Differential Effects of Developmental Cerebellar Abnormality on Cognitive and Motor Functions in the Cerebellum: An fMRI Study of Autism
Abstract
OBJECTIVE: Recent years have seen a revolution in views regarding cerebellar function. New findings suggest that the cerebellum plays a role in multiple functional domains: cognitive, affective, and sensory as well as motor. These findings imply that developmental cerebellar pathology could play a role in certain nonmotor functional deficits, thereby calling for a broader investigation of the functional consequences of cerebellar pathology. Autism provides a useful model, since over 90% of autistic cerebella examined at autopsy have shown well-defined cerebellar anatomic abnormalities. The aim of the present study was to examine how such pathology ultimately impacts cognitive and motor function within the cerebellum. METHOD: Patterns of functional magnetic resonance imaging (fMRI) activation within anatomically defined cerebellar regions of interest were examined in eight autistic patients (ages 14–38 years) and eight matched healthy comparison subjects performing motor and attention tasks. For the motor task, subjects pressed a button at a comfortable pace, and activation was compared with a rest condition. For the attention task, visual stimuli were presented one at a time at fixation, and subjects pressed a button to every target. Activation was compared with passive visual stimulation. RESULTS: While performing these tasks, autistic individuals showed significantly greater cerebellar motor activation and significantly less cerebellar attention activation. CONCLUSIONS: These findings shed new light on the cerebellar role in attention deficits in autism and suggest that developmental cerebellar abnormality has differential functional implications for cognitive and motor systems.
In recent years, the traditional view of cerebellar function has been seriously challenged. Long considered to be involved exclusively in motor coordination, new findings suggest that the cerebellum plays a role in multiple functional domains (e.g., references 1–9). Such findings have emerged largely from functional neuroimaging studies. One such study used functional magnetic resonance imaging (fMRI) to demonstrate a dissociation between cerebellar regions involved in attention and those involved in a simple motor task (5). Motor activation was localized to the anterior cerebellar hemisphere, whereas activation during a nonspatial selective attention task was localized to the superior posterior cerebellar hemispheres. Several other recent investigations have supported this finding by showing similar loci of cerebellar attention activation (e.g., references 7, 10–13).
While these findings help to broaden our view of cerebellar function, they also imply that cerebellar pathology can effect a variety of nonmotor functional deficits (14). This is a crucial implication in light of mounting evidence for cerebellar involvement in various neurologic and psychiatric conditions, including autism (15–17), attention deficit hyperactivity disorder (18), mood disorders (19), obsessive-compulsive disorder (20), and schizophrenia (21, 22). Such evidence calls for a broader investigation of the functional consequences of cerebellar pathology. Autism provides a useful model for such an inquiry, for it is a condition in which cerebellar pathology is particularly well defined.
Ninety-five percent of autistic cerebella examined at autopsy have shown cerebellar anatomic abnormalities (16, 23–30), and in all but one case, the abnormality was a reduction in the normal number of Purkinje cells; molecular abnormalities in the autistic cerebellum have also been reported in all cases examined (31–33). The Purkinje cell is one of the principal cortical neurons in the cerebellum and the only source of output from the cerebellar cortex (34). A crucial question, then, is how reduced numbers of this important element of circuitry ultimately impact cerebellar function. Studies that have used fMRI to show involvement of the normal cerebellum in attention would predict that cerebellar pathology is associated with attentional impairments, and such pathology-deficit associations have in fact been demonstrated in autism (35, 36). However, what is not known is whether the autistic cerebellum is in fact functioning abnormally during attention tasks.
The present investigation examined functional activation in the cerebella of autistic patients and healthy comparison subjects performing the attention and motor tasks from our initial study of normal cerebellar function (5). We employed an anatomical region-of-interest approach to analyzing data, allowing a detailed examination of functional integrity in regions of the cerebellar cortex known to be involved in attention operations. The general prediction was that reductions in viable cerebellar tissue due to an early reduction of Purkinje cells would result in significantly reduced cerebellar activation. This represents a first look at the potential impact of a developmental anomaly of the human cerebellum on patterns of cerebellar cognitive activation.
Method
Subjects
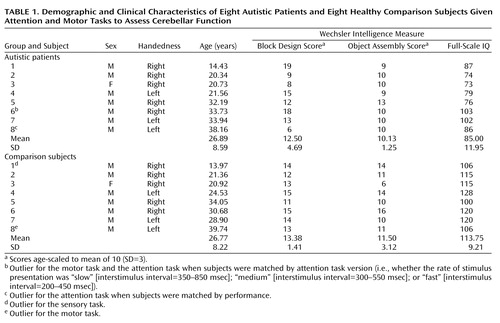
Participants (Table 1) were eight patients with autism (age range=14–38 years) and eight healthy comparison subjects (age range=13–39). Patients were diagnosed with autistic disorder as defined by DSM-IV and also fulfilled criteria from the Autism Diagnostic Interview—Revised (37) and the Autism Diagnostic Observation Schedule (38). None met criteria for Asperger’s disorder. All diagnoses were made by clinical psychologists within our laboratory and not by referring clinicians. No patient was positive for fragile X syndrome as determined by DNA or chromosomal analyses; none had a history of seizure; none had additional psychiatric or neurologic diagnoses; and none used psychotropic medication. IQ was evaluated with the WISC-R (39) or the WAIS-R (40), and results indicated that all subjects were nonretarded (i.e., full-scale IQ >70 [ Table 1]).
Healthy comparison subjects recruited from the community had no history of developmental, psychiatric, or neurologic disorders. Autistic and healthy subjects were matched for age, sex, and handedness but not IQ (Table 1). Groups are often matched for IQ to control for the effects of general cognitive functioning. However, in the case of autism, IQ is not a valid measure of such functioning because of the atypical profile of subtest performance (41). Moreover, for studies in which measures of anatomy or physiology are the dependent variables, ability measures are not appropriate matching variables for the simple reason that they, in turn, can be influenced by the dependent variables. Despite the negative implications of this practice (i.e., erroneously removing true effects and injecting spurious ones), it is often done. Thus, as an alternative, groups in the present study were matched for their scores on the Wechsler Block Design and Object Assembly subtests, measures of abilities that are typically spared in autistic individuals. Matched pair t tests (two-tailed) demonstrated that the groups did not differ significantly in age (t=0.12, df=7, p=0.91), Block Design score (t=–0.62, df=7, p=0.56), or Object Assembly score (t=–1.04, df=7, p=0.33).
The complete experimental protocol was approved by the Institutional Review Boards of San Diego Children’s Hospital Research Center, the University of California, San Diego, and San Diego State University. After complete description of the study to the subjects, written informed consent was obtained. Parents provided written consent for the two subjects under 18 to participate in the study, and these subjects provided verbal assent.
Experimental Procedures
Throughout all tasks, subjects held a joystick in their dominant hand. Pressing a button on the joystick with the thumb activated a fiber optic response device, and digitally recorded responses provided measures of performance accuracy and button-press frequency. A rear-projection screen was mounted at subjects’ feet and viewed through a mirror on the radiofrequency coil. Every 40 seconds over the course of 320 seconds, a one-word instruction appeared on the screen for 2 seconds, cueing subjects to alternate between the task and its control condition. Three tasks were employed.
Attention task
Circles, squares, or triangles in red, green, or blue were presented randomly one at a time at fixation for 100 msec. The attention task tested the ability to selectively attend and respond to targets (squares or red shapes, target probability=33%). Subjects were later matched separately according to accuracy (“performance match”) and task version (“task match”). Three versions of the task were administered, which varied in terms of the rate of stimulus presentation: “slow”=interstimulus intervals ranged from 350 to 850 msec; “medium”=interstimulus intervals=300–550 msec; “fast”=interstimulus intervals=200–450 msec. The fast version was identical to that used in our previous investigation of attention activation in the normal cerebellum (5). To control for the effects of visual sensory stimulation, this task was alternated with a passive visual stimulation condition, during which subjects observed the same stimuli but did not selectively attend or respond. The word “SQUARE” or “RED” at the onset of the task cued subjects as to how to attend and respond, and “STOP” cued them to observe the stimuli passively.
Motor task
To examine activation due to the motor response alone, each subject was administered a simple motor task that involved pressing the button repeatedly at a comfortable pace. “GO” cued subjects to begin pressing the button; “STOP” cued them to rest. All subjects were fully cooperative when performing this task, and no extraneous movements were observed.
Sensory task
Subjects were also administered a sensory task in order to examine possible cerebellar activation induced by visual stimulation in the absence of attentional demands. Here, subjects alternated between blocks of visual fixation on a white asterisk and blocks of passive viewing of the attention task stimuli.
MR imaging
Images were acquired on a GE Signa 1.5-T system with a local head gradient coil and an asymmetrical circular endcapped radiofrequency coil (Medical Advances, Milwaukee) designed to provide extended coverage of the posterior-inferior portions of the brain. In order to obtain functional data from comparable coronal slice locations in all subjects, we first acquired localizer images in the axial and sagittal planes; axial images confirmed limited head rotation, while sagittal images were used to designate coronal slice locations (Figure 1).
After shimming to reduce inhomogeneities in the magnetic field, echo-planar images were acquired with a single-shot gradient-recalled echo-planar pulse sequence (interleaved slice acquisition; repetition time [TR]=2500 msec; echo time [TE]=40 msec; flip angle=90°; matrix=64×64; field of view=24 cm; slice thickness=5 mm; slice gap=1 mm). While subjects alternated between the experimental and control conditions, a time series of 130 echo-planar images was acquired at five coronal slice locations through the cerebellum. Subsequent to functional imaging, 20 echo-planar phase map images per slice were also acquired. Finally, high-resolution images (three-dimensional magnetization prepared rapid gradient echo [MPRAGE] pulse sequence: TR=30 msec; TE=5 msec; flip angle=45°; matrix=256×192×128; field of view=24 cm; slice thickness=1.5–1.7 mm) were acquired during the same scan session for each subject.
Data Analysis
Measurement of cerebellar volume
To investigate possible group differences in cerebellar anatomy, we estimated the full cortical volume of the cerebellum for each subject. Cerebellar gray matter (excluding all white matter and CSF) was traced manually on every other MPRAGE image passing through the cerebellum. The total number of voxels within all tracings was then multiplied by twice the voxel volume to arrive at an estimate of total cerebellar gray matter volume.
Identification of anatomical regions of interest
Before functional analysis, regions of interest were manually traced on the MPRAGE image corresponding to each echo-planar slice location. These regions of interest, all located in the cerebellar cortex (i.e., tracing excluded white matter and CSF), were hemisphere lobule VI ipsilateral to the moving hand, contralateral lobule VI, and right and left superior hemisphere lobule VIIa (superior semilunar lobule) (Figure 1). These regions of interest were chosen because they are consistently active in normal subjects during attention tasks (5, 7, 10–13). Region of interest identification was guided by MRI atlases of the human cerebellum (42–44).
Identification of functional activity
To correct for image distortion due to magnetic field inhomogeneities, an unwarping algorithm (45) employing the echo-planar phase map images was applied to each dataset. Next, to correct for subject motion, a three-dimensional volume registration algorithm (46) was applied. Two indices of functional activity, the correlation coefficient and percent signal change, were then calculated by using AFNI (47). Before these calculations, the first two repetitions of each slice, which were acquired before magnetization reached equilibrium, were eliminated, and the global drift of the time course MR signal was orthogonally projected out of the data. The data were then correlated (48) with a set of nine hemodynamic model response functions, each one a boxcar wave with sloped sides approximating the delay between task onset and maximum signal change. These different functions allowed for subtle subject- and voxel-wise variations in the hemodynamic delay and for differences in the time of acquisition of each slice. Functions varied according to the delay in the onset of the hemodynamic response (0, 2.5, or 5 seconds) and the delay between this onset and maximum signal change (5, 7.5, or 10 seconds). The output of this analysis was the result of a voxel-by-voxel best fit with these models. Significantly activated voxels were those that exceeded a threshold r value equivalent to one-tailed p<0.05 with Bonferroni correction for multiple comparisons, the number of comparisons being equivalent to the total number of voxels within all regions of interest across all five slices (i.e., approximately 500 voxels). The resulting threshold was an r value of 0.33 (p<0.00001). In addition to this analysis, the percent change in MR signal between control and experimental blocks was calculated at all voxels. This measure was not dependent on any statistical thresholding procedure. However, only those voxels with a nonnegative percent signal change were included in this analysis.
Region of interest analysis
First, the anatomical area of each region of interest was measured in each of the five slices in which it appeared. Then, the activation area (i.e., the total area of all significantly active voxels) within each region of interest was calculated. These values were collapsed across slices to create an anatomical “volume” and an activation “volume,” and the ratio between these volumes was calculated to determine the percent volume active, a measure of “activation extent.” The mean percent signal change within each region of interest, a measure of “activation magnitude,” was also calculated. Because this study included both right- and left-handed subjects, the lobule VI regions of interest were analyzed according to whether they were ipsilateral or contralateral to the moving hand.
Results
Cerebellar Volume and Region of Interest Anatomy
Mean cerebellar gray matter volume was 106.1 cm3 (SD=22.4) for the autistic subjects and 109.9 cm3 (SD=12.9) for the healthy comparison subjects. Thus, whole cerebellar volume was slightly (approximately 4%) smaller overall in the autistic subjects. This difference was not statistically significant (z=–0.42, p=0.34, one-tailed Wilcoxon rank sum test), but it was similar in direction and magnitude to the statistically significant difference in cerebellar volume recently found in much larger samples of autistic and normal subjects (49). For seven out of nine regions of interest, anatomic volumes were also smaller in the autistic group. The degree of this difference ranged from 2% for left superior hemisphere lobule VIIa to 19% for the anterior hemisphere ipsilateral to the moving hand. This latter difference approached statistical significance (z=–1.54, p=0.06, one-tailed Wilcoxon rank sum test). In all other regions of interest, group differences were nonsignificant.
Behavioral Data
For the attention task, performance accuracy (i.e., percent correct target detections) did not differ between groups (autism mean=90.81, SD=6.88; healthy mean=91.98, SD=7.82) (z=–0.68, p=0.50, two-tailed Wilcoxon rank sum test). When subjects were matched by attention task version, autistic subjects were significantly less accurate than comparison subjects (mean=81.31 [SD=12.16] versus mean=91.98 [SD=7.82], respectively) (z=–2.03, p<0.05, two-tailed Wilcoxon rank sum test). As described earlier, the mean number of button presses across the four motor task blocks was recorded as an index of button press frequency, which did not differ significantly between groups (autism patients: mean=75.3, SD=18.8; healthy subjects: mean=58.9, SD=23.5) (z=–1.68, p=0.09, two-tailed Wilcoxon rank sum test). No subject pressed the button during the rest condition, and no other extraneous movements were observed.
Cerebellar Activation
Figure 2 provides a summary of the activation results from the three tasks at a single slice location. As in our previous investigation (5), healthy comparison subjects showed bilateral superior posterior cerebellar hemisphere activation during the attention task. A strong positive correlation between the size of cerebellar hemisphere lobule VIIa and activation extent in that same region was also observed in healthy and autistic subjects (Figure 3). Moreover, even further support for the role of this region in attention came from the strong positive correlation seen in autistic subjects between the anatomic size of this region and accuracy during the “fast” version of the attention task.
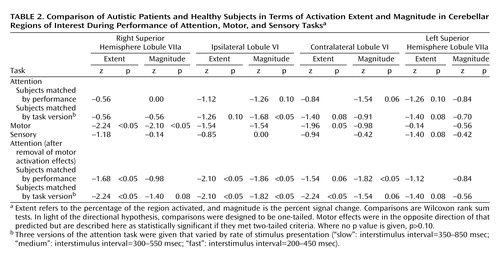
To address the question of normal differences in cerebellar cognitive and sensory activation, measures of attention and visual sensory activation were compared in the healthy subjects. Activation extent was significantly greater during the attention task in ipsilateral lobule VI (z=–2.1, p<0.05), contralateral lobule VI (z=–1.82, p<0.05), right superior hemisphere lobule VIIa (z=–1.69, p<0.05), and left superior hemisphere lobule VIIa (z=–1.82, p<0.05) (one-tailed Wilcoxon rank sum tests), while activation magnitude was significantly greater in ipsilateral lobule VI (z=–2.1, p<0.05) and left superior hemisphere lobule VIIa (z=–1.68, p<0.05) (one-tailed Wilcoxon rank sum tests).
Unlike their healthy counterparts, autistic patients showed minimal activation during the attention task (Figure 2), and this was reflected in the quantitative region of interest effects. Figure 4 shows that whether subjects were matched for performance or task version, activation in almost all regions of interest was greater in the healthy comparison subjects. Table 2 provides a summary of the group comparisons at all regions of interest for all tasks. For the performance match, the group differences in activation extent were not statistically significant, although this difference approached significance in the left superior hemisphere lobule VIIa. In terms of activation magnitude, the group difference approached significance in ipsilateral lobule VI and contralateral lobule VI. For the task match, activation extent was again not significantly reduced, although the difference approached significance in ipsilateral lobule VI, contralateral lobule VI, and the left superior hemisphere lobule VIIa. For activation magnitude, the group difference was statistically significant in lobule VI ipsilateral to the moving hand.
Group effects for the motor task were in the opposite direction from what was predicted and what was seen during the attention task. That is, autistic subjects showed a pattern of activation that was more diffuse than that seen in the healthy subjects (Figure 2 and Figure 5). This group difference in activation extent met criteria for statistical significance in the right superior hemisphere lobule VIIa and approached significance in contralateral lobule VI. Motor activation magnitude in the cerebella of the autistic patients was also significantly greater than that seen in healthy subjects in the right superior hemisphere lobule VIIa (Table 2). During the sensory task, trace amounts of cerebellar activation extent and magnitude were seen in both groups (Figure 2 and Figure 5).
Overall, the functional maps (Figure 2) suggested that cerebellar attention activation was markedly reduced in autistic patients, while region of interest analyses depicted less dramatic differences that did not always meet criteria for statistical significance. This may have been due in part to limitations in statistical power and the presence of outliers. To explore this possibility, data were also analyzed with more powerful parametric tests (t tests) following the removal of outliers. Outliers (Table 1) were identified by using the extreme Studentized deviate method (51), which adjusts the critical z score for defining outliers according to sample size; for the present data, this was a z of 2.13. When analyzed in this manner, performance-matched activation extent and magnitude during the attention task were significantly reduced in autistic subjects in contralateral lobule VI, with the differences approaching significance in ipsilateral lobule VI and left superior hemisphere lobule VIIa. When matched by task version, activation was significantly reduced in left superior hemisphere lobule VIIa, with contralateral lobule VI and right superior hemisphere lobule VIIa approaching significance.
Group differences in attention activation may have also appeared less robust than expected because relative decreases in attention activation in the autistic subjects were obscured by simultaneous increases in motor activation. To address this possibility, each subject’s motor activation value was subtracted from his or her attention activation value in each region of interest. Figure 6 and Table 21 show the results of the attention-minus-motor subtractions in the four regions of interest. When subjects were matched by performance, activation extent was significantly lower in the autistic subjects than in the healthy subjects in ipsilateral lobule VI and right superior hemisphere lobule VIIa, with the difference in contralateral lobule VI approaching significance. Activation magnitude was significantly lower in ipsilateral lobule VI and contralateral lobule VI. When subjects were matched by task version, activation extent in the autistic cerebellum was significantly reduced in ipsilateral lobule VI, contralateral lobule VI, and right superior hemisphere lobule VIIa, and this difference approached significance in left superior hemisphere lobule VIIa. Activation magnitude was significantly lower in ipsilateral lobule VI, with the difference in contralateral lobule VI and right superior hemisphere lobule VIIa approaching significance. Figure 7 shows the effects of subtracting motor from attention activation on the functional maps. For both the performance and task matches, healthy subjects continued to show activation in the anterior cerebellum and bilaterally in superior posterior cerebellar hemispheres, whereas autistic subjects showed virtually no cerebellar activation.
Discussion
Although the cerebellum’s fundamental function remains a mystery, the functional domains that the cerebellum serves are remarkably diverse (for review, see reference 52). We previously demonstrated that the cerebellum is active during attention operations independent of motor involvement (5). The present results replicate this finding, again demonstrating superior posterior cerebellar hemisphere involvement in nonspatial visual selective attention. The new finding that the size of cerebellar hemisphere lobule VIIa is strongly correlated with the amount of attention activation in that same region provides even further support for cerebellar involvement in attention operations. In contrast, minimal cerebellar activation was observed when subjects simply viewed visual stimuli but did not selectively attend or respond.
Attention-related cerebellar activation was lower in autistic patients than in healthy subjects whether or not groups were matched for performance, shedding new light on the cerebellar role in attention deficits in autism. Attentional impairments are among the most common forms of cognitive deficit in autism (53). Thus, their basis is of great importance to clinicians, teachers, and parents of autistic individuals. Neurobehavioral studies conducted over the course of the past decade have shown that deficits in specific attention operations are associated with abnormalities of cerebellar anatomy. For instance, autistic individuals have difficulty rapidly reallocating attentional resources to new sensory modalities (3, 54) and to new spatial locations (9, 35, 36, 55, 56). Such findings are more prominent in autistic patients with greater abnormality of the cerebellum (i.e., hypoplasia), and they are also seen in patients with acquired neocerebellar lesions (9, 54, 57). In addition, Lee and colleagues (33) have recently described abnormal nicotinic receptor composition in the cerebella of autistic individuals, which, as pointed out by those authors, is of great interest given the role of the nicotinic receptor in attention (58). Moreover, in the present study, decreased size of the right cerebellar hemisphere lobule VIIa in autistic subjects was associated with decreased accuracy in the attention task (Figure 3). However, the question of whether the autistic cerebellum is functioning abnormally during attention tasks has remained unanswered, until now.
The present study showed that cerebellar functioning is, in fact, abnormally reduced during a selective attention task, even when groups were matched for performance. Such abnormal activation in the context of normal performance might suggest that the autistic brain has developed compensatory mechanisms for performing the attention task. However, an alternative interpretation is that a fundamental cerebellar function is not required to perform this task and thus is not “missed” by the dysfunctional autistic cerebellum. We have previously proposed that the fundamental function of the cerebellum is to learn predictive relationships among sequences of events so that whenever an analogous sequence begins to unfold in real time, the cerebellum can generate predictions about upcoming events and prepare whichever neural systems are expected to be needed to respond appropriately to such information (3, 5, 14, 52, 59). Without this function, other systems continue to function but will do so suboptimally in situations where cerebellar prediction and preparation might otherwise aid performance.
In the attention task, the sequence of sensory events is randomly ordered and thus cannot be learned by the cerebellum. As such, the normal cerebellum might be active in attempting to learn and predict the sequence, but its activity cannot provide much useful preparatory output to neural systems involved in detecting and responding to upcoming target stimuli. Thus, although active, the normal cerebellum is not effectively aiding the rest of the central nervous system and therefore does not have a noticeable advantage over the autistic cerebellum in this context. In fact, autistic and neocerebellar lesion patients are typically not impaired on attention tasks similar to the one employed here (3). Why, then, were autistic subjects impaired on certain versions of the attention task in this study? In the fastest version of the attention task, interstimulus intervals ranged from 200 to 450 msec, but in previous studies that used a similar task, interstimulus intervals ranged from 450 to 1450 msec. Perhaps at this new high rate stimuli outpaced the basic sensory tracking abilities of the dysfunctional autistic cerebellum. That is, although the loss of preparatory output may not impede performance in this context, deficient sensory tracking (60) might do so when stimuli appear at such a rapid rate. Of course, it is also possible that supratentorial dysfunction played a role in this impaired performance, and as can be seen in the functional maps (Figure 2 and Figure 7), activation in cerebral cortex was also reduced in the autistic subjects.
The simplest and most straightforward interpretation regarding changes in cerebellar activation in autism is that a decreased volume of viable tissue due to a reduction in Purkinje cells will lead to abnormally reduced functional activation. While this might seem sufficient to explain the attention findings, it is clearly not sufficient when one considers the unexpected activation increases seen during a simple motor task. Although the autistic individuals pressed the button at a slightly faster rate than their healthy counterparts, this did not account for the large difference in activation, and button press frequency was in fact not correlated with greater activation in these subjects. One possible alternative explanation for the divergent findings is derived from the “crowding” concept of neuroplasticity (61). This concept was born out of the fact that patients with early lesions of the left cerebral hemisphere can show preserved language at the cost of other functions typically subserved by the right hemisphere. The notion is that intact tissue in the right hemisphere “takes over” the language functions but other functions are “crowded out” in the process.
One view regarding crowding is that more primitive functions, both phylogenetically and ontogenetically, are more likely to be spared and thus more likely to crowd out later developing ones (62). In the cerebellum, basic motor functions tend to be subserved by phylogenetically older regions (e.g., the paleocerebellum), while more advanced cognitive operations tend to involve newer regions (i.e., the neocerebellum) that evolved in parallel with parts of the cerebral cortex involved in those same functions (63). These more recently evolved regions of the cerebellum include superior posterior hemisphere regions that are active during attention tasks. We suggest that an early loss of Purkinje neurons might cause more primitive functions normally subserved by paleocerebellar regions to be displaced into the neocerebellum at the cost of tissue that subserves cognitive functions such as attention and language. Functional activation during simple motor tasks is normally most prominent in the paleocerebellum ipsilateral to movement (5, 64, 65). However, in the autistic cerebellum, such activation spreads to include contralateral and posterior regions not normally associated with simple motor tasks, including neocerebellar areas normally involved in attention. This might reflect such motor functions being taken over by newer regions of the cerebellum due to loss of Purkinje cells in the older ones. Reduced attention activation in these regions thus may reflect dual circumstances of Purkinje cell reduction (i.e., impaired function due to abnormal circuitry and crowding out of what remains by more primitive functions). In this context, it is interesting to recall that the region of interest with the greatest reduction in anatomical size was in fact the anterior hemisphere ipsilateral to the moving hand, the paleocerebellar region typically involved in simple motor tasks.
The present study is but a first look at the relationship between developmental anatomic defect and patterns of fMRI cognitive activation in the human cerebellum. Much more needs to be done to better understand this relationship. Cerebellar anatomic abnormality, whether developmental or acquired, often does not occur in isolation. Therefore, the interaction between multiple loci of abnormality, and how this relates to functional impairment, is an important area of investigation. In the present study, functional maps indicated that autistic subjects have additional reductions in activation of cerebral cortex, particularly in the parietal lobes (manuscript in progress). While we cannot rule out the possible influence of such changes on cerebellar activation through rich cerebro-ponto-cerebellar projections (66), one must also consider the potential influence of cerebellar functional changes on reductions in cerebral activation via reciprocal cerebello-thalamo-cortical projections. It is to be hoped that these and other questions will be addressed by future studies of autism and other disorders, ultimately leading to a broader understanding of the functional implications of cerebellar anatomic defect.
 |
 |
Presented in part at the 29th annual meeting of the International Neuropsychological Society, Chicago, Feb. 14–17, 2001. Received June 18, 2001; revision received May 13, 2002; accepted Aug. 29, 2002. From the Laboratory for Research on the Neuroscience of Autism, San Diego Children’s Hospital Research Center; and the Department of Neurosciences, School of Medicine, University of California, San Diego. Address reprint requests to Dr. Courchesne, Research on the Neuroscience of Autism, Children’s Hospital Research Center, 8110 La Jolla Shores Dr., Rm. 200, La Jolla, CA 92037; [email protected] (e-mail). Supported by NIMH grant MH-36840 (Dr. Courchesne) and by the McDonnell-Pew Center for Cognitive Neuroscience (Dr. Allen). The authors thank Josiah B. Ambrose for technical assistance.
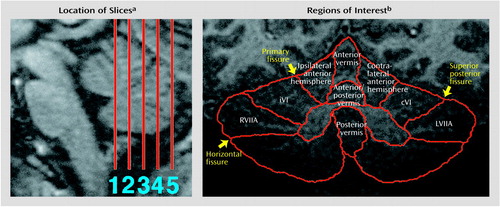
Figure 1. Slice Locations and Regions of Interest Used for the Analysis of Functional Magnetic Resonance Imaging (fMRI) Data From Eight Autistic Patients and Eight Healthy Comparison Subjects Given Attention and Motor Tasks to Assess Cerebellar Function
aSlices are shown on a midsagittal MR image of the brainstem and cerebellum from a single subject. Slice 1 was immediately posterior to the apex of the fourth ventricle, constraining slice 5 to a position at or near the caudal limit of the vermis.
bRegions of interest are shown on a coronal MR image of the cerebellum from a single subject. Vermis and anterior hemisphere regions were traced only for group comparisons of regional cerebellar anatomy (RVIIA=right superior hemisphere lobule VIIa; iVI=ipsilateral lobule VI; cVI=contralateral lobule VI; LVIIA=left superior hemisphere lobule VIIa).
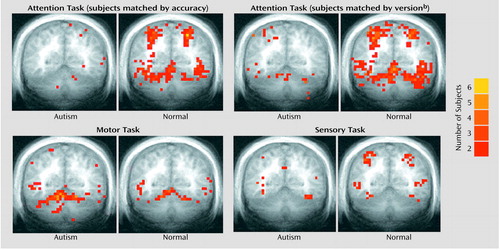
Figure 2. Sites of Cerebellar Activation During Performance of Attention, Motor, and Sensory Tasks in Seven Autistic Patients and Seven Healthy Comparison Subjectsa
aFor one autistic subject, a full cerebellar volume was not available. Thus, this subject’s brain could not be normalized, and he and his matched comparison subject were thus excluded from the functional maps. To create maps, each brain was spatially normalized according to the system of Talairach and Tournoux (50). All active voxels were then superimposed, thus displaying common sites of activation within the two groups. The color scale represents the number of subjects overlapping at any single voxel. Activation is overlaid on an averaged coronal anatomical image (Talairach y coordinate=–55). Ipsilateral (to the moving hand) cerebellum is to the reader’s left.
bThree versions of the attention task were given that varied by rate of stimulus presentation (“slow”: interstimulus interval=350–850 msec; “medium”: interstimulus interval=300–550 msec; “fast”: interstimulus interval=200–450 msec).
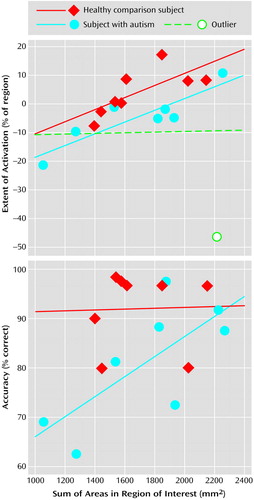
Figure 3. Correlation of the Area of Right Superior Hemisphere Lobule VIIa With Activationa and With Accuracyb During Performance of an Attention Task in Eight Autistic Patients and Eight Healthy Comparison Subjects
aPercentage of the right superior hemisphere lobule VIIa active during the attention task (subjects matched by performance) plotted against summed anatomical areas in that same region of interest for healthy subjects (r=0.72; t=2.54, df=6, p<0.05), autistic subjects (r=0.03; t=0.07, df=6, p>0.10), and autistic subjects minus one outlier (r=0.88; t=4.54, df=5, p<0.05).
bPerformance during the “fast” version of the attention task (interstimulus interval=200–450 msec) plotted against summed anatomical areas in right superior hemisphere lobule VIIa for autistic (r=0.72; t=2.54, df=6, p<0.05) and healthy (r=0.03; t=0.07, df=6, p>0.10) subjects. Motor activation effects were subtracted before plotting data.
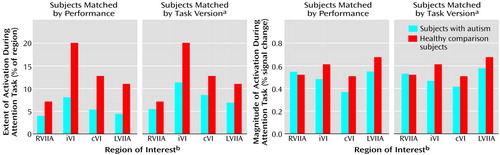
Figure 4. Extent and Magnitude of Activation in Cerebellar Regions of Interest in Eight Autistic and Eight Healthy Comparison Subjects During Performance of an Attention Task
aThree versions of the attention task were given that varied by rate of stimulus presentation (“slow”: interstimulus interval=350–850 msec; “medium”: interstimulus interval=300–550 msec; “fast”: interstimulus interval=200–450 msec).
bRVIIA=right superior hemisphere lobule VIIa; iVI=ipsilateral lobule VI; cVI=contralateral lobule VI; LVIIA=left superior hemisphere lobule VIIa.
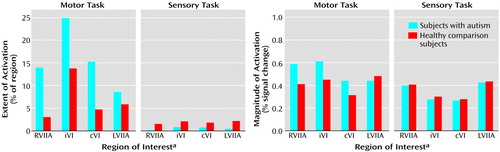
Figure 5. Extent and Magnitude of Activation in Cerebellar Regions of Interest in Eight Autistic Patients and Eight Healthy Comparison Subjects During Performance of Motor and Sensory Tasks
aRVIIA=right superior hemisphere lobule VIIa; iVI=ipsilateral lobule VI; cVI=contralateral lobule VI; LVIIA=left superior hemisphere lobule VIIa.
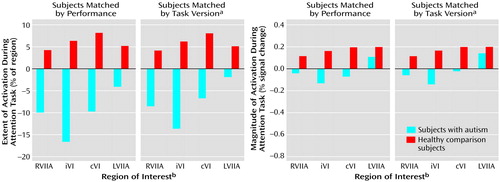
Figure 6. Extent and Magnitude of Activation in Cerebellar Regions of Interest in Eight Autistic and Eight Healthy Comparison Subjects During Performance of an Attention Task After Removal of Motor Activation Effects
aThree versions of the attention task were given that varied by rate of stimulus presentation (“slow”: interstimulus interval=350–850 msec; “medium”: interstimulus interval=300–550 msec; “fast”: interstimulus interval=200–450 msec).
bRVIIA=right superior hemisphere lobule VIIa; iVI=ipsilateral lobule VI; cVI=contralateral lobule VI; LVIIA=left superior hemisphere lobule VIIa.
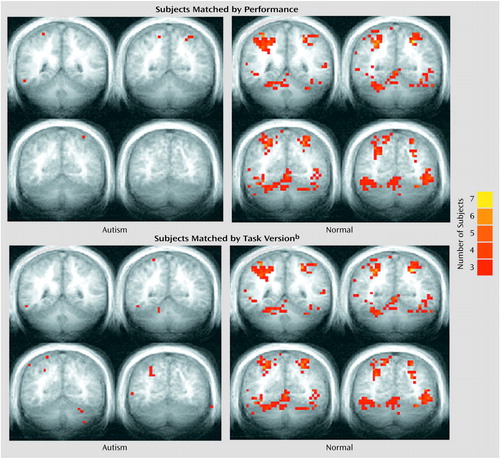
Figure 7. Sites of Cerebellar Activation During Performance of an Attention Task After Removal of Motor Activation Effects in Seven Autistic Patients and Seven Healthy Comparison Subjectsa
aFor one autistic subject, a full cerebellar volume was not available, and this subject and his matched comparison subject were thus excluded from the functional maps. Colors represent the number of subjects overlapping at any single voxel. Anterior-posterior Talairach y coordinates are –45, –51, –57, and –63.
bThree versions of the attention task were given that varied by rate of stimulus presentation (“slow”: interstimulus interval=350–850 msec; “medium”: interstimulus interval=300–550 msec; “fast”: interstimulus interval=200–450 msec).
1. Ivry RB, Keele SW: Timing functions of the cerebellum. J Cogn Neurosci 1989; 1:136-152Crossref, Medline, Google Scholar
2. Fiez JA, Petersen SE, Cheney MK, Raichle ME: Impaired non-motor learning and error detection associated with cerebellar damage. Brain 1992; 115:155-178Crossref, Medline, Google Scholar
3. Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, James HE, Haas RH, Schreibman L, Lau L: Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci 1994; 108:848-865Crossref, Medline, Google Scholar
4. Gao J-H, Parsons LM, Bower JM, Xiong J, Li J, Fox PT: Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 1996; 272:545-547Crossref, Medline, Google Scholar
5. Allen G, Buxton RB, Wong EC, Courchesne E: Attentional activation of the cerebellum independent of motor involvement. Science 1997; 275:1940-1943Crossref, Medline, Google Scholar
6. Paradiso S, Andreasen NC, O’Leary DS, Arndt S, Robinson RG: Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol 1997; 10:1-8Medline, Google Scholar
7. Le HT, Pardo JV, Hu X: 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 1998; 79:1535-1548Crossref, Medline, Google Scholar
8. Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. Brain 1998; 121:561-579Crossref, Medline, Google Scholar
9. Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA: Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci 1999; 19:5632-5643Crossref, Medline, Google Scholar
10. Rees G, Frackowiak R, Frith C: Two modulatory effects of attention that mediate object categorization in human cortex. Science 1997; 275:835-838Crossref, Medline, Google Scholar
11. Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL: A common network of functional areas for attention and eye movements. Neuron 1998; 21:761-773Crossref, Medline, Google Scholar
12. Coull JT, Nobre AC: Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 1998; 18:7426-7435Crossref, Medline, Google Scholar
13. Casey BJ, Thomas KT, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA: Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA 2000; 97:8728-8733Crossref, Medline, Google Scholar
14. Allen G, Courchesne E: The cerebellum and non-motor function: clinical implications. Mol Psychiatry 1998; 3:207-210Crossref, Medline, Google Scholar
15. Courchesne E: Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 1997; 7:269-278Crossref, Medline, Google Scholar
16. Kemper TL, Bauman M: Neuropathology of infantile autism. J Neuropathol Exp Neurol 1998; 57:645-652Crossref, Medline, Google Scholar
17. Courchesne E, Pierce K: Autism, in Encyclopedia of the Human Brain vol 1. Edited by Ramachandran VS. San Diego, Calif, Academic Press, 2002, pp 321-342Google Scholar
18. Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL: Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1996; 53:607-616Crossref, Medline, Google Scholar
19. Soares JC, Mann JJ: The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86-106Crossref, Medline, Google Scholar
20. Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, O’Sullivan RL, Shera DM, Rauch SL, Keuthen N, Rosen BR, Caviness VS, Filipek PA: Cerebral structural abnormalities in obsessive-compulsive disorder: a quantitative morphometric magnetic resonance imaging study. Arch Gen Psychiatry 1998; 53:625-632Crossref, Google Scholar
21. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985-9990Crossref, Medline, Google Scholar
22. Katsetos CD, Hyde TM, Herman MM: Neuropathology of the cerebellum in schizophrenia—an update: 1996 and future directions. Biol Psychiatry 1997; 42:213-224Crossref, Medline, Google Scholar
23. Williams RS, Hauser SI, Purpura D, DeLong R, Swisher CN: Autism and mental retardation. Arch Neurol 1980; 37:749-753Crossref, Medline, Google Scholar
24. Bauman ML, Kemper TL: Developmental cerebellar abnormalities: a consistent finding in early infantile autism. Neurology 1986; 36(suppl 1):190Google Scholar
25. Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, Ritvo A: Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry 1986; 143:862-866Link, Google Scholar
26. Arin D, Bauman ML, Kemper TL: The distribution of Purkinje cell loss in the cerebellum in autism. Neurology 1991; 41(suppl 1):307Google Scholar
27. Fehlow P, Bernstein K, Tennstedt A, Walther F: Autismus infantum und exzessive aerophagie mit symptomatischem magakolon und ileus bei einem fall von ehlers-danlos-syndrom (Infantile autism and excessive aerophagy with symptomatic megacolon and ileus in a case of Ehlers-Danlos syndrome). Padiatr Grenzg 1993; 31:259-267Medline, Google Scholar
28. Bauman ML, Kemper TL: Neuroanatomic observations of the brain in autism, in The Neurobiology of Autism. Edited by Bauman ML, Kemper TL. Baltimore, MD, Johns Hopkins University Press, 1994, pp 119-145Google Scholar
29. Guerin P, Lyon G, Barthelemy C, Sostak E, Chevrollier V, Garreau B, Lelord G: Neuropathological study of a case of autistic syndrome with severe mental retardation. Dev Med Child Neurol 1996; 38:203-211Crossref, Medline, Google Scholar
30. Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P: A clinicopathological study of autism. Brain 1998; 121:889-905Crossref, Medline, Google Scholar
31. Fatemi SH, Halt AR, Stary JM, Realmuto GM, Jalali-Mousavi M: Reduction in anti-apoptotic protein Bcl-2 in autistic cerebellum. Neuroreport 2001; 12:929-933Crossref, Medline, Google Scholar
32. Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J: Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 2001; 57:1618-1628Crossref, Medline, Google Scholar
33. Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E: Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 2002; 125:1483-1495Crossref, Medline, Google Scholar
34. Ito M: The Cerebellum and Neural Control. New York, Raven Press, 1984Google Scholar
35. Townsend J, Harris NH, Courchesne E: Visual attention abnormalities in autism: delayed orienting to location. J Int Neuropsychol Soc 1996; 2:541-550Crossref, Medline, Google Scholar
36. Harris NS, Courchesne E, Townsend J, Carper R, Lord C: Neuroanatomic contributions to slowed orienting of attention in children with autism. Brain Res Cogn Brain Res 1999; 8:61-71Crossref, Medline, Google Scholar
37. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659-685Crossref, Medline, Google Scholar
38. Lord C, Rutter M, Goode S, Heemsburgen J, Jordan H, Mawhood L, Schopler E: Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 1989; 19:185-212Crossref, Medline, Google Scholar
39. Wechsler D: Wechsler Intelligence Scale for Children—Revised. San Antonio, Tex, Psychological Corp, 1974Google Scholar
40. Wechsler D: Wechsler Adult Intelligence Scale—Revised. New York, Psychological Corp, 1981Google Scholar
41. Lincoln AJ, Courchesne E, Kilman BA, Elmasian R, Allen M: A study of intellectual abilities in high-functioning people with autism. J Autism Dev Disord 1988; 18:505-524Crossref, Medline, Google Scholar
42. Courchesne E, Press GA, Murakami J, Berthoty D, Grafe M, Wiley CA, Hesselink JR: The cerebellum in sagittal plane—anatomic-MR correlation, 1: the vermis. AJNR Am J Neuroradiol 1989; 10:659-665Google Scholar
43. Press GA, Murakami J, Courchesne E, Berthoty D, Grafe M, Wiley CA, Hesselink JR: The cerebellum in sagittal plane—anatomic-MR correlation, 2: the cerebellar hemispheres. AJNR Am J Neuroradiol 1989; 10:667-676Google Scholar
44. Press GA, Murakami J, Courchesne E, Grafe M, Hesselink JR: The cerebellum, 3: anatomic-MR correlation in the coronal plane. AJNR Am J Neuroradiol 1990; 11:41-50Medline, Google Scholar
45. Reber P: Correction of off resonance-related distortion in echo-planar imaging using EPI-based field maps. Magn Reson Med 1998; 39:328-330Crossref, Medline, Google Scholar
46. Cox RW, Jesmanowicz A: Real-time 3D image registration for functional MRI. Magn Reson Med 1999; 42:1014-1018Crossref, Medline, Google Scholar
47. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162-173Crossref, Medline, Google Scholar
48. Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS: Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 1993; 30:161-173Crossref, Medline, Google Scholar
49. Courchesne E, Karns C, Davis H, Ziccardi R, Carper R, Tigue Z, Chisum H, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas R, Akshoomoff N, Courchesne RY: Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57:245-254Crossref, Medline, Google Scholar
50. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
51. Iglewicz B, Hoaglin DC: How to Detect and Handle Outliers. Milwaukee, American Society for Quality, 1993Google Scholar
52. Courchesne E, Allen G: Prediction and preparation, fundamental functions of the cerebellum. Learn Mem 1997; 4:1-35Crossref, Medline, Google Scholar
53. Allen G, Courchesne E: Attention function and dysfunction in autism. Front Biosci 2001; 6:D105-D119Google Scholar
54. Akshoomoff NA, Courchesne E: A new role for the cerebellum in cognitive operations. Behav Neurosci 1992; 106:731-738Crossref, Medline, Google Scholar
55. Townsend J, Courchesne E, Egaas B: Slowed orienting of covert visual-spatial attention in autism: specific deficits associated with cerebellar and parietal abnormality. Dev Psychopathol 1996; 8:563-584Crossref, Google Scholar
56. Belmonte M: Abnormal attention in autism shown by steady-state visual evoked potentials. Autism 2000; 4:269-285Crossref, Google Scholar
57. Akshoomoff NA, Courchesne E: Intramodality shifting attention in children with damage to the cerebellum. J Cogn Neurosci 1994; 6:388-399Crossref, Medline, Google Scholar
58. Mirza NR, Stolerman IP: The role of nicotinic and muscarinic acetylcholine receptors in attention. Psychopharmacology (Berl) 2000; 148:243-250Crossref, Medline, Google Scholar
59. Akshoomoff NA, Courchesne E, Townsend J: Attention coordination and anticipatory control, in The Cerebellum and Cognition. Edited by Schmahmann JD. San Diego, Calif, Academic Press, 1997, pp 575-598Google Scholar
60. Paulin MG: The role of the cerebellum in motor control and perception. Brain Behav Evol 1993; 41:39-50Crossref, Medline, Google Scholar
61. Teuber HL: Why two brains? in The Neurosciences: Third Study Program. Edited by Schmitt FO, Worden FG. Cambridge, Mass, MIT Press, 1974, pp 71-74Google Scholar
62. Nass R, Stiles J: Neurobehavioral consequences of congenital focal lesions, in Pediatric Behavioral Neurology. Edited by Frank Y. Boca Raton, Fla, CRC Press, 1996, pp 149-178Google Scholar
63. Leiner HC, Leiner AL, Dow RS: The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res 1991; 44:113-128Crossref, Medline, Google Scholar
64. Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS: Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 1995; 73:373-386Crossref, Medline, Google Scholar
65. Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH: Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci 1997; 17:9675-9685Crossref, Medline, Google Scholar
66. Schmahmann JD: From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 1996; 4:174-198Crossref, Medline, Google Scholar



