Anomalous Somatosensory Cortical Localization in Schizophrenia
Abstract
OBJECTIVE: Schizophrenia has been reported to be associated with altered localization of regions responding to sound in the auditory cortex, suggesting possible cortical reorganization accompanying the disorder, but it is not clear whether such disturbances are present in any primary sensory cortex other than the auditory. This study examines magnetoencephalographic localization of field sources evoked by tactile stimulation generated in area 3b of the primary somatosensory cortex in patients with schizophrenia compared with normal subjects. METHOD: A total of 29 subjects participated; 14 were patients with schizophrenia, and 15 were normal comparison subjects. Equivalent current dipole generators in both hemispheres were used to model the sources of the 50-msec latency somatosensory evoked field (M50) components produced by contralateral tactile mechanoreceptor stimulation of the tip of the index finger. RESULTS: Compared with normal subjects, patients with schizophrenia demonstrated reversed asymmetry (left anterior to right) and relatively anterior and inferior displacement of the M50 equivalent current dipole location in the left hemisphere. CONCLUSIONS: Cerebral lateralization and localization of the M50 distinguished patients with schizophrenia from normal subjects. These findings suggest the possibility of anatomical displacement and/or disturbed organization of the primary sensory cortex in schizophrenia.
When individuals listen to auditory stimuli such as short tones, active regions of auditory cortex in the superior temporal gyri can be localized by magnetoencephalography (MEG). Such regions most often demonstrate interhemispheric asymmetry, being slightly farther anterior in the right than in the left hemisphere. Many patients with schizophrenia, however, fail to demonstrate such asymmetry, showing activated sources that are either symmetrical or exhibit reversed asymmetry (1–5). This anomalous asymmetry does not appear to reflect abnormal placement of Heschl’s gyri, which are normally lateralized (right anterior to left) in subjects with and without schizophrenia (6). Anomalous asymmetry has been reported for evoked responses thought to be generated by both the secondary (4) and the primary (7) auditory cortex. It has been suggested that such anomalous asymmetry may reflect auditory cortical disorganization or reorganization that may accompany schizophrenic disorders (6). In fact, independent evidence exists for this in terms of apparently altered tonotopic organization in the auditory cortex of patients with schizophrenia (8).
To our knowledge, however, possible disturbed asymmetry in other primary sensory cortical regions in schizophrenia has not been reported to date. In this study, we examine the relative locations associated with activation of the somatosensory cortex in the left and right hemispheres of normal subjects and patients with schizophrenia. For this purpose, we activated cutaneous mechanoreceptors in the pad of the index finger using a touch stimulus and recorded magnetic evoked fields generated in the contralateral primary somatosensory cortex in a group of patients with schizophrenia and in a similar group of normal subjects.
Method
Subject Selection
We studied 14 patients with schizophrenia (10 men) and 15 adult comparison subjects (seven men). In the schizophrenia group, 11 patients were Caucasian, two were Hispanic, and one had a mixed racial/ethnic heritage. In the comparison group, 14 subjects were Caucasian and one reported mixed racial/ethnic heritage. All of the patients with schizophrenia were in outpatient treatment, and all were medicated. Diagnosis was determined by using the Structured Clinical Interview for DSM-IV (SCID) (9) and a review of medical records. SCIDs were performed by two of us (M.R. and J.C.). Exclusionary criteria included any medical illness affecting CNS function, neurological disorder, history of head trauma, and current substance abuse. Comparison subjects were community volunteers recruited to participate in our neuroimaging studies of psychosis. Comparison subjects met the same exclusionary criteria, had no axis I disorder, and had no first-degree relative with an axis I diagnosis.
All but one of the subjects were right-handed as determined by the Annett Handedness Scale (10). After the procedures were fully explained, written informed consent was obtained from all subjects in accordance with the guidelines of the Colorado Multiple Institutional Review Board. Thirteen of the 14 patients with schizophrenia were taking medications for which chlorpromazine equivalency has been reported (11); their mean dose at the time of study was 517.31 mg/day (SD=437.33). Seven patients with schizophrenia were taking risperidone, three olanzapine, one haloperidol, two clozapine, one fluphenazine, two perphenazine, four benztropine, three lithium, one carbamazepine, and one clonazepam (some patients were taking more than one medication). Demographic information for the subjects is given in Table 1.
Stimuli
A pressure pulse was presented to the volar distal phalanx of the first digit by means of a 1-cm-diameter rubber bladder encased in a plastic housing lightly taped to the finger (somatosensory stimulator, 4-D Neuroimaging, San Diego, Calif.). The bladder was activated pneumatically through a 2-m length of 3-mm polyurethane tubing. The valves and their custom-designed control electronics, interfaced to a personal computer parallel port, were located outside the magnetically shielded room. Diaphragm extension, duration, and initial displacement time were measured by attaching a small steel fragment to the center of a test stimulator (13) and then measuring the magnetic signature of the device with the neuromagnetometer. The time of onset of bladder inflation was adjusted to correspond to time zero in the recording window. Duration of stimulation was set to 200 msec. Stimuli were repeated every 4 seconds, with a total of 200 trials recorded and averaged from each hemisphere. Recordings were made from the hemisphere contralateral to the hand stimulated.
MEG Recording
Magnetic field data were obtained with a 37-channel Magnes I biomagnetometer (4-D Neuroimaging); concentric rings of first-order axial gradiometers (coil diameter=2 cm, baseline=5 cm) were used. Contralateral recordings were made with subjects lying on a nonmagnetic bed. To maintain alertness during recordings, subjects were watching and listening to a self-selected videotaped movie on a monitor about 4 m distant. Data were collected by using a 16-bit analog-to-digital converter with a sampling rate of 1041.7 Hz over a 250-msec window with a 50-msec prestimulus period. Analog filters were set at 200 Hz low-pass and 1 Hz high-pass. The 4-D Neuroimaging Magnes SCP version 1.6 software package was used for all recordings. Fiducial points (left and right preauricular points and the nasion) were digitized by using the Polhemus 3SPACE (Colchester, Vt.) tracker.
Coil locations and orientations were then expressed in the coordinate system defined as follows: Y axis along the line between the preauricular points, positive to the left; X axis perpendicular to the Y axis at the midpoint and contained in the plane formed by the nasion and preauricular points; and Z axis perpendicular to the same plane, starting at the midpoint and positive in the upward direction. The instrument was typically positioned with the center channel near an X coordinate of 1 cm and a Z coordinate of 8 to 9 cm. During recording, field topography was inspected on the building average, and the component at about 50 msec after stimulus delivery was required to have a balanced distribution with roughly equal numbers of ingoing and outgoing field measurements. If this condition was not met initially, the magnetometer was repositioned and the recording repeated.
MEG Analysis
Following recording, the data files were converted to Scan (NeuroSoft, Inc., El Paso, Tex.) 4.0 data format. Before averaging, we visually edited epoch files trial by trial for eye-blink, head movement, and other artifacts. There was no between-group difference in the number of trials rejected (for comparison subjects, mean=24, and for patients with schizophrenia, mean=17) (F=0.76, df=1, 27, p>0.35). Averaged data were digitally (forward-backward Butterworth) low-pass filtered (24 dB/octave) at 50 Hz and baseline-corrected to the mean of the prestimulus window. Source localization analysis was performed across the poststimulus window from 30 to 80 msec by using a single moving equivalent current dipole in a conductive sphere model (14) and a sliding 5-msec-wide time window at 1-msec increments for each hemisphere. The mean B-field amplitude for each channel in the 5-msec window (starting 2.5 msec before and ending 2.5 msec after each time point) was computed by averaging the samples in this interval.
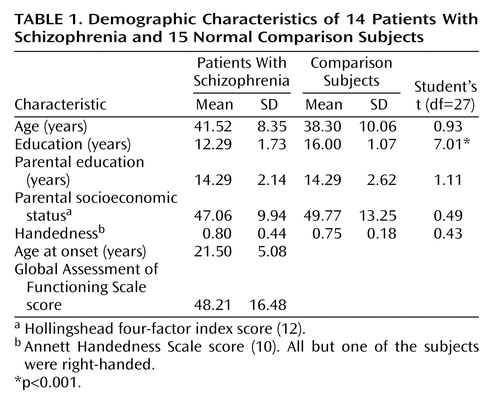
Custom software (Megeeg) was used to solve the inverse problem at each time point. Megeeg provides 95% confidence intervals and accounts for the fields associated with volume currents (15). The source location estimates chosen for farther statistical analysis were determined by using both dipole orientation and goodness of fit calculated by dividing the root mean square of the residual error by the root mean square of the data. Orientation was computed as the inverse tangent of the ratio of the source strength Z and X vectors, with the additional constraint that angles are positive as the dipole rotates rearward, regardless of hemisphere. Within the time window, the lowest fit (best goodness of fit) consistent with an orientation between 50° and 150° (posteriorly directed) was selected. Examples of the M50 evoked field component, associated field topography, and equivalent current dipole location on a sagittal magnetic resonance imaging slice are given in Figure 1.
Statistical Analysis
All statistical analyses were performed in Statistica 5.3 (Tulsa, Okla.). Significance tests were two-tailed and evaluated for significance at 0.05 alpha. For analysis of variance (ANOVA) designs, type III sums of squares were used. Differences between groups on demographic variables (age, education, and handedness) were examined with independent Student’s t tests.
The MEG source parameters (X, Y, Z, orientation, and source strength), goodness of fit of the single equivalent current dipole model, and source latency were then subjected to independent two-by-two, mixed-model ANOVA evaluations, with group as a between-subjects variable and hemisphere as a within-subjects variable.
Results
Source Parameters
For the X coordinate location, the group main effect was not significant (F=2.98, df=1, 27, p=0.10). The group-by-hemisphere interaction term was significant, however, indicating a difference between the groups that was more prominent in the left hemisphere than the right (F=4.60, df=1, 27, p<0.05). For the specific test of the source lateralization hypothesis in the anterior-posterior X coordinate, an asymmetry measure (right minus left X coordinate) was employed as the dependent variable in an independent Student’s t test. The asymmetry measure was significantly different between the normal and schizophrenia groups (t=2.14, df=27, p<0.05), suggesting differential lateralization of the sources between the groups. Schematic side and top-down views of the M50 source location estimate are given in Figure 2.
For the Y coordinate location, there was a potential but nonsignificant hemisphere main effect (F=2.99, df=1, 27, p=0.10), suggesting that the left hemisphere sources for both groups were more lateral in relation to the midline than the right hemisphere sources. The group-by-hemisphere interaction term was significant, however, indicating that the potential hemisphere difference in lateral source location was true only for the schizophrenia group (F=4.44, df=1, 27, p<0.05) (Figure 2).
For the Z coordinate location, only the group main effect term was significant (F=8.45, df=1, 27, p<0.008), suggesting that the source locations for the comparison subjects were located superior to those of the patients with schizophrenia. There were no significant effects found for the three remaining source measures (latency, orientation, and strength).
Discussion
These findings suggest not only anomalous (reversed) asymmetry of touch-activated sources in the primary somatosensory cortex in patients with schizophrenia but also a left-hemisphere relatively anterior and inferior displacement of such activity in these patients. Thus, explanations must be sought for both findings, and both anatomical and functional domains should be considered. The only previous MEG studies to our knowledge that described evidence of altered somatosensory cortical asymmetry in subjects with major mental illness used electrical stimulation of the median nerve at the wrist (16, 17). These reports described expected asymmetry (right anterior to left) of M20 sources in control subjects (16) but reversed asymmetry in subjects with schizoaffective disorder (17). A similar study (18) reported evidence of normal asymmetry in subjects with bipolar disorder who had not experienced psychosis in the context of their illness but also reported reversed asymmetry in subjects with bipolar disorder who had experienced psychosis in the past but were not psychotic at the time of recording. Electrical stimulation, which although extremely short in duration (<1 msec) and quite effective in eliciting a prominent contralateral M20 evoked response generated in area 3b of the postcentral gyrus (19, 20), is nonetheless an unnatural stimulus in which the specific nerve fibers being stimulated cannot be controlled and a large number of fibers from both superficial and deep receptors may be activated (21).
In this study, we chose instead to use a tactile stimulus that is more specific in both the nature of the peripheral receptors and in the somatosensory cortical region being activated. This tactile response appears to be generated in area 3b of the hand region on the postcentral gyrus in the somatotopically organized primary somatosensory cortex (22, 23) and most likely represents the activity of cutaneous mechanoreceptors of the single slowly adapting and rapidly adapting Meissner corpuscle mechanoreceptive afferent fibers (24). Several studies using a single equivalent current dipole have uniformly estimated that source activity of the M50 arises in S1 (25, 26). The intrinsic nature of the stimulus results in a less precise repeatability of stimulus onset (∼2 msec), and the 20-msec latency response is often not observed. Rather, the predominant evoked field response has a latency in the range of 46–101 msec (based on maximum amplitude) (27), with a source orientation opposite that of the N20 (28).
Our findings would be compatible with anatomical displacement of the S1 somatosensory cortex, which might be anticipated to be associated with anatomical displacement of the central sulcus as well as postcentral and precentral gyri. However, no evidence of that has yet been reported in schizophrenia. One might ask, for example, whether the relatively anterior displacement of somatosensory sources could be related to a relative decrease in frontal lobe volume, also reported in schizophrenia (29, 30). Again, we would expect evidence of gross neuroanatomically evident S1 displacement, which remains lacking. We note, however, that the normal human brain exhibits a counterclockwise “torque” in which right frontal regions tend to extend farther anterior and to the left (counterclockwise) and left posterior regions farther posterior and to the right.
A recent meta-analysis (31) reported that torque appears to be one of the measures of brain asymmetry significantly diminished in patients with schizophrenia, which Crow (32) suggested may be related to the gene protocadherin XY in the Xq21.3/Y region of homology in the X and Y chromosomes in humans. Whether brain torque may relate in some way to asymmetry in somatosensory (or, for that matter, auditory) laterality measures of sources of cortical evoked fields remains to be determined, but it would appear to be a testable hypothesis.
Sensory cortical reorganization accompanying schizophrenia cannot be excluded as a contributory explanation for our findings. Evidence of auditory cortical reorganization in schizophrenia has been obtained on the basis of MEG-based metrics (6). Somatosensory as well as primary motor cortical reorganization is known to occur in the face of altered sensory input or experience (33–35), and recent MEG findings (26) suggest that merely shifting attention to cutaneous tactile receptors may result in functional somatosensory cortical reorganization within a matter of minutes.
A final alternative explanation might examine the fact that cutaneous input is also directly addressed to the caudal portion of the primary motor cortex (M1) in the precentral gyrus (36). This raises the possibility that, for reasons yet unknown, such influences might be operative in an altered manner in schizophrenia, resulting in an apparent anterior displacement of M50 source estimates to motor regions. Patients with schizophrenia have recently been shown to demonstrate impaired control of smooth execution of certain motor functions involving the hand (37). This finding is compatible with impairments in cortico-cerebellar-thalamic circuits as discussed in theories related to cognitive dysmetria in schizophrenia (38). Whether altered (increased or dysregulated) somatosensory input to motor cortex might be involved in such conceptualizations remains to be determined.
All of our patients with schizophrenia were medicated, and the possible influences from medication effects cannot be determined definitely on the basis of our data. It is well-known that antipsychotic agents and other psychoactive agents can influence frequency and amplitude measures of background, evoked EEG (39–41), and evoked MEG (42–44) activity. Although amplitude and latency measures can be affected, especially for later components, there is at present no evidence that early primary cortical-generated somatosensory responses can be affected by medication in such a manner as we report. We found no difference in estimated source strength between groups, which might be expected to be the variable most sensitive to medication effects.
In summary, these findings demonstrate apparent alterations in estimates of tactile source location from the primary somatosensory cortex in patients with schizophrenia, raising the possibility of anatomical displacement and/or disturbed organization of the primary sensory cortex in this disorder.
 |
Received Feb. 6, 2003; revision received April 30, 2003; accepted May 7, 2003. From the Biomagnetic Imaging Laboratory, Department of Psychiatry, University of Colorado Health Sciences Center, Denver. Address reprint requests to Dr. Reite, Department of Psychiatry, Box C268-68, UCHSC, 4200 East 9th Ave., Denver, CO 80262; [email protected] (e-mail). Supported by NIMH grant MH-47476.
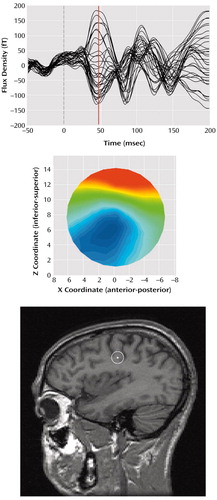
Figure 1. Representative Left Hemisphere Field Waveforms Evoked by Tactile Stimulation, Field Topography, and Current Estimated Location of the Equivalent Dipolea
aTop: time zero is the onset of diaphragm displacement. Center: field topography is shown at a latency of 47 msec (red line in top graph). Bottom: the equivalent current dipole estimated location (white marker) is superimposed on a sagittal magnetic resonance imaging slice for the same subject with a 95% confidence volume (white circle).
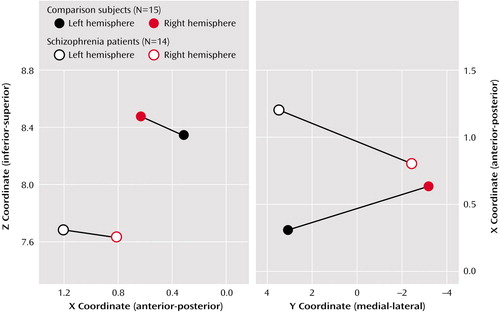
Figure 2. Estimated Locations of Equivalent Current Dipoles in the X-Z Plane and the X-Y Planea
aShown in the X-Z plane (side view) and the X-Y plane (top-down view, on the right).
1. Reite M, Teale P, Goldstein L, Whalen J, Linnville S: Late auditory sources may differ in the left hemisphere of schizophrenic patients: a preliminary report. Arch Gen Psychiatry 1989; 46:565–572Crossref, Medline, Google Scholar
2. Hajek M, Boehle C, Huonker R, Volz HP, Nowak H, Schrott PR, Sauer H: Abnormalities of auditory evoked magnetic fields in the right hemisphere of schizophrenic females. Schizophr Res 1997; 24:329–332Crossref, Medline, Google Scholar
3. Tiihonen J, Katila H, Pekkonen E, Jaaskelainen IP, Huotilainen M, Aronen HJ, Ilmoniemi R, Rasanen P, Virtanen J, Salli E, Karhu J: Reversal of cerebral asymmetry in schizophrenia measured with magnetoencephalography. Schizophr Res 1998; 30:209–219Crossref, Medline, Google Scholar
4. Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC: Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Arch Gen Psychiatry 1997; 54:433–440Crossref, Medline, Google Scholar
5. Rockstroh B, Kissler J, Mohr B, Eulitz C, Lommen U, Wienbruch C, Cohen R, Elbert T: Altered hemispheric asymmetry of auditory magnetic fields to tones and syllables in schizophrenia. Biol Psychiatry 2001; 49:694–703Crossref, Medline, Google Scholar
6. Rojas DC, Teale P, Sheeder J, Simon J, Reite M: Sex-specific expression of Heschl’s gyrus functional and structural abnormalities in paranoid schizophrenia. Am J Psychiatry 1997; 154:1655–1662Abstract, Google Scholar
7. Teale P, Carlson J, Rojas DC, Reite M: Reduced laterality of the source locations for generators of the auditory steady state field in schizophrenia. Biol Psychiatry (in press)Google Scholar
8. Rojas DC, Bawn SD, Carlson JP, Arciniegas DB, Teale PD, Reite ML: Alterations in tonotopy and auditory cerebral asymmetry in schizophrenia. Biol Psychiatry 2002; 52:32–39Crossref, Medline, Google Scholar
9. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
10. Annett M: Left, Right, Hand and Brain: The Right Shift Theory. Hillsdale, NJ, Lawrence Erlbaum Associates, 1985Google Scholar
11. Lehman AF, Steinwachs DM: Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull 1998; 24:1–10Crossref, Medline, Google Scholar
12. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
13. Mertens M, Lutkenhoner B: Efficient neuromagnetic determination of landmarks in the somatosensory cortex. Clin Neurophysiol 2000; 111:1478–1487Crossref, Medline, Google Scholar
14. Sarvas J: Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol 1987; 32:11–22Crossref, Medline, Google Scholar
15. Goldstein L, Teale P, Reite M: Monte Carlo study of confidence region accuracy for MEG dipole inverse solutions, in Advances in Biomagnetism. Edited by Williamson SJ, Hoke M, Stroink G, Kotani M. New York, Plenum, 1989, pp 555–558 Google Scholar
16. Wikstrom H, Roine RO, Salonen O, Aronen HJ, Virtanen J, Ilmoniemi R, Huttunen J: Somatosensory evoked magnetic fields to median nerve stimulation: interhemispheric differences in a normal population. Electroencephalogr Clin Neurophysiol 1997; 104:480–487Crossref, Medline, Google Scholar
17. Reite M, Teale P, Rojas DC, Sheeder J, Arciniegas D: Schizoaffective disorder: evidence for reversed cerebral asymmetry. Biol Psychiatry 1999; 46:133–136Crossref, Medline, Google Scholar
18. Reite M, Teale P, Rojas DC, Arciniegas D, Sheeder J: Bipolar disorder: anomalous brain asymmetry associated with psychosis. Am J Psychiatry 1999; 156:1159–1163Abstract, Google Scholar
19. Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S, Yoshimoto T: Neuromagnetic evidence of pre- and post-central cortical sources of somatosensory evoked responses. Electroencephalogr Clin Neurophysiol 1996; 100:44–50Crossref, Medline, Google Scholar
20. Kamada K, Oshiro O, Takeuchi F, Kuriki S, Houkin K, Iwasaki Y, Abe H: Identification of central sulcus by using somatosensory evoked magnetic fields and brain surface MR images: three dimensional projection analysis. J Neurol Sci 1993; 116:29–33Crossref, Medline, Google Scholar
21. Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R: Somatosensory homunculus as drawn by MEG. Neuroimage 1998; 7:377–386Crossref, Medline, Google Scholar
22. Kakigi R, Hoshiyama M, Shimojo M, Naka D, Yamasaki H, Watanabe S, Xiang J, Maeda K, Lam K, Itomi K, Nakamura A: The somatosensory evoked magnetic fields. Prog Neurobiol 2000; 61:495–523Crossref, Medline, Google Scholar
23. Hoechstetter K, Rupp A, Stancak A, Meinck HM, Stippich C, Berg P, Scherg M: Interaction of tactile input in the human primary and secondary somatosensory cortex—a magnetoencephalographic study. Neuroimage 2001; 14:759–767Crossref, Medline, Google Scholar
24. Srinivasan MA, LaMotte RH: Tactile discrimination of shape: responses of slowly and rapidly adapting mechanoreceptive afferents to a step indented into the monkey fingerpad. J Neurosci 1987; 7:1682–1697Medline, Google Scholar
25. Yang TT, Gallen CC, Schwartz B, Bloom FE: Noninvasive somatosensory homunculus mapping in humans by using a large-array biomagnetometer. Proc Natl Acad Sci USA 1993; 90:3098–3102Crossref, Medline, Google Scholar
26. Iguchi Y, Hoshi Y, Hashimoto I: Selective spatial attention induces short-term plasticity in human somatosensory cortex. Neuroreport 2001; 12:3133–3136Crossref, Medline, Google Scholar
27. Druschky K, Kaltenhauser M, Hummel C, Druschky A, Pauli E, Huk WJ, Stefan H, Neundorfer B: Somatotopic organization of the ventral and dorsal finger surface representations in human primary sensory cortex evaluated by magnetoencephalography. Neuroimage 2002; 15:182–189Crossref, Medline, Google Scholar
28. Biermann K, Schmitz F, Witte OW, Konczak J, Freund HJ, Schnitzler A: Interaction of finger representation in the human first somatosensory cortex: a neuromagnetic study. Neurosci Lett 1998; 251:13–16Crossref, Medline, Google Scholar
29. Selemon LD, Kleinman JE, Herman MM, Goldman-Rakic PS: Smaller frontal gray matter volume in postmortem schizophrenic brains. Am J Psychiatry 2002; 159:1983–1991Link, Google Scholar
30. Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ: Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 2002; 159:1497–1505Link, Google Scholar
31. Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A: Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry 2001; 178:344–351Crossref, Medline, Google Scholar
32. Crow TJ: Handedness, language lateralisation and anatomical asymmetry: relevance of protocadherin XY to hominid speciation and the aetiology of psychosis: point of view. Br J Psychiatry 2002; 181:295–297Crossref, Medline, Google Scholar
33. Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E: Increased cortical representation of the fingers of the left hand in string players. Science 1995; 270:305–307Crossref, Medline, Google Scholar
34. Blake DT, Byl NN, Merzenich MM: Representation of the hand in the cerebral cortex. Behav Brain Res 2002; 135:179–184Crossref, Medline, Google Scholar
35. Xerri C, Merzenich MM, Jenkins W, Santucci S: Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex 1999; 9:264–276Crossref, Medline, Google Scholar
36. Tanji J, Wise SP: Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 1981; 45:467–481Medline, Google Scholar
37. Delevoye-Turrell Y, Giersch A, Danion J-M: Abnormal sequencing of motor actions in patients with schizophrenia: evidence from grip force adjustments during object manipulation. Am J Psychiatry 2003; 160:134–141Link, Google Scholar
38. Andreasen NC: A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999; 56:781–787Crossref, Medline, Google Scholar
39. Roemer RA, Shagass C: Replication of an evoked potential study of lateralized hemispheric dysfunction in schizophrenics. Biol Psychiatry 1990; 28:275–291Crossref, Medline, Google Scholar
40. Czobor P, Volavka J: Quantitative electroencephalogram examination of effects of risperidone in schizophrenic patients. J Clin Psychopharmacol 1993; 13:332–342Crossref, Medline, Google Scholar
41. Centorrino F, Price BH, Tuttle M, Bahk W-M, Hennen J, Albert MJ, Baldessarini RJ: EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry 2002; 159:109–115Link, Google Scholar
42. Canive JM, Lewine JD, Edgar JC, Davis JT, Miller GA, Torres F, Tuason VB: Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacol Bull 1998; 34:101–105Medline, Google Scholar
43. Sperling W, Vieth J, Martus M, Demling J, Barocka A: Spontaneous slow and fast MEG activity in male schizophrenics treated with clozapine. Psychopharmacology (Berl) 1999; 142:375–382Crossref, Medline, Google Scholar
44. Rosburg T, Kreitschmann-Andermahr I, Ugur T, Nestmann H, Nowak H, Sauer H: Tonotopy of the auditory-evoked field component N100m in patients with schizophrenia. J Psychophysiol 2000; 14:131–141Crossref, Google Scholar



