Progressive Decrease of Left Superior Temporal Gyrus Gray Matter Volume in Patients With First-Episode Schizophrenia
Abstract
OBJECTIVE: Smaller temporal lobe cortical gray matter volumes, including the left superior temporal gyrus, have been reported in magnetic resonance imaging (MRI) studies of patients with chronic schizophrenia and, more recently, in patients with first-episode schizophrenia. However, it remains unknown whether there are progressive decreases in temporal lobe cortical gray matter volumes in patients with first-episode schizophrenia and whether similarly progressive volume decreases are present in patients with affective psychosis. METHOD: High-spatial-resolution MRI scans at initial hospitalization and 1.5 years later were obtained from 13 patients with first-episode schizophrenia, 15 patients with first-episode affective psychosis (mainly manic), and 14 healthy comparison subjects. MRI volumes were calculated for gray matter of superior temporal gyrus and for the amygdala-hippocampal complex. RESULTS: Patients with first-episode schizophrenia showed significant decreases in gray matter volume over time in the left superior temporal gyrus compared with patients with first-episode affective psychosis or healthy comparison subjects. This progressive decrease was more pronounced in the posterior portion of the left superior temporal gyrus (mean=9.6%) than in the anterior portions (mean=8.4%). No group differences in the rate of change over time were present in other regions. CONCLUSIONS: These findings demonstrate a progressive volume reduction of the left posterior superior temporal gyrus gray matter in patients with first-episode schizophrenia but not in patients with first-episode affective psychosis.
Abnormalities in temporal lobe structures, including the superior temporal gyrus and amygdala-hippocampal complex, play a crucial role in dysfunction of auditory and language processing and in memory in patients with schizophrenia (1). Studies of magnetic resonance imaging (MRI) techniques have also demonstrated a gray matter volume reduction in temporal lobe regions of interest, which are associated with auditory hallucinations, thought disorder, and memory dysfunction (see reviews [2–4]).
Moreover, several investigators have also reported a volume reduction of temporal lobe gray matter regions of interest in patients with first-episode schizophrenia, including the superior temporal gyrus (5, 6) and hippocampus (6, 7). Our laboratory noted smaller left posterior superior temporal gyrus (8) and smaller left planum temporale and total (left plus right) Heschl’s gyri gray matter volumes (9) in patients with first-episode schizophrenia that were not present in patients with first-episode affective (mainly manic) psychosis. On the other hand, both groups showed smaller left-side posterior amygdala-hippocampal complex volumes (8). This different pattern of gray matter reductions supports the hypothesis that schizophrenia and affective psychoses are different disorders, although it does not rule out the possibility of epistatic or environmentally induced variations of the same genetic etiology.
As noted, our group (8) demonstrated a gray matter volume reduction of the left posterior superior temporal gyrus that was present at the first episode and specific to patients with schizophrenia. Here we evaluate progression of this abnormality over time. We note that early cross-sectional studies reported differences in the prevalence and severity of ventricular enlargement and medial temporal lobe gray matter volume reductions between patients with first-episode and patients with chronic schizophrenia (10–12), although there are as yet too few first-episode superior temporal gyrus studies to permit a similar comparison. Cross-sectional P300 event-related-potential studies have demonstrated an age-related (13) or duration-of-illness-related (14) latency prolongation in schizophrenic patients, which is compatible with a progressive process.
However, only longitudinal studies can provide definitive evidence about progression. Ideally, longitudinal studies should 1) use high-spatial-resolution MRI technology (currently 1.5-mm contiguous slices), 2) examine several regions of interest, 3) segment gray and white matter (this procedure provides the most accurate measure [3–4]), 4) start the longitudinal study group at the first episode, and 5) demonstrate specificity to schizophrenia vis-à-vis another functional psychosis with a frequently chronic course, such as manic or unipolar psychosis.
A number of investigators have pioneered longitudinal MRI studies of temporal lobe structures in schizophrenia patients (5, 15–19), although the findings have been somewhat controversial. Mathalon et al. (19) demonstrated that male patients with chronic schizophrenia exhibited more volume decline than healthy comparison subjects in bilateral posterior superior temporal gyrus gray matter. Additionally, Jacobsen et al. (17) reported that patients with childhood-onset schizophrenia showed significantly greater decreases than healthy comparison subjects in the right temporal lobe, superior temporal gyrus, and left hippocampal volumes. Other studies of patients with first-episode schizophrenia, however, indicated no faster decreases than those of healthy comparison subjects in the hippocampus (15, 18) or (unsegmented) temporal lobes (15, 16). Keshavan et al. (5) reported a reversal of volume reduction in the initial gray matter superior temporal gyrus with neuroleptic treatment after 1 year of follow-up in patients with first-episode schizophrenia. However, to our knowledge, no study to date has evaluated progressive changes in superior temporal gyrus and amygdala-hippocampal complex gray matter volumes in patients with first-episode schizophrenia as contrasted with those in patients with first-episode affective psychosis.
We here report longitudinal data indicating a progressive reduction in gray matter volume of the left posterior superior temporal gyrus in patients with schizophrenia compared with those of patients with affective psychosis and healthy comparison subjects.
Method
Subjects
Thirteen patients with first-episode schizophrenia (three women), 15 patients with first-episode affective psychosis (one woman), and 14 healthy comparison subjects (one woman) participated in this study (Table 1). The patients were recruited from the inpatients at McLean Hospital, a private psychiatric hospital affiliated with Harvard Medical School. Healthy comparison subjects were recruited through newspaper advertisement. Our earlier study of current regions of interest at baseline MRI scan (8) included 51 subjects (17 with schizophrenia, 16 with affective psychosis, and 18 healthy comparison). Twenty-six of these subjects (10 with schizophrenia, nine with affective psychosis, and seven healthy comparison) agreed to participation in a second scan and were included in the present study. The remaining 16 subjects were newly recruited. The rate of nonparticipation in the second scan (dropout rate) did not differ significantly among the groups (χ2=2.34, df=2, p=0.31). After a complete description of the study, written informed consent was obtained from all participants.
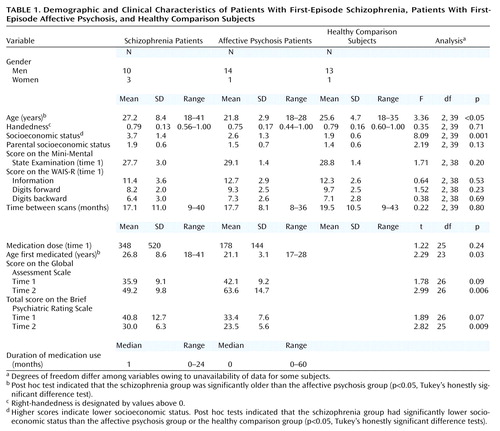
The protocols for diagnosis and clinical evaluations have been described in detail elsewhere (8, 9). Briefly, the patients and healthy comparison subjects met criteria for age (18–55 years), IQ (above 75), right-handedness (handedness score >0 on the Edinburgh Inventory [20]), and negative history for seizures, head trauma with loss of consciousness, neurological disorder, and any lifetime history of alcohol or other drug dependence. Healthy comparison subjects also had no axis I psychiatric disorder or a first-degree relative with axis I psychiatric disorder, according to the nonpatient version of the Structured Clinical Interview for DSM-IV (SCID). These criteria were applied at both the initial scan and rescan.
The patients were diagnosed on the basis of DSM-IV criteria by using the SCID interview, information from medical records, and diagnoses confirmed at the follow-up interview. Schizophrenia subtypes included 11 paranoid, one disorganized, and one undifferentiated. The affective psychosis group (all psychotic) included 13 bipolar disorder patients in a manic phase and two major depressive (unipolar) disorder patients. Omitting the two unipolar patients did not alter the statistical results. Consistent with the literature (8, 9, 15, 18), “first episode” was operationally defined as the first psychiatric hospitalization. Median duration of psychotropic medication before the MRI was short. At the time of the first scan, the patients were variously receiving typical neuroleptics (six schizophrenia and nine affective psychosis), atypical neuroleptics (two schizophrenia and five affective psychosis), or both (three schizophrenia and no affective psychosis); mood stabilizers (lithium [one schizophrenia and five affective psychosis], sodium valproate [three schizophrenia and five affective psychosis]); and only drugs other than neuroleptics or mood stabilizers (one schizophrenia and one affective psychosis), while typical/atypical neuroleptic status for one schizophrenic subject was unknown because of enrollment in a double-blind olanzapine/haloperidol crossover protocol. Between scans, hospital records and self-reports indicated that the patients were receiving neuroleptics (typical [one schizophrenia and no affective psychosis], atypical [nine schizophrenia and five affective psychosis, or both [no schizophrenia and one affective psychosis]); mood stabilizers (lithium [three schizophrenia and five affective psychosis], sodium valproate [five schizophrenia and seven affective psychosis]); and only drugs other than neuroleptics or mood stabilizers (one schizophrenia and one affective psychosis). Two schizophrenia and one affective psychosis patients had discontinued medication, while medication status for one affective psychosis subject was unknown.
Clinical evaluations both at time 1 and time 2 included the total and four syndrome factors (21) of the Brief Psychiatric Rating Scale (BPRS), the Mini-Mental State Examination (MMSE), the information and digits-forward and digits-backward subscales of the Wechsler Adult Intelligence Scale—Revised (WAIS-R), and the Global Assessment Scale (GAS) (22). The interrater reliabilities for diagnosis and clinical ratings in our laboratory settings, repeated over several different time periods, have been high (kappa>0.90).
MRI Acquisition and Processing
MRI scans were acquired with the same 1.5-T scanner (GE Medical Systems, Milwaukee) and the same acquisition protocol at time 1 and time 2. The MRI acquisition protocol and the postprocessing of images have been described in detail elsewhere (8, 9). Briefly, a 1.5-mm thickness coronal series of contiguous spoiled gradient/recall acquisition images (TR=35 msec, TE=5 msec, voxel dimensions=0.9375×0.9375×1.5 mm) was used for delineating and measuring temporal lobe regions. Second, an axial series of contiguous double-echo (proton density and T2-weighted) images (TR=3000 msec, TE=30 and 80 msec, voxel dimensions=0.9375×0.9375×3.0 mm) was used to evaluate total intracranial contents. Images were aligned by using the line between the anterior and posterior commissures and the sagittal sulcus to correct head tilt and were also resampled to make voxels isotropic (sides measured 0.9375 mm) (9). This procedure did not significantly alter the region-of-interest volumes reported in our earlier study (8), which did not realign/resample (decrease: mean=0.6%).
Regions of Interest
Temporal lobe gray matter regions of interest (the superior temporal gyrus and amygdala-hippocampal complex) were outlined manually on a workstation by a rater who was blind to knowledge of diagnosis or time of scan (initial or retest) by employing the established procedure in our previous report (8) (see Figure 1). Three raters who were blind to group membership independently drew regions of interest on five subjects, resulting in a high interrater reliability (left/right superior temporal gyrus, intraclass correlation coefficients [ICC]=0.99/0.99; left/right amygdala-hippocampal complex, ICC=0.99/0.98).
Statistical Analyses
Group differences in volume change
We evaluated change in regions of interest over time by using the percent of change as the dependent variable. Change (%) was calculated with the following formula: 100×([absolute volume at time 2]–[absolute volume at time 1]/[absolute volume at time 1]). A repeated measures analysis of variance (ANOVA) with group (schizophrenia, affective psychosis, or healthy comparison) as the between-subjects factor and region (superior temporal gyrus or amygdala-hippocampal complex) and side (left or right) as the within-subjects factors was performed. In the case of significant interactions between group, region, and hemisphere, each region was compared separately. In the case of significant group differences in the specific region of interest, we further conducted group comparison separately for the anterior and posterior subdivisions. For comparative purposes, we also used Wilcoxon’s signed rank nonparametric test for change in volume for each region of interest and group (significance level: p≤0.0048 [with Bonferroni correction, 0.05/12]).
Age, interscan interval, and intracranial contents
Neither patient group differed significantly in age from the healthy comparison group, but the schizophrenia patients were significantly older than the affective psychosis patients. The interscan interval (time between initial MRI scan and rescan) was not significantly different between groups. The statistical conclusions reported remained the same when analysis of covariance (ANCOVA) with age and/or interscan interval were adopted as covariates or when an age-matched subgroup was tested (schizophrenia subjects: N=12, affective psychosis subjects [manic only]: N=13, healthy comparison subjects: N=14). Moreover, the use of percent change per year (total percent change/[interscan interval (month)/12]) as the dependent variable did not alter the statistical conclusions (this measure produced a mean percent change for the left posterior superior temporal gyrus: schizophrenia subjects, –9.5%; affective psychosis subjects, 0.9%; healthy comparison subjects, 0.6%).
The intracranial contents volume was not significantly different between groups (repeated measures ANCOVA with age as the covariate: F=0.77, df=2, 38, p=0.47). The intracranial contents volume for all groups averaged about 0.6% smaller at time 2 than at time 1 (F=4.91, df=1, 38, p<0.04). This small change was not different among groups (F=0.95, df=2, 38, p=0.40). Additionally, intracranial contents changes were not correlated with any of the changes in regions of interest for any groups. Moreover, the statistical conclusions reported did not change when the relative volume ([absolute region of interest volume/intracranial contents]×100) was used in calculating the change nor when intracranial contents change was treated as a covariate.
Correlational analysis
Spearman’s r was used in exploratory analyses of the correlations between the change in each region of interest and initial or changes in clinical measures. Additionally, we also computed and tested averaged measures for time 1 and time 2 as a potential compensation for fluctuations in symptoms and functioning in early illness. Considering the use of multiple comparisons, we conservatively used p≤0.001 as the cutoff for statistical significance. Moreover, for any region of interest in which there was a significant group difference in change, correlations between change and interscan interval were also evaluated for each group.
Results
Volume Changes
Age, socioeconomic status, parental socioeconomic status, age first medicated, duration of medication treatment, medication dose, and intracranial contents volume at time 1 or time 2 did not correlate with any of the region-of-interest volumes at time 1 or time 2 or with the change in any of the region-of-interest volumes in either patient group.
Although Shapilo-Wilk tests indicated that percent changes in the left and right amygdala-hippocampal complex in schizophrenia patients were not normally distributed (W=0.86, df=13, p<0.05; W=0.84, df=13, p=0.002, respectively), there was no violation of sphericity for the repeated measures ANOVA as evaluated by Mauchly’s test for sphericity (Mauchly’s W=1.0). For confirmatory purposes, we also used arcsine transformations, which did not alter the statistical results described.
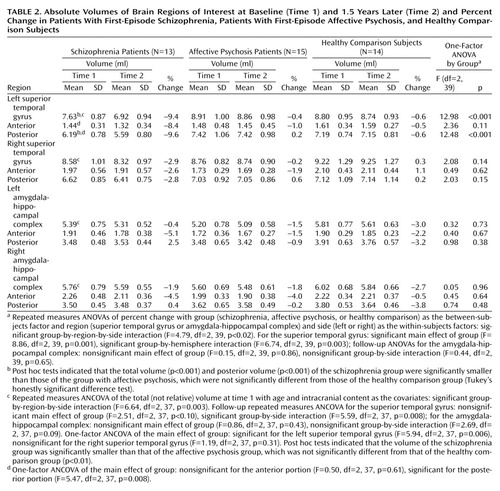
Groups were not significantly different in overall change in volume across regions (F=0.86, df=2, 39, p=0.43) (Table 2, Figure 2). However, a significant group-by-region interaction (F=4.79, df=2, 39, p<0.02) and a significant group-by-region-by-side interaction (F=4.79, df=2, 39, p<0.02) indicated that a group difference was present in at least one region. Thus, analyses were conducted separately on the superior temporal gyrus and amygdala-hippocampal complex. Groups did not differ significantly regarding the amygdala-hippocampal complex (F=0.15, df=2, 39, p=0.86), and there was no group-by-side interaction (F=0.44, df=2, 39, p=0.65).
In marked contrast, groups were significantly different regarding the superior temporal gyrus (F=8.86, df=2, 39, p=0.001), and the degree of group difference was significantly different between hemispheres (F=6.74, df=2, 39, p=0.003). We next compared the left and right superior temporal gyrus separately. Groups were significantly different regarding the left superior temporal gyrus (F=12.98, df=2, 39, p<0.001), with schizophrenia patients showing a significantly faster volume decrease than both affective psychosis patients and healthy comparison subjects (p<0.001, Tukey’s honestly significant difference test). On the other hand, groups were not different regarding the right superior temporal gyrus (F=2.08, df=2, 39, p=0.14).
To further isolate the locus of change in the superior temporal gyrus, we next performed group comparisons separately for the anterior and posterior portions of the left superior temporal gyrus using the one-factor ANOVA. Significant group differences were observed for the left posterior superior temporal gyrus (F=12.48, df=2, 39, p<0.001), with schizophrenia patients showing more change (9.6% decrease) than both affective psychosis patients and healthy comparison subjects (p<0.005, Tukey’s honestly significant difference). Group differences did not reach significance for the left anterior superior temporal gyrus (F=2.36, df=2, 39, p=0.11) (8.4% decrease for schizophrenia patients).
Wilcoxon’s signed rank nonparametric test revealed that only the left superior temporal gyrus in the schizophrenia group showed a significant volume change (z=–2.90, df=12, p=0.004; 11 of 13 patients showed decreases). Moreover, separate anterior and posterior superior temporal gyrus subdivision analyses showed that the schizophrenia patients had a significant change (decrease) in the left posterior superior temporal gyrus (z=–2.97, df=12, p=0.003; 11 of 13 had decreases) but that change in the left anterior superior temporal gyrus only approached significance (z=–1.89, df=12, p=0.06; 8 of 13 had a decrease). In contrast, eight of 15 affective psychosis subjects (z=–0.23, df=14, p=0.82) and seven of 14 healthy comparison subjects (z=–0.53, df=13, p=0.59) showed decreases in volume in the left superior temporal gyrus. For the remaining regions of interest, there were no significant changes for any of the groups (z=–1.85 to –0.23, df=12–14, p=0.06–0.86).
Correlations Between Volumes and Clinical Measures
For the patient groups, region-of-interest volume changes over time were not significantly associated with initial, average, or changes on the total or factor BPRS, the MMSE, the subscales of the WAIS-R, or the GAS. Of note, the left posterior superior temporal gyrus change did not significantly correlate with the interscan interval in the schizophrenia group (rs=0.285, N=13, p=0.35), driven by the fact that six of the eight patients who showed more than 10% decreases had a short interscan interval (9–11 months). In contrast, in the other groups, the left posterior superior temporal gyrus change was negatively correlated with interscan interval (the longer the interscan interval, the more change) in both the affective psychosis group (rs=–0.576, N=15, p<0.03) and the healthy comparison group (rs=–0.585, N=14, p<0.03), and both of these correlations were significantly different from that of the schizophrenia group (p<0.04, Fisher’s z transformation).
Discussion
To our knowledge, this is the first study to demonstrate a progressive reduction in left superior temporal gyrus gray matter volume in schizophrenia patients in the first 1.5 years after their first hospitalization. These results support the presence of a regionally selective progressive process in the pathophysiology of schizophrenia that is specific to schizophrenia patients as contrasted with patients with affective psychosis. Although posterior superior temporal gyrus—and not anterior superior temporal gyrus—change attained significance, we note it is possible that a larger study group might show anterior superior temporal gyrus significance. A conservative summary is that the change is selective to the left superior temporal gyrus relative to the right and is likely more pronounced in the posterior portions of the left superior temporal gyrus.
The present findings of superior temporal gyrus region-of-interest volumes at time 1 are in accordance with our earlier study (8), replicating the specificity of smaller left posterior superior temporal gyrus volume in schizophrenia (for results, see captions to Table 2) and showing similar results for the left posterior amygdala-hippocampal complex, although they did not reach significance. While some studies of temporal lobe morphometry in schizophrenia have not shown a left-lateralized reduction (6), in the present group of first-episode schizophrenia patients, the left posterior superior temporal gyrus not only showed a smaller volume at time 1 but also showed a 9.6% further volume reduction over 1.5 years that was also specific to schizophrenia psychosis. It is possible that the left anterior superior temporal gyrus might also show a statistically significant progressive decrease with a larger study group size or that the left anterior superior temporal gyrus might show a somewhat delayed or slower progression than the left posterior superior temporal gyrus. These possibilities could be tested in future studies with a larger group and a follow-up over a longer period of time.
The present study’s rate of 9.6% for progressive change in the left posterior superior temporal gyrus in schizophrenia patients is about twice the rate of change of about 3% per year found in chronic schizophrenia patients in the study by Mathalon et al. (19). Moreover, this large volume decrease appeared especially prominent in the first year, and the time course was significantly different compared with the affective psychosis and healthy comparison groups in which small and statistically nonsignificant volume reductions linearly increased with time. These data are compatible with a progressive process that is especially severe during the early stage of schizophrenia, while the affective psychosis and healthy comparison groups showed time-related changes in volume that might be expected in normal aging. Such a curvilinear decay of volume during the first year of overt psychosis that asymptotes within a year or two would be consistent with data showing less change over time in chronic schizophrenia (19) and also consistent with the presence of an active phase of cortical deterioration early in the disease. This hypothesis of a more severe volume reduction early in the illness, however, can only be definitively tested through further repeated scans of the present cohort and of additional subjects.
The precise neurobiological mechanism underlying this progressive, perhaps neurodegenerative, change in the left posterior superior temporal gyrus is unknown. However, there is a growing body of work implicating abnormal excitatory amino acid neurotransmission in schizophrenia, possibly mediated through a deficit in recurrent inhibition (23–25). Recent in vivo MRI spectroscopy findings have also suggested excitatory amino acid abnormalities in schizophrenia patients (26, 27). Although controversial, this mechanism could be a possible cause of ongoing, use-dependent cellular damage through excitotoxic effects, a mechanism that would strongly support the use of neuroleptic treatment to suppress overexcitation as well as psychosocial intervention. Alternately, there may be an abnormality in the normal synaptic pruning mechanism of late adolescence whereby schizophrenia patients overly reduce their dendritic arborization (28), reflected in gray matter volume reductions.
Previous findings from follow-up MRI studies evaluating temporal lobe structures in patients with first-episode schizophrenia have been controversial. For example, DeLisi et al. (15), using 5-mm-thick MRI slices with 2-mm gaps between slices, reported no volume changes over time in amygdala-hippocampal complex or temporal lobe volume. In contrast, Gur et al. (16), using 5-mm MRI slices with no gap, reported temporal lobe volume changes in both schizophrenia patients and healthy comparison subjects after a 2–3-year follow-up period. However, neither of these studies segmented gray and white matter separately, and thus a direct comparison with our study is not possible. Keshavan et al. (5), using 2.6-mm axial MRI slices with no gap, reported a reversal (9.0% increase) of superior temporal gyrus gray matter volume reduction with neuroleptic treatment after 1 year of follow-up in first-episode schizophrenia patients, while their healthy comparison subjects also had an increase of 6.9%. Although a paired t test comparing time 1 and time 2 scans was significant only in the schizophrenia group, no group-by-time ANOVA interaction was reported, and thus the possibility cannot be ruled out regarding unknown factors leading to a general increase in measured superior temporal gyrus volume in both healthy comparison and schizophrenia subjects in their study.
In this study, clinical measures were not significantly correlated with the percent change of the any of the region-of-interest volumes in either patient group. Mathalon et al. (19) reported that temporal gray matter volume decline was related to greater BPRS total and negative symptom scores in their group of chronic schizophrenia patients after a mean interscan interval of 4 years. The mean 1.5-year interscan interval in our study may not be long enough for symptom correlations to emerge, as the symptom profile of patients early in the disorder may be in flux. Further studies will be also necessary to investigate the relationship between morphological changes and auditory, language, and memory function involving temporal lobe structures.
In a follow-up MRI study of schizophrenia, the use of medicated patients inevitably raises the question of whether progressive effects are possibly due to medication or to the illness itself. While the present subject group was small, the available data may be useful to present. The percent change of left posterior superior temporal gyrus volume between schizophrenia patients who received neuroleptics between scans (N=10, change, mean=–9.3%) and those who did not (N=3, mean=–10.6%) was not statistically different (Mann-Whitney U=12.00, df=11, p=0.61). We caution that (obviously) the groups were very small and that, in this naturalistic study, it was not possible to control prescan or interscan medication type or dose or to monitor medication compliance other than through hospital records and patient accounts. Since only one subject was taking typical neuroleptics between scans, comparison with subjects taking atypical neuroleptics was not possible.
However, our use of neuroleptic-medicated patients with first-episode affective psychosis may help in disambiguating whether the progressive volume decrease in schizophrenia may be at least partly independent of medication effects. In fact, the affective psychosis patients who received neuroleptics between scans (N=6, change: mean=1.7%) and those who did not (N=8, mean=–0.9%) did not differ from the healthy comparison subjects in percent change of the left posterior superior temporal gyrus volume (affective psychosis subjects taking neuroleptics versus healthy comparison subjects: Mann-Whitney U=30.00, df=18, p=0.32: affective psychosis subjects not taking neuroleptics versus healthy comparison subjects: Mann-Whitney U=52.00, df=20, p=0.79). Moreover, these two subgroups of patients with affective psychosis were not different from each other in percent change regarding the left posterior superior temporal gyrus volume (Mann-Whitney U=14.00, df=12, p=0.20). This argues against a single determinative effect of neuroleptics on gray matter volume. While mood stabilizers, especially lithium, are another potential source of medication effects (29), exclusion of the eight patients receiving lithium between scans did not alter the statistical conclusion reported here.
Regarding the dropout rate, out of the 51 subjects reported in our earlier study (8), more than half (N=26) had rescans used in the present study, and of importance, the dropout rate did not differ significantly among groups. Nor was the dropout rate significantly different among subjects above or below the median value of the left posterior superior temporal gyrus volume in the Hirayasu et al. study (8) for either the schizophrenia, affective psychosis, or healthy comparison groups (p=1.00, p=0.62, p=0.34, respectively, Fisher’s exact test). Additionally, we note that the region-of-interest volume profile of the subjects in the earlier study was almost exactly replicated in the present study, further suggesting the absence of selection bias in the subjects receiving two scans.
In conclusion, the left posterior superior temporal gyrus gray matter volume reduction over time in our group of subjects with first-episode schizophrenia—but not in the group with first-episode affective psychosis—suggests that a progressive process in this specific brain region and in the early stage of the illness may play a crucial role in the pathophysiology of schizophrenia.
 |
 |
Presented in part at the 39th annual meeting of the Society for Psychophysiological Research, Granada, Spain, Oct. 6–10, 1999, and the 38th annual meeting of the American College of Neuropsychopharmacology, Acapulco, Dec. 12–16, 1999. Received July 20, 2001; revision received May 9, 2002; accepted July 18, 2002. From the Laboratory of Neuroscience, Clinical Neuroscience Division, Department of Psychiatry, Boston VA Healthcare System, Brockton Division, Harvard Medical School; the Surgical Planning Laboratory, MRI Division, Brigham and Women’s Hospital, Boston; the Department of Radiology, Harvard Medical School, Boston; and the Cognitive Neuroscience Laboratory, McLean Hospital, Belmont, Mass. Address reprint requests to Dr. McCarley, Department of Psychiatry (116A), Boston VA Healthcare System, Brockton Division, Harvard Medical School, 940 Belmont St., Brockton, MA 02301; [email protected] (e-mail). Supported in part by Department of Veterans Affairs Merit Awards (to Drs. Shenton and McCarley), a Middleton Award from the Department of Veterans Affairs (to Dr. McCarley), NIMH grants (MH-01110 and MH-50747 to Dr. Shenton, MH-40799 to Dr. McCarley, RR-11747 from the Division of Research Resources to Dr. Kikinis, and IP41PR13218 to Dr. Jolesz ), the Welfide Medicinal Research Foundation, Japan (to Dr. Kasai), and the Uehara Memorial Foundation, Japan (to Dr. Kasai). The authors thank Marie Fairbanks for administrative support, Elizabeth David for research assistance, and Dr. Paul G. Nestor for advice on data analysis.
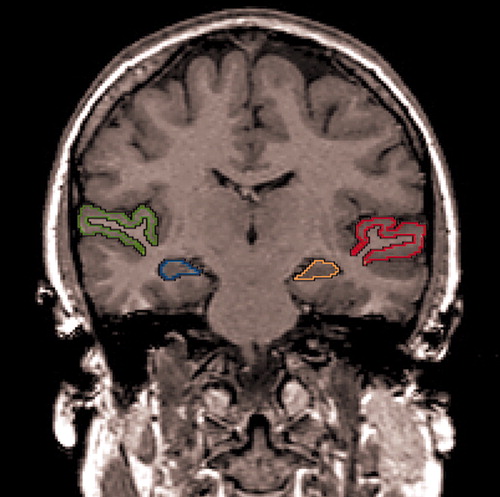
Figure 1. Delineation of the Posterior Superior Temporal Gyrus and Amygdala-Hippocampal Complex Regions of Interest in a Coronal Brain Slicea
aThe gray matter of the superior temporal gyrus is labeled red on the subject’s left and green on the subject’s right. The gray matter of the amygdala-hippocampal complex is orange on the subject’s left and blue on the subject’s right.
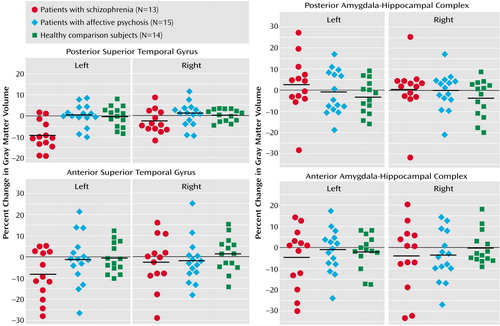
Figure 2. Percent Change Over 1.5 Years in Absolute Volumes of Regions of Interest in the Superior Temporal Gyrus and Medial Temporal Lobe Structures in Patients With First-Episode Schizophrenia, Patients With First-Episode Affective Psychosis, and Healthy Comparison Subjectsa
aHeavy black lines indicate means.
1. Blakemore SJ, Frith CD: Functional neuroimaging studies of schizophrenia, in Brain Mapping: The Disorders. Edited by Mazziotta JC, Toga AW, Frackowiak RSJ. San Diego, Academic Press, 2000, pp 523-544Google Scholar
2. Pearlson GD: Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1203-1229Crossref, Medline, Google Scholar
3. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099-1119Crossref, Medline, Google Scholar
4. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:149-200Crossref, Google Scholar
5. Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW: Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998; 32:161-167Crossref, Medline, Google Scholar
6. Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC: Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57:769-775Crossref, Medline, Google Scholar
7. Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D: Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:133-141Crossref, Medline, Google Scholar
8. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley R: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384-1391Link, Google Scholar
9. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 2000; 57:692-699Crossref, Medline, Google Scholar
10. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first-episode schizophrenia. Psychiatry Res 1990; 35:1-13Crossref, Medline, Google Scholar
11. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236-246Crossref, Medline, Google Scholar
12. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JM, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531-537Crossref, Medline, Google Scholar
13. O’Donnell BF, Faux SF, McCarley RW, Kimble MO, Salisbury DF, Nestor PG, Kikinis R, Jolesz FA, Shenton ME: Increased rate of P300 latency prolongation with age in schizophrenia. Arch Gen Psychiatry 1995; 52:544-549Crossref, Medline, Google Scholar
14. Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A: P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry 2000; 47:413-427Crossref, Medline, Google Scholar
15. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res Neuroimaging 1997; 74:129-140Crossref, Medline, Google Scholar
16. Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC: A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 1998; 55:145-152Crossref, Medline, Google Scholar
17. Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Lenane MC, Rapoport JL: Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry 1998; 155:678-685Link, Google Scholar
18. Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R: Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psychiatry 2001; 49:487-499Crossref, Medline, Google Scholar
19. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A: Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58:148-157Crossref, Medline, Google Scholar
20. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
21. Overall JE, Hollister LE, Pichot P: Major psychiatric disorders: a four-dimensional model. Arch Gen Psychiatry 1967; 16:146-151Crossref, Medline, Google Scholar
22. Endicott J, Spitzer RL, Fleiss JL, Cohen J: The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33:766-771Crossref, Medline, Google Scholar
23. Coyle JT: The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry 1996; 3:241-253Crossref, Medline, Google Scholar
24. McCarley RW, Hsiao JK, Freedman R, Pfefferbaum A, Donchin E: Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr Bull 1996; 22:703-725Crossref, Medline, Google Scholar
25. McCarley RW, Niznikiewicz MA, Salisbury DF, Nestor PG, O’Donnell BF, Hirayasu Y, Grunze H, Greene RW, Shenton ME: Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. Eur Arch Psychiatry Clin Neurosci 1999; 249(suppl 4):69-82Google Scholar
26. Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, Canaran G, Rylett RJ, Neufeld RW: Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1997; 54:959-965Crossref, Medline, Google Scholar
27. Goff DC, Hennen J, Lyoo IK, Tsai G, Wald LL, Evins AE, Yurgelun-Todd DA, Renshaw PF: Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biol Psychiatry 2002; 51:493-497Crossref, Medline, Google Scholar
28. McGlashan TH, Hoffman RE: Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 2000; 57:637-648Crossref, Medline, Google Scholar
29. Moore GJ, Bebchuk JM, Wilds IB, Chen G, Menji HK: Lithium-induced increase in human brain grey matter. Lancet 2000; 356:1241-1242Crossref, Medline, Google Scholar



