, Medline, Google Scholar
29. Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH: Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet 1996; 12:78-80Crossref, Medline, Google Scholar
30. Swanson JM, Sunohara GA, Kennedy JL, Regino R, Fineberg E, Wigal T, Lerner M, Williams L, LaHoste GJ, Wigal S: Association of the dopamine receptor D4 (DRD4) gene with a refined phenotype of attention deficit hyperactivity disorder (ADHD): a family-based approach. Mol Psychiatry 1998; 3:38-41Crossref, Medline, Google Scholar
31. Faraone SV, Doyle AE, Mick E, Biederman J: Meta-analysis of the association between the 7-repeat allele of the dopamine D4 receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 2001; 158:1052-1057Link, Google Scholar
32. Manor I, Tyano S, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP: The short DRD4 repeats confer risk to attention deficit hyperactivity disorder in a family-based design and impair performance on a continuous performance test (TOVA). Mol Psychiatry 2002; 7:790-794Crossref, Medline, Google Scholar
33. Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP: Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999; 8:621-624Crossref, Medline, Google Scholar
34. Kagan J: Galen’s Prophecy. New York, Basic Books, 1994Google Scholar
35. Biederman J: The diagnosis and treatment of adolescent anxiety disorders. J Clin Psychiatry 1990; 51(suppl):20-26; discussion, 51(suppl):50-53; correction, 51:440Google Scholar
36. Stein MB, Chartier MJ, Kozak MV, King N, Kennedy JL: Genetic linkage to the serotonin transporter protein and 5HT2A receptor genes excluded in generalized social phobia. Psychiatry Res 1998; 81:283-291Crossref, Medline, Google Scholar
37. Hirschhorn JN, Lindgren CM, Daly MJ, Kirby A, Schaffner SF, Burtt NP, Altshuler D, Parker A, Rioux JD, Platko J, Gaudet D, Hudson TJ, Groop LC, Lander ES: Genomewide linkage analysis of stature in multiple populations reveals several regions with evidence of linkage to adult height. Am J Hum Genet 2001; 69:106-116Crossref, Medline, Google Scholar
38. Jorm AF, Prior M, Sanson A, Smart D, Zhang Y, Easteal S: Association of a functional polymorphism of the serotonin transporter gene with anxiety-related temperament and behavior problems in children: a longitudinal study from infancy to the mid-teens. Mol Psychiatry 2000; 5:542-547Crossref, Medline, Google Scholar
39. Ebstein RP, Auerbach JG: Dopamine D4 receptor and serotonin transporter promoter polymorphisms and temperament in early childhood, in Molecular Genetics and the Human Personality. Edited by Benjamin J, Ebstein RP, Belmaker RH. Washington, DC, American Psychiatric Publishing, 2002, pp 137-149Google Scholar
40. Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R: DRD4 related to infant attention and information processing: a developmental link to ADHD? Psychiatr Genet 2001; 11:31-35Crossref, Medline, Google Scholar
41. Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH: Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Mol Psychiatry 1998; 3:238-246Crossref, Medline, Google Scholar
42. Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, Ebstein R: Dopamine D4 receptor (D4DR) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Mol Psychiatry 1999; 4:369-373Crossref, Medline, Google Scholar
43. Welkowitz LA, Struening EL, Pittman J, Guardino M, Welkowitz J: Obsessive-compulsive disorder and comorbid anxiety problems in a national anxiety screening sample. J Anxiety Disord 2000; 14:471-482Crossref, Medline, Google Scholar
44. Davidson JR: Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry 2001; 62(suppl 11):46-50; discussion, 62(suppl 11):51-52Google Scholar
45. Zanardi R, Benedetti F, Di Bella D, Catalano M, Smeraldi E: Efficacy of paroxetine in depression is influenced by a functional polymorphism within the promoter of the serotonin transporter gene. J Clin Psychopharmacol 2000; 20:105-107Crossref, Medline, Google Scholar
46. Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG, Carroll BJ: Serotonin transporter gene polymorphism and antidepressant response. Neuroreport 2000; 11:215-219Crossref, Medline, Google Scholar
47. Cook EH Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL: Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry 1997; 2:247-250Crossref, Medline, Google Scholar
48. Smalley SL, McCracken J, Tanguay P: Autism, affective disorders, and social phobia. Am J Med Genet 1995; 60:19-26Crossref, Medline, Google Scholar
49. Ewens WJ, Spielman RS: The transmission/disequilibrium test: history, subdivision and admixture. Am J Hum Genet 1995; 57:455-464Crossref, Medline, Google Scholar
50. Spielman RS, McGinnis RE, Ewens WJ: Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 1993; 52:506-516Medline, Google Scholar
51. Pritchard JK, Stephens M, Donnelly P: Inference of population structure using multilocus genotype data. Genetics 2000; 155:945-959Medline, Google Scholar
52. Bacanu SA, Devlin B, Roeder K: The power of genomic control. Am J Hum Genet 2000; 66:1933-1944Crossref, Medline, Google Scholar
53. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG: Replication validity of genetic association studies. Nat Genet 2001; 29:306-309Crossref, Medline, Google Scholar
54. Gottesman II, Wolfgram DL: Schizophrenia Genesis: The Origins of Madness. New York, WH Freeman, 1991Google Scholar



