Perception of Complex Sounds: Abnormal Pattern of Cortical Activation in Autism
Abstract
OBJECTIVE: Bilateral temporal hypoperfusion at rest was recently described in autism. In normal adults, these regions are activated by listening to speech-like sounds. To investigate auditory cortical processing in autism, the authors performed a positron emission tomography activation study. METHOD: Regional cerebral blood flow was measured in five autistic adults and eight comparison subjects during rest and while listening to speech-like sounds. RESULTS: Similar to the comparison subjects, autistic patients showed a bilateral activation of the superior temporal gyrus. However, an abnormal pattern of hemispheric activation was observed in the autistic group. The volume of activation was larger on the right side in the autistic patients, whereas the reverse pattern was found in the comparison group. The direct comparison between the two groups showed that the right middle frontal gyrus exhibited significantly greater activation in the autistic group. Conversely, the left temporal areas exhibited lessactivation in autistic patients. CONCLUSIONS: These findings suggest that abnormal auditory cortical processing is implicated in the language impairments and the inadequate response to sounds typically seen in autism.
Autism is a severe pervasive developmental disorder characterized by impairments in reciprocal social relationships, verbal and nonverbal communication, and the ability to play and to develop interests outside of stereotypic preoccupations (1). Autism is also characterized by disturbances in the perception and modulation of sensory information, especially in the auditory domain. For example, young autistic children are often initially misdiagnosed as deaf (2). Two independent studies (3, 4) have reported bilateral hypoperfusion at rest in the temporal lobes of children with primary autism. In our study (3), abnormalities were centered on the multimodal superior temporal sulcus and in the auditory cortex of the superior temporal gyrus (3). However, up to now only two auditory functional brain imaging studies have been performed in autistic patients (5, 6). Thus, in the present positron emission tomography (PET) activation study, we further test auditory cortical processing in autism by using a passive listening task of complex speech-like auditory stimuli. We have previously shown in healthy subjects that these stimuli activate large areas of the superior temporal cortices (7), which are selectively involved in the initial “acoustic” stage of speech perception (8). These stimuli are never recognized as speech and are therefore unlikely to be explicitly processed by semantic language systems. Therefore, with this paradigm, the putative cortical activation differences between autistic patients and healthy subjects may reflect basic anomalies of cortical prelinguistic auditory processing rather than consequences of abnormal language development.
Method
We studied five adults (four men and one woman) with a primary autistic disorder (mean age=19.1 years [SD=4.5]; mean IQ=64 [SD=5]). Autism was diagnosed according to DSM-IV criteria. Autism was confirmed in all patients with the Autism Diagnostic Interview—Revised (9) (social interaction score: mean=25, SD=3.8; nonverbal communication score: mean=10.4, SD=2.5; stereotypy score: mean=6.2, SD=2.3; age at onset: mean=3.6 years, SD=1.3). Four patients had typical autistic speech abnormalities (verbal perseveration, stereotypy, echolalia, abnormal prosody, neologism), and one patient had no communicative speech. We excluded from this study patients with infectious, metabolic, neurological, or genetic diseases and those with abnormal EEG or MRI results. All patients were medication free for at least 1 month before the PET scan.
Eight healthy male university students were volunteers for this study (mean age=21.9 years, SD=3.3). They had no history of neurological or psychiatric disorders.
All autistic and comparison subjects had normal auditory functioning as assessed by an audiogram. An ethics committee approved this study. Written informed consent from the patients’ parents and from the healthy subjects was obtained after the procedure had been fully explained.
Relative regional cerebral blood flow (rCBF) was determined from the distribution of radioactivity measured with PET (ECAT-EXACT-HR+) after bolus intravenous injection of H215O (10). The experimental protocol included three rCBF measurements carried out in a single session, performed at 10-minute intervals. The first measurement occurred during the rest condition, and the second and the third occurred during passive listening to complex speech-like synthetic sounds. During each auditory stimulation, started 25 seconds before image acquisition, stimuli were delivered binaurally at a 68-dB sound pressure level and a 1-second inter-onset interval.
We used a subset of the synthetic speech-like auditory stimuli previously published elsewhere (7). Briefly, these stimuli contain spectral maxima (like speech formants) changing in time. They consist of complex sounds with a central 200-msec steady-state period surrounded by initial and final changes in frequency of the spectral maxima. Their acoustic structure was very similar to consonant-vowel-consonant, but as stated, normal volunteers never recognized them as speech sounds.
The rCBF images were analyzed with statistical parametric mapping software (SPM 96) used for image realignment, transformation into standard stereotactic anatomical space, smoothing (15 mm), and statistical analysis (11). State-dependent differences in global flow were covaried out by using proportional scaling. Comparisons across conditions were made with the t statistic subsequently transformed into the normally distributed z statistic by using a multistudy design. The resulting z maps were thresholded at p<0.001 corrected at p<0.05 for multiple comparisons.
Two statistical analyses of activation were performed: a within-group comparison of activation for listening to complex sounds versus the rest condition and a between-group comparison of activation.
Results
Passive listening to speech-like sounds versus the rest condition was assessed in each group independently. In the healthy comparison subjects, there was bilateral superior temporal cortex activation with a left-biased asymmetry (z>3.09, df=22, p<0.001). Five peaks of activation were detected along the left superior temporal cortex, whereas only two peaks were detected in the right superior temporal gyrus (Brodmann’s area 22). The volume of the activation was larger in the left hemisphere (32 cm3) than in the right (20 cm3). No peak of activation was detected outside the temporal lobes. This pattern of activation has been previously reported (7).
There was also bilateral superior temporal cortex activation in the autistic patients, but with a reverse right-biased asymmetry (z>3.09, df=22, p<0.001). Five peaks were detected in the right superior temporal cortex and three peaks in the left superior temporal gyrus. The volume of activation was larger in the right hemisphere (55 cm3) than in the left (19 cm3). In addition, six foci of activation were detected in the right frontal lobe and two in the left precentral gyrus.
The direct comparison showed a statistically significant difference between the autistic and the healthy comparison subjects in two brain areas (z>3.09, df=22, p<0.001). The right middle frontal gyrus (Brodmann’s areas 9, 9/46, and 10) exhibited significantly greater activation in the autistic patients than in the comparison subjects. In addition, the posterior part of the left middle and inferior temporal gyrus (Brodmann’s area 21) exhibited significantly less activation in the autistic patients than in the comparison subjects (Figure 1).
Discussion
In this study, we investigated auditory cortical processing in patients with autism by using stimuli designed to investigate early cortical stages of auditory speech processing. In healthy subjects, these stimuli induce bilateral activation of secondary auditory areas located in the lateral belt of the auditory cortex (7), which are selectively involved in the initial acoustic steps of language processing (8). In the present study, we observed an abnormal pattern of activation in the autistic patients. At the level of the auditory temporal cortex, our data suggest a reverse hemispheric dominance, since the volume of activation was larger in the right auditory cortex of autistic subjects, whereas the reverse pattern was found in the comparison subjects. It is of interest that the abnormal right dominance was confirmed by analysis of other brain areas. Autistic patients strongly activated the right middle frontal gyrus but not the comparison subjects, and the difference between both groups was statistically significant. Furthermore, the posterior part of the left middle temporal gyrus, a region that is involved in word processing (12), was significantly less activated in autistic patients than in the comparison group. This temporal region is also presumed to act as an interface between word perception and long-term representations of familiar words in memory (13).
These speech-like stimuli elicited an abnormal global cortical mapping in autistic patients characterized by hypoactivation of the left temporal word processing network and also by an emergence of an abnormal right frontotemporal network. Right-biased cortical processing has been previously reported in autism with other types of auditory stimuli (5, 6). In healthy subjects, the right middle frontal gyrus is activated by an auditory attentional task, i.e., discrimination of sound duration (14). Therefore, greater activation in the right middle frontal cortex, a region that integrates a cortical “attentional network,” may be implicated in the abnormal and often unexplained behavioral responses to sounds that are one of the most pronounced signs in autism.
The right shift of the global cortical mapping of auditory stimuli and reduced recruitment of the left temporal region in response to complex sounds in autism may be implicated in the abnormal behavioral responses to sounds and language acquisition impairment, both characteristic of autism. As auditory stimuli are perceived as strange electronic noises but never as speech, we speculate that the observed abnormal pattern of auditory activation in autism may reflect a fundamental alteration of the auditory cortical processing that leads to an abnormal early stage of language development rather than reflecting the consequences of abnormal language development. In addition, a similar right-shift pattern of activation was described by Muller et al. (6) in autistic men listening to human speech sounds. Our results are also in accordance with auditory evoked potential findings in autistic patients of a right hemispheric dominance in the processing of verbal and nonverbal auditory stimuli (15).
In conclusion, these preliminary results extend to the auditory domain previous findings of abnormal visual cortical pattern of activation in patients with autism (16–18), suggesting a disorganization in the establishment of global cortical networks.
Received Jan. 31, 2003; revision received April 10, 2003; accepted April 24, 2003. From ERM0205, INSERM-CEA-Service Hospitalier Frédéric Joliot, Direction des Sciences du Vivant, Département de Recherche Médicale, Commisariat à l’Energie Atomique; the Service de Radiologie Pédiatrique, Necker Enfants Malades, Assistance Publique-Hopitaux de Paris; the University of Montreal Department of Psychology, Montreal; the Service de Pédopsychiatrie, Hôpital Robert Debré, Assistance Publique-Hopitaux de Paris; the Institut National de la Santé et de la Recherche Médicale, Tours, France; and the Service des Urgences Cérébro-Vasculaires, Groupe Hospitalier Pitié-Salpêtrière, Assistance Publique-Hopitaux de Paris. Address reprint requests to Dr. Zilbovicius, Commisariat à l’Energie Atomique, Service Hospitalier Frédéric Joliot, 4 place du Général Leclerc, 91406, Orsay, France; [email protected] (e-mail). Supported by funding from the France-Télecom Foundation and the France Foundation. The authors thank the nurses and technical staff of the Orsay Brain Imaging Center for their assistance and the families for their cooperation.
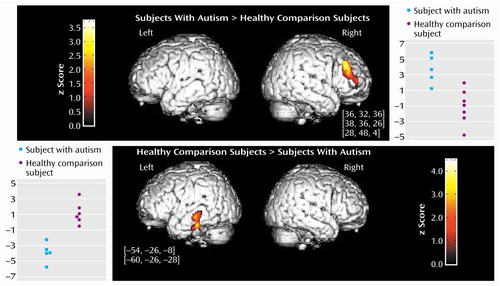
Figure 1. Differences in Regional Cerebral Blood Flow (rCBF) Changes Between Subjects With Autism (N=5) and Healthy Comparison Subjects (N=8) Listening to Speech-Like Soundsa
aThe right middle frontal cortex (Brodmann’s areas 9, 9/46, and 10) was significantly more activated in autistic patients than in the comparison subjects (z=3.09, df=22, p<0.001). The left middle and inferior temporal gyrus (Brodmann’s area 21) was significantly less activated in the autistic patients than in the comparison subjects (z=3.09, df=22, p<0.001). The x, y, and z stereotaxic coordinates of activation peaks are shown in brackets. Diagrams indicate individual rCBF changes (auditory activation minus rest condition) for autistic patients and healthy comparison subjects (some data points missing because of overlap).
1. Kanner L: Autistic disturbances of affective contact. Nerv Child 1943; 2:217–250Medline, Google Scholar
2. Rapin I, Katzman R: Neurobiology of autism. Ann Neurol 1998; 43:7–14Crossref, Medline, Google Scholar
3. Zilbovicius M, Boddaert N, Belin P, Poline J-B, Remy P, Mangin J-F, Thivard L, Barthélémy C, Samson Y: Temporal lobe dysfunction in childhood autism: a PET study. Am J Psychiatry 2000; 157:1988–1993Link, Google Scholar
4. Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M: Abnormal regional blood flow in childhood autism. Brain 2000; 123:1838–1844Crossref, Medline, Google Scholar
5. Garreau B, Zilbovicius M, Guerin P, Samson Y, Syrota A, Lelord G: Effects of auditory stimulation on regional cerebral blood flow in autistic children. Developmental Brain Dysfunction 1994; 7:119–128Google Scholar
6. Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Manger TJ, Chugani HT: Brain mapping of language and auditory perception in high functioning autistic adults: a PET study. J Autism Dev Disord 1999; 29:19–31Crossref, Medline, Google Scholar
7. Thivard L, Belin P, Zilbovicius M, Poline JB, Samson Y: A cortical region sensitive to auditory spectral motion. Neuroreport 2000; 11:2969–2972Crossref, Medline, Google Scholar
8. Samson Y, Belin P, Thivard L, Boddaert N, Crozier S, Zilbovicius M: Auditory perception and language: functional imaging of speech sensitive auditory cortex. Rev Neurol 2001; 157:837–846Medline, Google Scholar
9. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659–685Crossref, Medline, Google Scholar
10. Fox PT, Mintun MA, Raichle ME, Herscovitch P: A non-invasive approach to quantitative functional brain mapping with H215O and positron emission tomography. J Cereb Blood Flow Metab 1984; 4:329–333Crossref, Medline, Google Scholar
11. Friston K, Holmes AP, Worsley KJ, Poline JB, Heather JD, Frackowiak R: Statistical parametric mapping in functional imaging: a general linear approach. Hum Brain Map 1995; 2:189–210Crossref, Google Scholar
12. Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ: Hearing and saying: the functional neuroanatomy of auditory word processing. Brain 1996; 119:919–931Crossref, Medline, Google Scholar
13. Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA: Separate neural subsystems within “Wernicke’s area.” Brain 2001; 124:83–95Crossref, Medline, Google Scholar
14. Belin P, McAdams S, Thivard L, Smith B, Savel S, Zilbovicius M, Samson S, Samson Y: The neuroanatomical substrate of sound duration discrimination. Neuropsychologia 2002; 40:1956–1964Crossref, Medline, Google Scholar
15. Bruneau N, Roux S, Adrien JL, Barthelemy C: Auditory associative cortex dysfunction in children with autism: evidence from late auditory evoked potentials (N1 wave-T complex). Clin Neurophysiol 1999; 110:1927–1934Crossref, Medline, Google Scholar
16. Schultz RT, Gauthier I, Klin A, Fullbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC: Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340Crossref, Medline, Google Scholar
17. Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG: The functional neuroanatomy of social behavior: changes in cerebral blood flow when people with autistic disorder process facial expression. Brain 2000; 123:2203–2212Crossref, Medline, Google Scholar
18. Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E: Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain 2001; 124:2059–2073Crossref, Medline, Google Scholar



