Olfactory Function in Mild Cognitive Impairment and Alzheimer’s Disease: An Investigation Using Psychophysical and Electrophysiological Techniques
Abstract
OBJECTIVE: To clarify the olfactory deficit hypothesis regarding Alzheimer’s disease, the authors compared olfactory function in patients with Alzheimer’s disease, subjects with mild cognitive impairment, and healthy comparison subjects. METHOD: Olfactory function of 14 patients with mild Alzheimer’s disease, eight subjects with mild cognitive impairment, and eight healthy age-matched comparison subjects was assessed with both psychophysical tests and olfactory event-related potentials. RESULTS: Group comparison of the psychophysical test results showed a significant main effect of diagnosis for odor detection threshold, odor discrimination, and odor identification. These results correlated only partially with those obtained from olfactory event-related potentials. Seven Alzheimer’s disease patients and four with mild cognitive impairment showed no olfactory event-related potentials, suggesting hyposmia, while all comparison subjects had clearly discernible responses. Patients with Alzheimer’s disease were significantly more likely to be nonresponders. In the four Alzheimer’s disease patients and four subjects with mild cognitive impairment who had clear electrophysiological responses, amplitudes and latencies of the various event-related potential components were normal, i.e., similar to those of the comparison subjects, although 12 of the 14 Alzheimer’s disease patients and seven of the eight mildly impaired subjects were classified as functionally anosmic with psychophysical methods. CONCLUSIONS: The electrophysiological results confirm prior findings of olfactory dysfunction in patients with Alzheimer’s disease and preclinical Alzheimer’s disease. Investigations of larger study groups with detailed cognitive examination and postmortem diagnosis may resolve the intriguing possibility of early diagnosis and discrimination of Alzheimer’s disease subtypes through chemosensory event-related potentials in addition to existing biomarkers.
Brain areas involved in olfactory function are situated in medial temporal regions that undergo early neuropathological change in Alzheimer’s disease (1–3). Correspondingly, patients with Alzheimer’s disease are expected to develop an olfactory dysfunction early in the disease, a hypothesis supported by an increasingly large body of research (4–7). Olfactory dysfunction in Alzheimer’s disease correlates with disease progression (8, 9), aids in the differential diagnosis of Alzheimer’s disease versus major depression (10), and may be clinically useful as an early diagnostic marker in predicting incident Alzheimer’s disease in high-risk individuals (11–13).
An olfactory deficit is typically assessed by using tests of odor identification, discrimination, and detection-threshold sensitivity. These psychophysical tests require active cooperation of the test subject and various cognitive functions, including working memory and semantic categorization. Therefore, cognitive impairment in Alzheimer’s disease may significantly mediate olfactory test performance. In a more objective way, olfactory sensory physiology can be assessed with complex electrophysiological and functional brain imaging measures. A study of olfactory-evoked regional cerebral blood flow using positron emission tomography showed low activation in the piriform cortex of Alzheimer’s disease patients (14). Electrophysiological studies of patients with Down’s syndrome (15), which is neuropathologically similar, and individuals who are positive for apolipoprotein E4 (16) demonstrated abnormal olfactory event-related potentials. The few direct studies of olfactory event-related potentials in Alzheimer’s disease have shown contradictory results. Sakuma et al. (17) found abnormal potentials despite the absence of psychophysical olfactory dysfunction, whereas Hawkes and Shepard (18) reported normal potentials in four patients, although the odor identification scores were abnormal.
The purpose of the present study was to confirm and extend the olfactory deficit hypothesis of Alzheimer’s disease by using an objective electrophysiological measure. A further aim was to investigate the olfactory function in subjects with mild cognitive impairment (19). Mild cognitive impairment is a recently defined clinical term referring to a cognitive decline between those of normal aging and Alzheimer’s disease and is believed to indicate high risk for the development of probable Alzheimer’s disease. Mild cognitive impairment is recognized as suitable for the evaluation of early diagnostic markers of Alzheimer’s disease (19).
Method
Subjects
The study included patients with Alzheimer’s disease, subjects with mild cognitive impairment, and a healthy age-matched comparison group. All subjects were recruited from outpatients in the Memory Clinic of the Department of Psychiatry and Psychotherapy of the University Hospital Frankfurt am Main, Germany. The research protocol was approved by the local ethics commission, and written informed consent was obtained from each patient, his or her caregiver (spouse or adult child), and each comparison subject before any examination procedures were performed.
Routine diagnostic assessment at the Memory Clinic involves a physician cooperating with a multiprofessional team (neurologists, psychiatrists, psychologists). The procedures include a detailed medical history from both the patient and the spouse or relative; general physical, neurological, and psychiatric examinations; and laboratory testing that comprises a CBC with differential counts, syphilis and Lyme borreliosis screening, and measures of serum electrolytes, liver and renal function, cholesterol status, thyroid function, and serum vitamin B12 and folate levels. Technical diagnostic procedures include a structural magnetic resonance imaging (MRI) scan of the brain and functional assessment with [18F]fluorodeoxyglucose positron emission tomography (FDG PET). The FDG PET scan is performed in a resting state after 12 hours of fasting and is visually analyzed for Alzheimer-typical patterns of hypometabolism (20). A trained neuropsychologist administers the following tests: the Mini-Mental State Examination (MMSE) (21), the test battery of the Consortium to Establish a Registry for Alzheimer’s Disease (22), and the Short Cognitive Performance Test (23). All available information is reviewed by the multiprofessional team, which makes a consensus diagnosis that includes a rating of disease severity according to the Global Deterioration Scale (24) and the Clinical Dementia Rating (25), in order to distinguish between mild cognitive impairment and Alzheimer’s disease and to document possible progression on follow-up.
All included patients were seen at least twice during semiannual follow-up visits. For patients with Alzheimer’s disease, the inclusion criteria were age greater than 50 years, diagnosis of dementia made on the basis of ICD-10 criteria, and diagnosis of probable Alzheimer’s disease according to criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (26). Patients with disease severity ranging from very mild to moderate, corresponding to a Global Deterioration Scale rating of 3 to 4, and an MMSE score higher than 15 were accepted. Stable general health and the ability to read and write as well as to follow basic test instructions were further prerequisites. Fourteen patients with Alzheimer’s disease were recruited, five men and nine women. Their mean age was 72.2 years (SD=5.7, range=57–81), their mean MMSE score was 23.3 (SD=3.1, range=16–27), and the mean disease severity according to the Global Deterioration Scale was 3.7.
Recruitment of the patients with mild cognitive impairment followed current conceptual criteria for amnestic mild cognitive impairment (19) and required memory complaints corroborated by an informant, impaired memory function for age and education, preserved general cognitive function, and intact activities of daily living. Further inclusion criteria were age greater than 50, stable general health, and the diagnosis of “questionably demented” (score=0.5) according to the Clinical Dementia Rating (25). Eight subjects were recruited, five men and three women. Their mean age was 72.5 years (SD=5.0, range=65–79), and their mean MMSE score was 28.7 (SD=1.4, range=27–30). All of the included patients with mild cognitive impairment demonstrated temporoparietal hypometabolism in functional imaging with PET, characteristic of beginning Alzheimer’s disease (20).
The comparison group was recruited from age-matched spouses of the cognitively impaired subjects. These individuals underwent an evaluation similar to that just described, including a general medical history, neurological examination, and brief neuropsychological testing with the MMSE. Subjects qualified as healthy comparison subjects if, in the opinion of the clinician, they were functioning normally in daily life and did not have any sign of cognitive impairment. Eight male comparison subjects were included. Their mean age was 73.9 years (SD=9.4, range=53–83), and their mean MMSE score was 29.9 (SD=0.4, range=29–30).
The exclusion criteria for all participants were 1) any current or past history of neurological or psychiatric illness other than mild cognitive impairment or Alzheimer’s disease, including stroke, head trauma, affective disorders, and structural evidence of stroke or major perfusion deficits (MRI evidence of cortical stroke, subcortical lacunae or infarcts, extensive periventricular white matter changes, or other radiological changes indicative of a probable vascular dementia according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Association Internationale pour la Recherche et l"Enseignement en Neurosciences [27]); 2) history of alcohol or substance dependence (ICD-10 criteria); 3) current or past history of smoking; 4) endocrinological disorder; 5) any unstable medical condition; or 6) acute rhinitis, active allergy, or any history of obstructive nasal disease or nasal-sinus surgery. All participants were right-handed.
Psychophysical Testing of Olfactory Function
Before the session to evaluate event-related potentials, all participants were tested for chemosensory functioning by means of a commercially available psychophysical olfactory test battery; normative data in relation to age and gender and information on the battery’s validity in comparison to established measures of olfactory sensitivity have been published (28, 29). It comprises three tests of olfactory function: threshold for detection of butanol odor, odor discrimination, and odor identification. Odors are presented in felt-tip pens 14 cm long with a 1.3-cm inner diameter. Instead of dye, the pen’s tampon is saturated with a liquid odorant. For odor presentation, the cap was removed by the experimenter and the pen’s tip was placed 2 cm in front of both nostrils for approximately 3 seconds. An interval of at least 30 seconds between odors minimized olfactory desensitization.
Odor thresholds were assessed by using n-butanol. Sixteen dilution steps were established in a geometric series, starting from 4% n-butanol (with aqua conservata as solvent). The threshold was determined by employing a multiple staircase method, with a triple-force design, i.e., three pens were presented in a randomized order, two containing the solvent and one with the odorant at a certain dilution. The subject’s task was to identify the odor-containing pen. After correct identification in two successive trials, a higher-dilution step was attempted. If the subject failed to identify the odor, a lower dilution was repeated. The mean of the last four reversal points was used as the threshold estimate. The scores could range between 0 and 16; results show a normal distribution in the general population (29).
In the odor-discrimination task, triplets of pens were presented in a randomized order, with two containing the same odorant and the third a different odorant. The subject’s task was to determine which pen differed. As a total of 16 triplets were presented, the scores could range from 0 to 16.
Odor identification was assessed by presentation of 16 common odors (orange, leather, fish, cinnamon, peppermint, banana, apple, lemon, licorice, turpentine, garlic, coffee, rose, pineapple, clove, and aniseed). The subjects were free to sample each odor as often as necessary before selecting one out of four descriptors from a list specifically assigned to each odor. Again, the scores could range from 0 (none correctly recognized) to 16.
The results of the three subtests were summarized in the composite score (threshold plus discrimination plus identification), resulting from addition of the three subtest scores. A composite score of 15 or less is used to identify functional anosmia (29).
Olfactory and Trigeminal Event-Related Potentials
Monomodal chemosensory nasal stimulation was performed by the use of a sophisticated stimulus apparatus (Olfactometer OM2S, Burghart Instruments, Wedel, Germany) that allows the application of chemical stimuli without concomitant stimulation of mechanoreceptors or thermoreceptors (30–32). This was achieved by embedding chemical stimuli of 200-msec duration in a constantly flowing air stream (8 liters/minute) applied to the nasal mucosa by a cannula with an inner diameter of 3 mm inserted approximately 1 cm into the nostril beyond the nasal valve area. The temperature and humidity of the air stream were kept constant (36.5°C, 80% relative humidity). The rise time of the stimulus concentration was less than 20 msec. Hydrogen sulfide (H2S), 8 ppm, was used for olfactory stimulation, since it specifically stimulates the olfactory system without simultaneous trigeminal activation (33). In contrast, carbon dioxide (CO2), 50% volume/volume, was used for specific stimulation of trigeminal nociceptors, in order to control for systematic errors and to confirm an intact nasal sensitivity. It produces a painful stinging sensation (34). During each session, 20 stimuli of H2S and CO2 each were applied to the right nostril with an interstimulus interval of 40 seconds to avoid habituation. The procedure lasted approximately 40 minutes. In keeping with published recommendations for the recording of olfactory event-related potentials (32, 35), the session started with H2S. Among the reasons for this procedure is that vigilance decreases during sessions. Thus, olfactory event-related potentials, as the measure of highest interest, were recorded first, while trigeminal event-related potentials were recorded later during the sessions. The subject was seated in an air-conditioned room that was darkened and acoustically shielded to minimize other sensory stimuli; the patient’s movements were monitored through a video camera system. The complete procedure was performed while the subject breathed naturally.
The EEG was recorded from five positions of the international 10/20 system (Cz, C3, C4, Fz, and Pz) referenced to linked earlobes (A1, A2). Blink artifacts were monitored from an additional site (Fp2). Stimulus-linked EEG segments of 2,048 msec were digitally recorded at a frequency of 250 Hz (band-pass filter 0.2–15 Hz). Event-related potentials were obtained by off-line averaging of at least 15 digitized EEG segments. The baseline event-related potential was set at the mean of the EEG record during the pretrigger period of 548 msec. Records contaminated by eye blinks (>50 μV at the Fp2 lead) or other disturbances (e.g., high-frequency motor artifacts) were discarded during off-line visual inspection of single trials by a trained observer (T.H.). Only participants with at least 15 remaining records per session were considered for further evaluation.
Furthermore, an event-related potential was considered absent if it was not possible to distinguish a clear response from the background noise in an artifact-free recording. Subjects without a clear response to trigeminal stimulation with CO2, suggesting either a procedural error or generalized pathology of the nasal mucosa, were excluded from further evaluation. The presence of clear evoked potentials in response to CO2 combined with an indiscernible response to specific olfactory stimulation with H2S was considered to be indicative of anosmia (32).
There is discussion about the nomenclature of chemosensory event-related potentials (32). In this study the peaks were named P1, N1, and P2, in keeping with the widely accepted nomenclature of Evans et al. (35). On average, the first positive peak (P1) in this study occurred at position Pz after a latency of 395 msec, followed by a major negativity (N1) at 515 msec and a late positive complex (P2) at 802 msec. Peak amplitudes N1 and P2 (measured in relation to prestimulus baseline) and latencies N1 and P2 (in relation to stimulus onset) were evaluated by an experienced rater (T.H.), who was blinded to the subjects’ diagnoses. On the basis of these measurements, the peak-to-peak amplitudes A-P1N1 and A-N1P2, base-to-peak amplitudes A-P1, A-N1, and A-P2, and peak latencies T-P1, T-N1, and T-P2 were subjected to statistical analysis.
Statistical Analysis
The analysis focused on group differences between the patients with Alzheimer’s disease, subjects with mild cognitive impairment, and healthy comparison group.
Age, odor detection threshold, odor discrimination, odor identification, and each event-related potential measure (amplitude and latency at each electrode site) was subjected to univariate analysis of variance (ANOVA); diagnosis was treated as a between-subjects factor with three levels (Alzheimer’s disease, mild cognitive impairment, and healthy). The alpha level was set at 0.05. In case of significance, ANOVA was followed by univariate post hoc comparisons to identify the specific diagnostic group for which the factor differed significantly. For this purpose, correlated unpaired t tests with Bonferroni adjustment for multiple comparisons were performed.
In order to test the statistical significance of the differences in the various rates of present or absent olfactory event-related potentials and the significance of the gender difference between the diagnostic groups, the nonparametric Fisher’s exact test was conducted. The analysis was processed with the SPSS 9.0 package (Chicago, SPSS).
Results
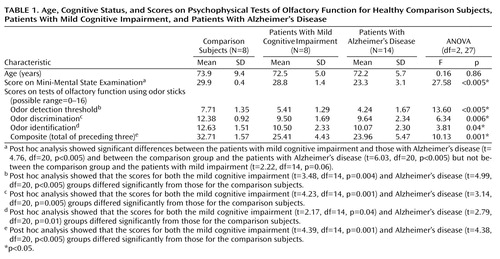
The patients with Alzheimer’s disease, subjects with mild cognitive impairment, and comparison group did not differ in terms of age (F=0.16, df=2, 27, p=0.86). The comparison group contained a significantly higher proportion of men (eight of eight) than the Alzheimer’s disease group (five of 14) (p=0.008, Fisher’s exact test). The MMSE score was a significant between-group factor (Table 1). Post hoc analysis of the MMSE scores showed significant differences between the patients with mild cognitive impairment and those with Alzheimer’s disease and between the comparison group and the patients with Alzheimer’s disease but not between the comparison group and the patients with mild impairment (Table 1).
Psychophysical Tests of Chemosensory Function
All 30 subjects completed the assessment with the psychophysical test battery. A significant main effect of diagnosis was identified for scores on all three individual tests as well as for the composite score (Table 1). Post hoc analysis showed that the scores for both the mild cognitive impairment and Alzheimer’s disease groups differed significantly from those for the comparison subjects (Table 1). According to the normative data derived with the odor stick battery of tests (29), the composite score for olfactory functioning indicated that all of the comparison subjects had normal olfactory function, while seven of the eight subjects with mild cognitive impairment and 12 of the 14 Alzheimer’s disease patients were classified as having hyposmia.
Olfactory and Trigeminal Event-Related Potentials
With all 30 subjects the complete stimulation procedures were performed. Only traces with a clear response to the trigeminal irritant CO2 and negligible artifacts were considered for further evaluation. Of these 30 sessions, one was excluded because of excessive contamination by eye blinks (one comparison subject) and three were excluded because of missing discernible responses to the trigeminal irritant CO2 (three patients with Alzheimer’s disease).
Of the remaining seven comparison subjects, all had clearly discernible responses to both gases. Of the eight subjects with mild cognitive impairment, four had distinct olfactory event-related potentials in response to H2S, while four had no discernible response, interpreted as representing anosmia. Of the 11 Alzheimer’s disease patients with valid data, four had distinct event-related potential responses to H2S; in the remaining seven patients a response to H2S was absent (Figure 1).
A Fisher’s exact test of the difference in the rates of missing responses to H2S between the diagnostic groups demonstrated that the subjects with Alzheimer’s disease were significantly more likely to be nonresponders to H2S than were the comparison subjects (p=0.02). The rates of absent olfactory event-related potentials in the groups with mild cognitive impairment and Alzheimer’s disease were similar (p=0.30).
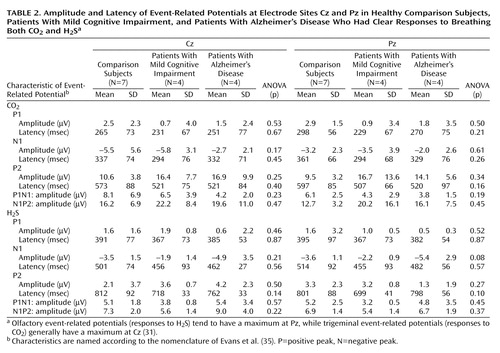
The remaining traces from subjects with clear event-related potential responses to both gases (seven of seven comparison subjects, four of eight subjects with mild cognitive impairment, four of 11 Alzheimer’s disease patients) were compared with regard to the effect of the between-subject factor diagnosis. ANOVA was used for each event-related potential component, i.e., amplitudes (A-P1N1, A-N1P2, A-N1, A-P1, A-P2) and latencies (T-P1, T-N1, T-P2), at each electrode site (Cz, C3, C4, Fz, and Pz) and for each stimulation modality. For all components analyzed, there was no significant effect of diagnosis on either H2S or CO2 trigeminal event-related potentials. Table 2 shows grand means at the electrode sites Cz and Pz. They indicate that there was no major difference in chemoreception between the healthy comparison subjects and the subgroup of patients with Alzheimer’s disease and mild cognitive impairment in whom olfactory event-related potentials were present. Furthermore, of these four subjects with Alzheimer’s disease and four with mild impairment who demonstrated normal sensory responses to stimulation, three in each group had been classified as having hyposmia or functional anosmia according to previous psychophysical testing.
Discussion
Our results are partially consistent with an olfactory dysfunction hypothesis derived from previous studies. Both subjects with mild cognitive impairment and patients with mild Alzheimer’s disease showed significantly lower olfactory functioning than age-matched comparison subjects in all three psychophysical tests using odor sticks: odor detection threshold, odor discrimination, and odor identification. The verification of previous psychophysical results for Alzheimer’s disease (4–9) was expected and underscores the validity and reliability of the rather new test of nasal chemosensory performance based on pen-like odor-dispensing devices (28, 29).
In subgroups of patients with mild cognitive impairment and Alzheimer’s disease, the psychophysical test results were confirmed by assessment with olfactory event-related potentials. In a substantial number of subjects in both of these groups, a response to olfactory stimulation with H2S was missing, whereas all of the age-matched comparison subjects had clearly discernible responses. This finding is in concordance with abnormal event-related potentials found in patients with related diseases, such as Down’s syndrome (15), in patients with idiopathic Parkinson’s disease (36), and in subjects at high risk of developing Alzheimer’s disease (16).
Magnetoencephalographic techniques have localized cortical generators of olfactory event-related potentials to the medial temporal cortex (37) and generators of trigeminal event-related potentials to the secondary somatosensory cortex (38). Therefore, the missing response to olfactory stimulation despite a positive response to trigeminal stimulation could be explained by the specific regional atrophy found in Alzheimer’s disease (1, 2). The results are also consistent with PET findings on the olfactory-evoked glucose metabolic rate in Alzheimer’s disease, which showed significantly less activation of the medial temporal lobe (39).
In contrast, in the subgroups in which a clear event-related potential response to olfactory stimulation was discernible, the analysis of the various event-related potential components did not demonstrate major abnormalities with regard to latency and amplitude, according to comparison with the healthy age-matched subjects. It is important to add that both groups with and without response to olfactory stimulation were clinically homogeneous concerning disease severity and neuropsychological deficits. In addition, in six of the eight subjects with mild cognitive impairment or Alzheimer’s disease who had normal olfactory event-related potentials, the scores from the tests with odor sticks were abnormal, replicating a discrepancy observed previously by Hawkes and Shepard (18). Several explanations for the lack of discrimination power of the event-related potentials are possible.
One possibility is that cognitive impairment, inherent to Alzheimer’s disease and mild cognitive impairment, significantly mediates psychophysical test results and could lead to discrepancies between the results of active olfactory tests and passive chemosensory stimulation. Clarification necessitates an exact evaluation of the various deficits of cognitive functioning in Alzheimer’s disease and their correlation with the individual event-related potential responses.
Normal aging is a significant factor in decreased olfactory function (40) and correlates with abnormalities in olfactory event-related potentials (41). A larger study group would be necessary to distinguish between age-related and disease-related changes. Further, gender was not balanced. Older men demonstrate greater abnormalities than older women in olfactory event-related potentials (42). However, all of the comparison subjects were male, which further lowers the possibility of finding significant differences.
In order to adapt the already complex experimental environment to the constraints of cognitively impaired subjects with mild Alzheimer’s disease, it was not possible to perform a parallel control of attention, for instance with a tracking task used in many laboratories (32) or a technique for control of breathing, during the event-related potential procedures. Olfactory event-related potentials do, however, respond to allocation and diversion of attention (43), while olfactory function can be modulated by breathing technique (44).
Neuropathological validation of the clinical diagnosis was not performed in the study. Review of necropsies of patients with dementia reveal that isolated disease entities are uncommon in aged demented patients. Rather, cerebrovascular disease often coexists with the histological features of Alzheimer’s disease (45), and clinical diagnostic criteria often fail to identify patients with Lewy body pathology (46). Both disease entities may represent further uncontrolled confounding factors.
In summary, the results confirm prior findings of olfactory dysfunction in Alzheimer’s disease and preclinical Alzheimer’s disease, i.e., mild cognitive impairment, based on psychophysical olfactory tests and further suggest that the abnormal performance is not only due to an impaired working memory and deficits in semantic categorization. The olfactory deficits are supported by missing electrophysiological response to olfactory stimulation, likely to be linked to the specific neuropathological development in medial temporal regions, including the primary olfactory cortex. A final clarification must involve correlation of objective olfactory functioning with individual cognitive deficits and postmortem neuropathological findings. Investigations of larger study groups will be necessary to assess the clinical utility and predictive and discrimination value of chemosensory event-related potentials in comparison to existing biomarkers of Alzheimer’s disease.
 |
 |
Received July 25, 2002; revision received March 19, 2003; accepted April 10, 2003. From the Department of Psychiatry and Psychotherapy I, University Hospital, Johann Wolfgang Goethe-University of Frankfurt am Main. Address reprint requests to Dr. Peters, Department of Psychiatry and Psychotherapy I, University Hospital, Johann Wolfgang Goethe-University of Frankfurt am Main, Heinrich-Hoffmann-Strasse 10, 60528 Frankfurt am Main, Germany; juergen. [email protected] (e-mail).
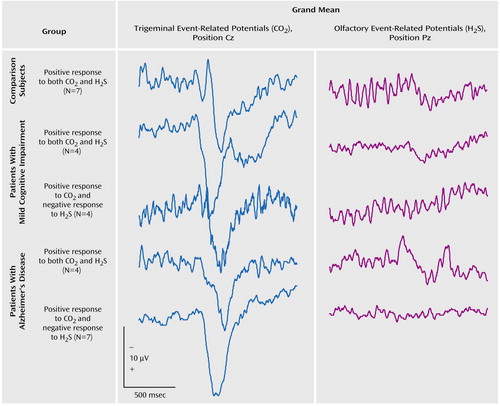
Figure 1. Grand Means of Event-Related Potentials Following Trigeminal and Olfactory Stimulation With CO2 and H2S, Respectively, in Healthy Comparison Subjects, Patients With Mild Cognitive Impairment, and Patients With Alzheimer’s Diseasea
aStimuli were presented 548 msec after the beginning of the recordings.
1. Braak H, Braak E: Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991; 82:239–259Crossref, Medline, Google Scholar
2. Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT: Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 1996; 16:4491–4500Crossref, Medline, Google Scholar
3. Kovacs T, Cairns NJ, Lantos PL: Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport 2001; 12:285–288Crossref, Medline, Google Scholar
4. Koss E, Weiffenbach JM, Haxby JV, Friedland RP: Olfactory detection and recognition in Alzheimer’s disease (letter). Lancet 1987; 1:622Crossref, Medline, Google Scholar
5. Doty RL, Reyes PF, Greogor T: Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull 1987; 18:597–600Crossref, Medline, Google Scholar
6. Serby M, Larson P, Kalstein D: The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiatry 1991; 148:357–360Link, Google Scholar
7. Mesholam RI, Moberg PJ, Mahr RN, Doty RL: Olfaction in neurodegenerative disease. Arch Neurol 1998; 55:84–90Crossref, Medline, Google Scholar
8. Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR: Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging 1990; 11:465–469Crossref, Medline, Google Scholar
9. Nordin S, Almkvist O, Berglund B, Wahlund LO: Olfactory dysfunction for pyridine and dementia progression in Alzheimer disease. Arch Neurol 1997; 54:993–998Crossref, Medline, Google Scholar
10. McCaffrey RJ, Duff K, Solomon GS: Olfactory dysfunction discriminates probable Alzheimer’s dementia from major depression: a cross validation and extension. J Neuropsychiatry Clin Neurosci 2000; 12:29–33Crossref, Medline, Google Scholar
11. Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R: Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry 2000; 157:1399–1405Link, Google Scholar
12. Bacon AW, Bondi MW, Salmon DP, Murphy C: Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann NY Acad Sci 1998; 855:723–731Crossref, Medline, Google Scholar
13. Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB: Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E e4 status. Neurology 1999; 53:1480–1487Crossref, Medline, Google Scholar
14. Kareken DA, Doty RL, Mosnik D, Chen SH, Farlow MR, Hutchins GD: Olfactory-evoked regional cerebral blood flow in Alzheimer’s disease. Neuropsychology 2001; 15:18–29Crossref, Medline, Google Scholar
15. Wetter S, Murphy C: Individuals with Down’s syndrome demonstrate abnormal olfactory event-related potentials. Clin Neurophysiol 1999; 110:1563–1569Crossref, Medline, Google Scholar
16. Wetter S, Murphy C: Apolipoprotein E e4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiol Aging 2001; 22:439–447Crossref, Medline, Google Scholar
17. Sakuma K, Nakashima K, Takahashi K: Olfactory evoked potentials in Parkinson’s disease, Alzheimer’s disease and anosmic patients. Psychiatry Clin Neurosci 1996; 50:35–40Crossref, Medline, Google Scholar
18. Hawkes CH, Shepard BC: Olfactory evoked responses and identification tests in neurological disease. Ann NY Acad Sci 1998; 855:608–615Crossref, Medline, Google Scholar
19. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B: Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Medline, Google Scholar
20. Herholz K: FDG PET and differential diagnosis of dementia. Alzheimer Dis Assoc Disord 1995; 9:6–16Crossref, Medline, Google Scholar
21. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
22. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–1165Crossref, Medline, Google Scholar
23. Erzigkeit H: The SKT—a short cognitive performance test as an instrument for the assessment of clinical efficacy of cognition enhancers, in Diagnosis and Treatment of Senile Dementia. Edited by Bergener M, Reisberg B. New York, Springer-Verlag, 1989, pp 164–174Google Scholar
24. Reisberg B, Ferris SH, De Leon MJ, Crook T: The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139Link, Google Scholar
25. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL: A new clinical scale for the staging of dementia. Br J Psychiatry 1982; 140:566–572Crossref, Medline, Google Scholar
26. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM: Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
27. Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo J-M, Brun A, Hofman A, Moody DM, O’Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Berejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmenn A, Scheinberg P: Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993; 43:250–260Crossref, Medline, Google Scholar
28. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G: “Sniffin’ Sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory thresholds. Chem Senses 1997; 22:39–52Crossref, Medline, Google Scholar
29. Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T: Multicenter investigation of 1036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and odor thresholds. Eur Arch Otorhinolaryngol 2000; 257:205–211Crossref, Medline, Google Scholar
30. Kobal G: Pain related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain 1985; 22:151–163Crossref, Medline, Google Scholar
31. Kobal G, Hummel C: Cerebral chemosensory evoked potentials of the human nasal mucosa elicited by chemical stimulation. Electroencephalogr Clin Neurophysiol 1988; 71:241–250Crossref, Medline, Google Scholar
32. Hummel T, Kobal G: Olfactory event-related potentials, in Methods in Chemosensory Research. Edited by Simon SA, Nicolelis MAL. Washington, DC, CRC Press, 2002, pp 429–464Google Scholar
33. Kobal G, Hummel T: Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope 1998; 108:1033–1035Crossref, Medline, Google Scholar
34. Thürauf N, Hummel T, Kettenmann B, Kobal G: Nociceptive and reflexive responses recorded from the human nasal mucosa. Brain Res 1993; 629:293–299Crossref, Medline, Google Scholar
35. Evans WJ, Kobal G, Lorig T, Prah JD: Suggestions for collection and reporting of chemosensory (olfactory) event-related potentials. Chem Senses 1993; 18:751–756Crossref, Google Scholar
36. Barz S, Hummel T, Pauli E, Majer M, Lang CJG, Kobal G: Chemosensory event-related potentials in response to trigeminal and olfactory stimulation in idiopathic Parkinson’s disease. Neurology 1997; 49:1424–1431Crossref, Medline, Google Scholar
37. Kettenmann B, Hummel C, Stefan H, Kobal G: Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses 1997; 22:493–502Crossref, Medline, Google Scholar
38. Huttunen J, Kobal G, Kaukoranta E, Hari R: Cortical responses to painful CO2–stimulation of nasal mucosa: a magnetoencephelographic study in man. Electroencephalogr Clin Neurophysiol 1986; 64:347–349Crossref, Medline, Google Scholar
39. Buchsbaum MS, Kesslack JP, Lynch G, Chui H, Wu J, Sicotte N, Hazlett E, Teng E, Cotman CW: Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Arch Gen Psychiatry 1991; 48:840–847Crossref, Medline, Google Scholar
40. Schiffmann SS: Taste and smell losses in normal aging and diseases. JAMA 1997; 278:1357–1362Crossref, Medline, Google Scholar
41. Hummel T, Barz S, Pauli E, Kobal G: Chemosensory event-related potentials change with age. Electroencephalogr Clin Neurophysiol 1998; 108:208–217Crossref, Medline, Google Scholar
42. Morgan CD, Covington JW, Geisler MW, Polich J, Murphy C: Olfactory event-related potentials: older males demonstrate the greatest deficits. Electroencephalogr Clin Neurophysiol 1997; 104:351–358Crossref, Medline, Google Scholar
43. Pause B, Krauel K: Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int J Psychophysiol 2000; 36:105–122Crossref, Medline, Google Scholar
44. Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JDE: An impairment in sniffing contributes to the olfactory impairment in Parkinson’s disease. Proc Natl Acad Sci USA 2001; 98:4154–4159Crossref, Medline, Google Scholar
45. Jagust W: Untangling vascular dementia. Lancet 2001; 358:2097–2098Crossref, Medline, Google Scholar
46. McShane RH, Nagy Z, Esiri MM, King E, Joachim C, Sullivan N, Smith AD: Anosmia in dementia is associated with Lewy bodies rather than Alzheimer’s pathology. J Neurol Neurosurg Psychiatry 2001; 70:739–743Crossref, Medline, Google Scholar



