Increased Cortical GABA Concentrations in Depressed Patients Receiving ECT
Abstract
OBJECTIVE: Reduced γ-aminobutyric acid (GABA) concentrations have been reported in the plasma, CSF, and cortex of depressed subjects. Of interest is that ECT, one of the most effective treatments for severe refractory depression, produces considerable anticonvulsant effects that may be related to increased GABAergic transmission. The purpose of this study was to determine if cortical GABA concentrations increase following a course of ECT. METHOD: Occipital cortex GABA concentrations in eight depressed patients were measured by using proton magnetic resonance spectroscopy before and after a course of ECT. RESULTS: A significant increase in occipital cortex GABA concentrations was seen following ECT treatment of depression. CONCLUSIONS: Occipital cortex GABA concentrations increase two-fold following ECT. This suggests possible GABAergic involvement in ECT’s mechanism of anticonvulsant and antidepressant actions.
A compensatory increase in the function of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) has been proposed as one possible mechanism contributing to both the anticonvulsant and antidepressant actions of ECT (1). As an initial test of this hypothesis, we sought to ascertain if cortical GABA concentrations increase following a course of ECT. Proton magnetic resonance spectroscopy (1H-MRS) was employed to measure occipital cortex GABA concentrations in depressed patients before and after a course of ECT. The occipital cortex was chosen because of the technical difficulties involved in the measurement of cortical GABA concentrations and the fact that previous studies had demonstrated lower occipital GABA concentrations in both subjects with poorly controlled seizure disorders (2) and subjects with major depression (3).
Method
Ten subjects diagnosed with major depression and planning to receive treatment with ECT were enrolled in the study after providing written informed consent. Diagnosis of unipolar major depression was confirmed by a structured clinical interview (4) before initiation of the study protocol. All patients had been medication-free for at least 2 weeks before the study (only diphenhydramine was allowed for treatment of insomnia), with the exception of two subjects. One subject required four doses of thioridazine for severe anxiety; the second was maintained on her previous daily dose of risperidone (2 mg) after proving she was unable to tolerate the initiation of a medication washout. The mean age of the eight subjects (five men and three women) used in the analysis was 46.0 years (SD=5.3).
Assessments with a modified 25-item Hamilton Depression Rating Scale (5) and a Hopkins Verbal Learning Test (6) to measure memory impairment were given before the pretreatment 1H-MRS measurement and again before the posttreatment 1H-MRS measurement. The mean Hamilton depression scale score before initiation of ECT was 36.1 (SD=7.8). The posttreatment 1H-MRS measurement was done at least 1 day after completion of the final ECT session (five subjects were assessed after 1 day, and the other three were assessed after 2, 4, and 46 days).
ECT was performed with either a MECTA SR-2 or a SpECTrum 5000Q (MECTA Corp., Lake Oswego, Ore.) after a standardized ECT dose titration protocol (7) along with standard anesthetics and muscle relaxants. Seven of the eight subjects were treated with bilateral ECT. The mean number of treatments received was 8.5 (SD=2), with a stimulus intensity increase of 28.5 joules (SD=15.1) over the treatment course. Treatment was terminated solely on the basis of the clinical judgment of the treating physicians (J.J.C. or R.B.O.), who were not directly involved in the process of data acquisition or data analysis. The treating physicians (J.J.C. and R.B.O.) determined seizure duration on the basis of visual inspection of a two-lead EEG readout.
Occipital cortex GABA concentrations were determined according to the methods described by Rothman et al. (3, 8). Briefly, studies were performed by using a 2.1-T Oxford Magnet with a 1-m bore, equipped with a Bruker Avance spectrometer (Bruker Instruments, Billerica, Mass.). An 8-cm radiofrequency surface coil tuned to the 1H-MRS frequency of 89.43 MHz was used to center a 1.5×3×3-cm volume of interest on the midline of the occipital cortex, 2 cm deep from the dura. Automated first- and second-order shimming was used to optimize B0 homogeneity. Homonuclear editing at 3.0 ppm (chemical shift scale) C4 GABA resonance was performed using the J-editing pulse sequence described previously. Two subspectra of 128 scans each were subtracted to obtain a difference spectrum that isolated total GABA (combined measure of GABA and the GABA-containing dipeptide Homocarnosine). Localization techniques include three-dimensional, image-selected in vivo spectroscopy with outer volume suppression, selective excitation, and use of a surface spoiler coil. The spectral acquisition parameters were as follows: TR=3.39 seconds; TE=68 msec; sweep width=1,500 Hz; acquisition time=510 msec. The integral of the GABA peak was adjusted for the macromolecular contribution and compared with the total creatine signal for absolute quantification.
Comparisons of pre- and post-ECT measures were made by using a paired t test. Correlations between GABA concentration changes and changes in clinical measures or changes in seizure duration were made by using a simple regression analysis.
Results
Post-ECT GABA concentrations were significantly higher compared with pre-ECT concentrations (Figure 1) (t=–3.06, df=7, p<0.02). Mean GABA concentrations rose from 0.85 mmol/kg brain tissue (SD=0.34) to 1.51 (SD=0.48). Seven of the eight subjects demonstrated an increase in cortical GABA concentrations at the time of the post-ECT study, and four showed increases of greater than 85% over their pre-ECT levels.
As expected, a significant decrease in seizure duration was seen over the course of treatment (paired t test comparing initial with final treatment: t=3.65, df=7, p<0.01), with seven of the eight subjects having shorter seizure durations on the final treatment day than on the first treatment day. There was also a significant decrease in the level of depression severity following ECT (paired t test comparing pre- and post-ECT Hamilton depression scores: t=3.75, df=7, p<0.01). Two of the subjects were clinically considered to have had complete remissions, five of the subjects showed partial response, and one subject exhibited no response. However, with this limited study group size, no significant correlation was found between measures of clinical response and the change in cortical GABA concentrations. ECT also had a modest but significant deleterious effect on memory as measured by a mean 2-point decrease (SD=0.63) in Hopkins Verbal Learning Test scores (6) at the completion of the treatment course (t=6.36, df=6, p<0.005). Again, however, we were unable to demonstrate a significant correlation with the change in cortical GABA concentrations in this small patient group.
Discussion
The finding of higher occipital cortex GABA concentrations following ECT is consistent with earlier animal studies that demonstrated elevated GABA concentrations in several brain regions following repeated electroconvulsive seizures (9) and a single study that measured CSF concentrations in two patients receiving a course of ECT (10). The finding of ECT-related decreases in seizure duration is also consistent with earlier reports (11, 12) and suggests an ECT-induced decrease in cortical excitability. Rodent studies that have demonstrated the ability of electroconvulsive seizures to specifically attenuate seizure induction by GABA antagonists suggest a causative relationship between the ECT-induced rise in cortical GABA concentrations and the relative decrease in cortical excitability (13, 14). Although circumstantial, our finding of both increased cortical GABA concentrations and decreased seizure duration following a course of ECT is consistent with this hypothesis. However, the limited patient group size of this study did not afford us enough power to adequately assess the correlation between these measures.
Could ECT-induced increases in GABAergic transmission also contribute to the modality’s antidepressant effects? Sackeim et al. (1) originally postulated almost two decades ago that enhanced GABAergic function was a mechanism likely to be related to the antidepressant properties of ECT. The ECT-related rise in occipital cortex GABA concentrations demonstrated in this study supports this hypothesis, especially in light of our previous study demonstrating lower occipital cortex GABA concentrations in depressed subjects relative to healthy comparison subjects (3). The fact that occipital cortex GABA concentrations also increase following selective serotonin reuptake inhibitor treatment of depression (15) leads us to speculate that the enhanced GABAergic function may provide a common mechanism of antidepressant action. It is possible, however, that the increase in occipital cortex GABA concentrations following treatment reflects a more general marker of improved mood states. We cannot rule out the possibility that we are observing a practice effect or a time effect, since there was no control for repeated measures in these studies. However, previous studies by our group have shown little effect of repeated measures on occipital cortex GABA concentrations (16). Finally, the low pre-ECT concentrations of GABA could be related to a medication washout effect. However, we have not noticed any significant effect of time off medications on cortical GABA concentrations in our larger database of depressed subjects. Although the data are suggestive, larger study group sizes investigating additional brain regions will be required to clearly ascertain whether the increase in cortical GABA concentration is related to the degree of clinical response.
Presented in part at the seventh scientific meeting of the International Society for Magnetic Resonance in Medicine, Philadelphia, May 22–28, 1999, and the 38th annual meeting of the American College of Neuropsychopharmacology, Acapulco, Mexico, Dec. 12–16, 1999. Received April 4, 2002; revision received July 30, 2002; accepted Aug. 14, 2002. From the Departments of Psychiatry, Biomedical Engineering, Radiology, and Molecular Biophysics and Biochemistry, Yale University School of Medicine, New Haven, Conn. Address reprint requests to Dr. Sanacora, Clinical Neuroscience Research Unit, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, 34 Park St., New Haven, CT 06519; [email protected] (e-mail). Supported by grants from The Donaghue Medical Research Foundation (J.K., G.S.) and the Dana Foundation (G.S.), NIMH grant MH-01715 (G.S.), the Mental Health Clinical Research Center at Yale grant MH-30929 (J.K., G.S.), National Institute on Alcohol Abuse and Alcoholism grant AA-00261 (J.K.), and grants from the VA Research Enhancement Award Program and Yale Alcohol Center. The authors thank Lisa Roach, Michael Appel, Donna Fasula, and Matthew Wachen for their assistance.
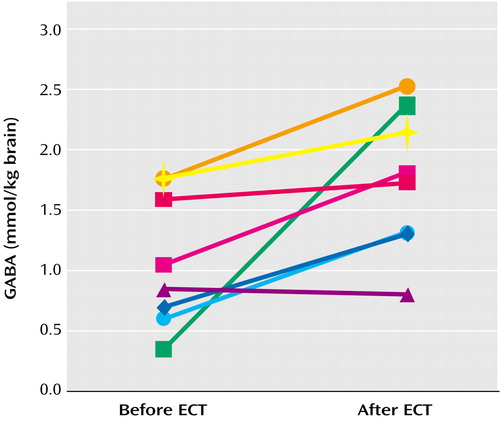
Figure 1. Occipital Cortex GABA Concentrations in Eight Depressed Subjects Before and After a Course of ECT
1.. Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor SR: Anticonvulsant and antidepressant properties of electroconvulsive therapy: a proposed mechanism of action. Biol Psychiatry 1983; 18:1301-1310Medline, Google Scholar
2.. Petroff OA, Rothman DL, Behar KL, Mattson RH: Low brain GABA level is associated with poor seizure control. Ann Neurol 1996; 40:908-911Crossref, Medline, Google Scholar
3.. Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OAC, Berman RM, Charney DS, Krystal JH: Reduced cortical γ-aminobutyric acid levels in depressed patients by proton magnetic spectroscopy. Arch Gen Psychiatry 1999; 56:1043-1047Crossref, Medline, Google Scholar
4.. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
5.. Mazure C, Nelson JC, Price LH: Reliability and validity of the symptoms of major depressive illness. Arch Gen Psychiatry 1986; 43:451-456Crossref, Medline, Google Scholar
6.. Brandt J: The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991; 5:125-142Crossref, Google Scholar
7.. Krystal AD, Dean MD, Weiner RD, Tramontozzi LA III, Connor KM, Lindahl VH, Massie RW: ECT stimulus intensity: are present ECT devices too limited? Am J Psychiatry 2000; 157:963-967Link, Google Scholar
8.. Rothman DL, Petroff OAC, Behar KL, Mattson RH: Localized 1H NMR measurements of γ-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA 1993; 90:5662-5666Crossref, Medline, Google Scholar
9.. Green AR: Changes in gamma-aminobutyric acid biochemistry and seizure threshold. Ann NY Acad Sci 1986; 462:105-119Crossref, Medline, Google Scholar
10.. Lipcsey A, Kardos J, Prinz G, Simonyi M: [Effect of electroconvulsive therapy on the GABA level in the cerebrospinal fluid]. Psychiatr Neurol Med Psychol (Leipz) 1986; 38:554-555 (German)Medline, Google Scholar
11.. Sackeim HA, Decina P, Portnoy S, Neeley P, Malitz S: Studies of dosage, seizure threshold, and seizure duration in ECT. Biol Psychiatry 1987; 22:249-268Crossref, Medline, Google Scholar
12.. Sackeim HA, Devanand DP, Prudic J: Stimulus intensity, seizure threshold, and seizure duration: impact on the efficacy and safety of electroconvulsive therapy. Psychiatr Clin North Am 1991; 14:803-843Crossref, Medline, Google Scholar
13.. Wielosz M, Stelmasiak M, Ossowska G, Kleinrok Z: Effects of electroconvulsive shock on central GABA-ergic mechanisms. Pol J Pharmacol Pharm 1985; 37:113-122Crossref, Medline, Google Scholar
14.. Nutt DJ, Cowen PJ, Green AR: Studies on the post-ictal rise in seizure threshold. Eur J Pharmacol 1981; 71:287-295Crossref, Medline, Google Scholar
15.. Sanacora G, Mason GF, Rothman DL, Krystal JH: Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002; 159:663-665Link, Google Scholar
16.. Petroff OAC, Hyder F, Mattson RH, Rothman DL: Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology 1999; 52:473-478Crossref, Medline, Google Scholar



