Olanzapine Effects on Auditory Sensory Gating in Schizophrenia
Abstract
OBJECTIVE: New-generation antipsychotics have been proposed to normalize the P50 sensory gating index in patients with schizophrenia. In the context of a double-blind comparison of olanzapine and haloperidol, the authors examined the effect of olanzapine on P50 suppression. METHOD: P50 measurements were obtained at baseline while patients were being treated with fluphenazine and after 12 weeks of double-blind treatment with either olanzapine (N=12) or haloperidol (N=12). RESULTS: There were no treatment group differences in P50 amplitude, latency, or sensory gating ratio. CONCLUSIONS: These results suggest that there is not a significant differential effect of olanzapine and haloperidol on the P50 sensory gating index in schizophrenia.
Sensory gating abnormalities may index a genetic vulnerability for schizophrenia that reflects abnormal hippocampal function (1). Adler et al. have demonstrated that normal sensory gating depends, in part, on nicotinic cholinergic modulation of inhibitory interneurons in a CA3 hippocampal circuit (1). Consistent with this model, gating abnormalities in schizophrenia patients and their family members have been shown to improve after administration of nicotine (2, 3) or donepezil (4). Dopaminergic, GABAergic, noradrenergic, and serotonergic influences on sensory gating have also been documented (1). Given the complexity of the possible pharmacological influences on sensory gating, it is important to evaluate antipsychotic medication effects on this putative vulnerability marker.
Conventional antipsychotic medications do not remediate sensory gating deficits for schizophrenia patients (5). New-generation antipsychotic medications such as clozapine (5–7), olanzapine (7), or risperidone (8) may be associated with improvements in sensory gating. However, to our knowledge medication effects have never been investigated in the context of a randomized clinical trial. This study was designed to compare the effects of olanzapine and haloperidol on the P50 sensory gating index in treatment-resistant patients with schizophrenia.
Method
Patients participating in a 16-week, double-blind, parallel-group comparison of olanzapine and haloperidol were invited to enter the study. Patients met DSM-IV criteria for either schizophrenia or schizoaffective disorder. All participants provided written informed consent prior to study entry. Subjects with concurrent substance abuse or alcoholism, organic brain disorder, or mental retardation were excluded. The age range of the patient population was 18 to 60. Patients had to have demonstrated an inadequate treatment response to conventional antipsychotics, defined as 1) a history of two or more ineffective trials of at least two different classes of conventional antipsychotics, and 2) failure to respond to a prospective 4-week open-label trial of fluphenazine (mean dose=20 mg/day, range=10–30) and benztropine (4 mg/day). Patients who exhibited a 30% improvement in either the BPRS positive symptom score or the SANS total score were excluded from the study. Patients who experienced a relapse or were intolerant of fluphenazine were not eligible for participation in the study.
Patients who fulfilled treatment resistance criteria and were clinically stable were randomly assigned to either olanzapine or haloperidol. The optimal dose for both olanzapine and haloperidol was 15 mg/day, and both medications had dose ranges of 10–30 mg/day. Benztropine, 4 mg/day, was prescribed to patients randomly assigned to the haloperidol condition to minimize extrapyramidal symptoms. Patients assigned to olanzapine received placebo benztropine.
Sensory gating measures were obtained from each participant at baseline, while patients were receiving fluphenazine and benztropine, and 12 weeks after randomization. P50 event-related potentials were recorded with tin electrodes (Electro-Cap International, Eaton, Ohio) placed at midline locations (FPz, Fz, FCz, Cz, CPz, Pz, Oz) referenced to linked earlobes during a paired-click procedure (75-dB clicks, 150 click pairs, 10-second interpair interval, 0.5-second interclick interval). Eye movements were monitored with an electrode placed at the outer canthus of the right eye, which was referred to the right frontal pole. Neurophysiological signals were sampled at 1000 Hz with a Neuroscan Synamp amplifier (200 Hz low pass, 1.0 Hz high pass) to yield 500-msec epochs (100-msec prestimulus window). Signal averaging was conducted offline to permit the rejection of single trials that contained movement artifact (rejection criterion of ±75 μV). P50 amplitude and latency measures were made on bandpass filtered (10–100 Hz, 12 dB) averages obtained from Cz. For the first click, P50 was defined as the largest positive-going wave occurring within 35–70 msec poststimulus. Amplitude was measured from the trough of the preceding wave to the P50 peak. The P50 latency window for the second click was set to within ±10 msec of the P50 latency for the first click. P50 amplitude measures were used to construct sensory gating ratios (P50 amplitude following the second click divided by the P50 amplitude following the first click).
Repeated-measures analysis of variance was used to evaluate the effects of time (baseline, endpoint) and medication (olanzapine, haloperidol) and their interaction. Chi-square was used to compare normal suppression rates at baseline and endpoint.
Results
Twenty-seven patients entered the study. One patient dropped out before study completion, one was not capable of giving informed consent at the second P50 measurement occasion, and the baseline data of one patient were lost because of equipment malfunction. Data for 24 patients with complete measurements are presented. The haloperidol and olanzapine groups did not differ in terms of gender (10 male subjects in each group) or age (mean=46.1 [SD=10.0] and 41.5 years [SD=8.1], respectively). The mean haloperidol dose at the end of the study was 17.3 mg/day (SD=6.2); the mean olanzapine dose at the end of the study was 20.4 mg/day (SD=5.8).
The normal P50 sensory gating ratio cutoff in our laboratory is 0.50. The mean baseline ratios were above this cutoff for both the olanzapine and the haloperidol groups (mean=0.61 [SD=0.29] and 0.60 [SD=0.27], respectively). Seven patients in each group (58%) exceeded the normal sensory gating ratio cutoff. There were no between-group differences in P50 amplitudes or latencies following the first or second click at baseline.
The end-of-study P50 amplitude and latency measures did not significantly differentiate olanzapine-treated from haloperidol-treated patients nor did the end-of-study sensory gating ratios (mean=0.57 [SD=0.26] and 0.68 [SD=0.26], respectively) (Figure 1). The time-by-drug interaction was not significant (F=0.68, df=1, 22, p=0.42). Six (50%) of the olanzapine-treated and four (33%) of the haloperidol-treated patients had normal sensory gating at endpoint. There was no significant group difference in the proportion of patients who had normal sensory gating at endpoint (χ2=0.35, df=1, p=0.55). There were no significant within-group changes or between-group differences in BPRS total score, BPRS positive symptom score, or SANS total score (all p values >0.20).
Discussion
Olanzapine was not significantly superior to haloperidol for the treatment of sensory gating impairments. The medication-by-time effect for the P50 measure of sensory gating was not significant, nor was there a significant group difference in the number of subjects whose P50 suppression normalized with treatment. The failure to detect a beneficial effect of haloperidol on the P50 sensory gating index is consistent with previous research (5).
The lack of a beneficial effect of olanzapine on the P50 sensory gating index differs from the findings of the Light et al. study (7), which suggested that patients treated with new-generation antipsychotics, including olanzapine (N=6), but not conventional antipsychotics, have normal P50 suppression. The difference between the two studies may be related to the use of a randomized, longitudinal design in the current study versus cross-sectional assessment in the latter study. The lack of longitudinal assessment in the Light et al. study precludes attribution of group differences in P50 suppression to medication effects. The failure to detect a beneficial effect of olanzapine on P50 suppression is unlikely to be due to the relatively small number of subjects, since there was only a 6% reduction in sensory gating ratio in the olanzapine-treated patients, which even with a substantially larger number of subjects would not be statistically significant.
Our results are also in contradistinction to the putative effect of clozapine on P50 suppression. In previous longitudinal studies, clozapine has been shown to improve P50 suppression in a group of treatment-resistant patients (5, 6). Improvement in sensory gating paralleled improvement in clinical status and decline in norepinephrine metabolite levels (5, 6). The differential effect of clozapine and olanzapine on sensory gating may be related to the higher relative affinity of clozapine compared with olanzapine for the α-2 adrenergic receptor (9). Alternatively, the lack of effect of olanzapine on P50 suppression could be related to the inclusion of treatment-resistant patients and the failure to observe a clinical response. However, there are several arguments against this explanation. First, there are a number of studies that have failed to show a relationship between P50 suppression and severity of negative or positive symptoms (7, 10, 11). Second, the beneficial effect of clozapine on P50 suppression was observed in treatment-resistant patients. Conley and colleagues (12) demonstrated that patients who fail to respond to olanzapine may respond to clozapine, which further argues for a differential effect of these drugs in this patient population.
Received Dec. 4, 2002; revision received March 28, 2003; accepted April 8, 2003. From the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland, Baltimore. Address correspondence to Dr. Buchanan, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228; [email protected] (e-mail). Supported in part by NIMH grants MH-40279 and MH-45074 and by the UMB-Novartis collaboration.
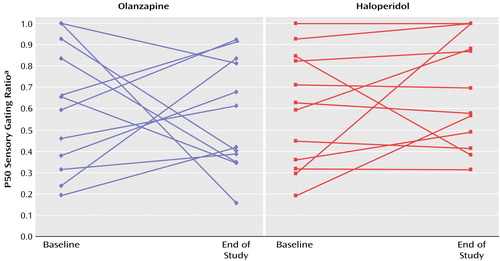
Figure 1. P50 Sensory Gating Ratio Changes in 24 Patients With Treatment-Resistant Schizophrenia Randomly Assigned to 12 Weeks of Double-Blind Treatment With Olanzapine or Haloperidol
aThe gating ratio is the P50 amplitude following the second auditory stimulus divided by the P50 amplitude following the first stimulus.
1. Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R: Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 1998; 24:189–202Crossref, Medline, Google Scholar
2. Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R: Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 1992; 32:607–616Crossref, Medline, Google Scholar
3. Adler LE, Hoffer LD, Wiser A, Freedman R: Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
4. Buchanan RW, Summerfelt A, Tek C, Gold J: An open-labeled trial of adjunctive donepezil for cognitive impairments in patients with schizophrenia. Schizophr Res 2003; 59:29–33Crossref, Medline, Google Scholar
5. Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R: Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Crossref, Medline, Google Scholar
6. Nagamoto HT, Adler LE, McRae KA, Huettl P, Cawthra E, Gerhardt G, Hea R, Griffith J: Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology 1999; 39:10–17Crossref, Medline, Google Scholar
7. Light GA, Geyer MA, Clementz BA, Cadenhead KS, Braff DL: Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am J Psychiatry 2000; 157:767–771Link, Google Scholar
8. Yee CM, Nuechterlein KH, Morris SE, White PM: P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol 1998; 107:691–698Crossref, Medline, Google Scholar
9. Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996; 14:87–96Crossref, Medline, Google Scholar
10. Adler LE, Waldo MC, Tatcher A, Cawthra E, Baker N, Freedman R: Lack of relationship of auditory gating defects to negative symptoms in schizophrenia. Schizophr Res 1990; 3:131–138Crossref, Medline, Google Scholar
11. Yee CM, Nuechterlein KH, Morris SE, White PM: P50 suppression in recent-onset schizophrenia: clinical correlates and risperidone effects. J Abnorm Psychol 1998; 107:691–698Crossref, Medline, Google Scholar
12. Conley RR, Tamminga CA, Kelly DL, Richardson CM: Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46:73–77Crossref, Medline, Google Scholar



