Parental Depression: Animal Models of an Adverse Life Event
Abstract
OBJECTIVE: This article reviews findings in preclinical research on the adverse impact of parental depression on the development of offspring, with emphasis on the relevance of this research for the psychiatric care of depressed parents. METHOD: The authors reviewed literature from the last 40 years reporting laboratory animal studies pertaining to the persistent effects of parental stress and parenting deficits on neurobehavioral and neurobiological development in offspring. RESULTS: Animal studies indicate that disrupted parenting produces a persistent, deleterious biobehavioral impact on offspring. Stressors, including maternal separation, variable foraging, and a variety of prenatal maternal challenges, produce offspring behaviors reminiscent of the cardinal features of anxiety and affective disorders. The stress paradigms also uniformly produce persistent hyperresponsivity in hypothalamic-pituitary-adrenal axis activity secondary to hypersecretion of corticotropin-releasing hormone. These findings bear striking similarities to findings for stress-related illnesses in humans, including major depression. CONCLUSIONS: Data from research on animal parenting reinforce the idea that parental mental illness may pose the first adverse life event for a child. A thorough risk-benefit assessment for the psychiatric care of parents of young children must consider the impact on the infant of exposure both to treatment and to parental illness. Preclinical data regarding the risk to offspring posed by untreated parental mental illness should be incorporated into clinical decision making in the treatment of parents with mental illness.
Modern psychiatry has consistently stressed the seminal role of early childhood experiences in shaping adult behavior. It must, however, be emphasized that a child does not develop in a psychological vacuum. Freud’s Oedipal conflict (1), Bowlby’s concepts regarding attachment (2), and Klein’s notion of infant splitting of the mother (3, 4) all suggest that parent-child interaction is critical to understanding the developing human psyche. The ongoing social debate regarding the adequacy of single parenting further underscores our societal conviction that good parenting, however defined, is crucial to optimal child development.
The children of depressed parents unfortunately are often ill-adapted to meet the myriad challenges posed by the modern world. They are more likely to experience psychiatric illness (5–9), with depressed adolescents being three times more likely than nondepressed adolescents to have had a depressed parent (10). Furthermore, the children of depressed parents experienced greater social, educational, behavioral, and vocational difficulties (7, 11, 12). Although this familial risk is in part genetically transmitted, other potential pathways of transmission include: 1) exposure to prenatal maternal neuroendocrine aberrations associated with depression during pregnancy, 2) exposure to heightened intrafamilial stress when a parent is depressed, and 3) exposure to the affective, behavioral, and cognitive alterations of the depressed parent (13).
Considerable attention has been focused on the impact of depression on parenting behavior. For example, a recent meta-analysis of 46 observational studies of depressed mothers demonstrated a moderate association of maternal depression with negative (i.e., hostile, coercive) parenting behaviors and disengaged parenting behaviors (14). Despite social changes leading to increased paternal participation in child rearing, there has been little investigation of the impact of depression on the parenting behaviors of fathers. One recent study did indicate that families with a depressed father are less likely to sustain positive communication (e.g., approval, assent, humor) than families with a depressed mother (15). Conversely, another study suggested that depressed fathers are less likely than depressed mothers to engage in negative parenting behaviors; however, it remains unclear whether this is a consequence of gender-specific differences in the manner of coping with depressive illness or the less taxing demands of parenting placed on fathers, who more commonly serve as secondary caregivers (16).
Alterations in the parenting behaviors of depressed parents may in turn adversely impact the psychosocial and neurobiological development of their children. A meta-analysis of the association between maternal mental health and the security of infant attachment, encompassing 35 studies and more than 200 mother-infant dyads, concluded that maternal depression, maternal stress, and an unsupportive partner are all associated with a greater risk of insecure infant attachment (17). The authors noted, however, that the heterogeneous distribution of the observed effects suggested that other as yet unidentified contextual factors help determine the probability that the child of a depressed mother develops an insecure attachment.
A few studies have demonstrated that neurobiological alterations are also evident in the children of depressed parents. For example, in two recent studies the preschool-age children of depressed mothers exhibited electroencephalographic alterations in frontal lobe activity that correlated with diminished empathy and other behavioral problems (18, 19). Neuroendocrine function may also be altered, as one study demonstrated higher serum cortisol concentration in the children of depressed mothers that was correlated with the severity of maternal depression (20).
Maternal depression during pregnancy also has potentially deleterious consequences for infant development. The newborns of mothers depressed during pregnancy have exhibited poorer motor skills during neurological examination (21). In addition, depression-associated changes in maternal hypothalamic-pituitary-adrenal (HPA) axis function during pregnancy have been shown to be predictive of similar biological alterations in newborn offspring (22). At 6 months of age, the children of euthymic mothers who had been depressed during pregnancy continued to exhibit heightened salivary cortisol responses to a standardized psychosocial stressor (23).
In summary, parental depression may represent a significant early adverse life event for the developing child. Improving our understanding of the mechanisms whereby parental depression conveys risk to the child is critical to the development of therapeutic guidelines for depressed parents. The progress of this research is hampered by the understandable ethical and logistical complexities of conducting neurobiological research involving at-risk children, but the extant preclinical literature contains a wealth of information, unfamiliar to many clinical psychiatrists, that may hasten our understanding of these processes. A variety of animal research models have been devised to investigate the impact of early life stress on development. Although early animal models of stress have most often been cited in the psychiatric literature for their similarities to the impact of childhood trauma (e.g., child abuse, exposure to war), a subset of these research models conferred stress on the young animal by interfering with parental care. Such animal models consequently may be relevant to a consideration of human conditions such as depression that hinder parenting efforts. This review summarizes the laboratory animal data paralleling the common clinical dilemma of parental depression and its potentially deleterious impact on child development.
Defining and Validating Animal Models of Human Behavior
The continuum of animal models used in psychiatric research ranges from animal assays to homologous models (24, 25). Animal assays provide an expeditious preclinical means to screen potential psychotropic medicines by instituting specific behavioral assessments after the administration of a candidate compound. Because the target animal behaviors need not be similar to any human parallel, animal assays have limited utility for the study of disease etiopathogenesis. Homologous models are instead predicated on the conviction that certain aspects of animal behavior and physiology mirror their human counterparts by virtue of a common evolutionary ancestry. The animal behaviors of interest are not simply analogous (i.e., mimicking a human behavior) but homologous (i.e., resembling a human behavior because they serve a similar function and are activated by similar biological processes). Homologous models therefore permit testing of hypotheses regarding human behavior at various levels within the phylogenetic order.
In a homologous model, the test animal is exposed to a laboratory stimulus that reliably induces a state approximating one or more attributes of some human condition. The stimulus that induces the homologous state may be either a biological intervention (e.g., pharmacological probe, surgical procedure) or an environmental manipulation (e.g., thermal extreme, psychosocial deprivation). When an environmental stressor is used as the laboratory stimulus, the similarity of both the stressor and its outcomes are critical to any valid application of the model to an understanding of human behavior.
Numerous sets of criteria have been developed for evaluating animal models. In one of the simplest and most widely referenced, McKinney and Bunney (26) contended that the validity of an animal model is contingent on its reproducibility (i.e., reliability) and its accuracy in portraying the phenomenology and treatment response of a corresponding human illness. In the context of psychiatric research, in which the nosology is predicated not on disease causality but on a descriptive phenomenology of illness, this emphasis on empirical evidence has proven most welcome.
More stringent criteria proposed by Willner (27) require that animal models exhibit face, predictive, and construct validity. Face validity refers to the phenomenological similarity between an animal model and a human syndrome. It should be remembered that behaviors may be analogous without being homologous. For example, certain avian species commonly kill their young for brood reduction during periods of nutritional stress. Although this avian behavior bears certain empirical similarities to human child abuse, few would argue that it is a relevant model for studying human infanticide. Predictive validity refers to the accuracy of a model in forecasting the course and outcome of a human syndrome. It has been argued that predictive validity is the only scientifically meaningful criterion by which to evaluate an animal model (25). Finally, construct validity represents the degree to which both the human syndrome and the animal model are unambiguously defined such that a rational theory can be constructed to explain and test theories regarding the etiopathogenesis of the disorder. It is ultimately the presence of homology that constitutes the construct validity of a purportedly homologous animal model.
Although there is widespread agreement that construct validity constitutes the defining property of a test, neither psychosocial nor neurobiological research has successfully met Willner’s call for construct validity. Because the pathophysiology of mental disorders remains obscure, the homology of an animal model to a human psychiatric condition cannot be absolutely demonstrated. An animal model’s homology can nevertheless be inferred, in the absence of a conclusive determination of construct validity, when evidence from the model’s face and predictive validity is consistent with a rational theoretical construct explaining how the model reflects a human condition.
Parenting and the Diathesis-Stress Model
Stress plays a pivotal role in the pathogenesis of many psychiatric (28) and medical illnesses (29–32). Individuals, however, display a heterogeneous susceptibility to the effects of stress. Whereas some are especially resilient, others demonstrate an endogenous vulnerability toward stress-induced illness. It is this predisposition to illness, or diathesis, that provides the foundation for the diathesis-stress model (33–37).
The diathesis-stress model has been applied to a host of psychiatric and medical illnesses. This model has also been applied to our understanding of parenting styles (38) and to the development of aberrant parenting behaviors (39). But what is the source of the diathesis? One contentious area of deliberation has been the relative contribution of inheritance and environment, the so-called nature-versus-nurture debate. Such arguments have historically been couched in absolute terms, but the diathesis-stress model permits a more balanced consideration. Because inherited and acquired factors contribute to vulnerability, this model becomes a useful structure for organizing concepts regarding the mechanisms by which parents convey the risk for illness to their offspring. The parental contribution to the vulnerability of offspring to psychiatric illness may occur through numerous pathways, most notably genetic transmission and altered parenting. Theoretically, the predisposition to illness should be demonstrable by using both psychological and biological measures. It is this conviction that the diathesis for illness may be measurable that underlies many applications of animal models to the study of human behavior.
Despite the absence of a definitive conclusion regarding their validity, homologous animal models do offer a means to explore the diathesis-stress model. Both inherited and acquired vulnerability to experimentally induced stress have been demonstrated by using animal models. The genetic diathesis has largely been demonstrated not by design but inadvertently as researchers have endeavored through selective breeding to develop animal strains that either more reliably or more robustly exhibit the target signs and symptoms resembling a human syndrome. The success, for example, in breeding animals that are more susceptible to stress-related behavioral alterations illustrates the contribution of genetic inheritance to the vulnerability to stress-related illness.
Animal models have also contributed to our understanding of environmentally acquired vulnerability to disease. Stress sensitization models have been used to demonstrate that exposure to one stressor can alter the response to subsequent milder stress exposures. For example, in a predator exposure model, rats placed briefly in a small enclosure with a cat later demonstrated increased anxiety-like behaviors that were detectable long after the initial exposure (40, 41). Similarly, animals exposed to a foot shock and then repeatedly introduced to the setting of that initial stressor developed an exaggerated acoustic startle (42). One frequent application of animal models relevant to the diathesis-stress model has been to study the biobehavioral sequelae of early life stress. In some instances, these models simply expose young animals to nonspecific noxious stimuli such as foot shock or restraint. Models of early adverse experiences have recently evolved to utilize psychosocial stressors. These models typically stress the young animal by modulating parent-offspring interaction and thereby reflect not only early life stress but more specifically conditions such as parental depression that hinder parenting.
In assessing the validity of such animal models, it is critical to establish an operational definition of the comparative human experience. As mentioned previously, the homology applies not only to parallels in early life stress but also to long-term outcome. The most extensively studied psychiatric vulnerability arising in the children of depressed parents has been for subsequent depression. Hence, the predictive validity of homologous animal models of parental depression ultimately depends on the accuracy of these models in recreating the persistent biobehavioral changes underlying a diathesis for depression.
Regarding the neurobiology of depression, existing research has emphasized monoamine neurotransmitter systems and the HPA axis. The classic HPA axis stress response commences with increases in hypothalamic corticotropin-releasing hormone (CRH) secretion from nerve terminals in the median eminence that in turn induce pituitary release of ACTH and ultimately increased adrenal secretion of cortisol. The role of HPA axis alterations in depression has been studied for more than 40 years, since it was first reported that depressed patients have increased concentrations of circulating adrenocortical hormones (43, 44). Cortisol nonsuppression in the dexamethasone suppression test reliably demonstrates an association between severity of depression and HPA axis hyperactivity, although clinical utility of such findings is limited because of poor sensitivity and specificity (45–48). Numerous lines of evidence, including elevated concentrations of CRH in CSF (49–53), blunted ACTH responses to administration of exogenous CRH (54–58), and heightened CRH mRNA expression in the hypothalamus (59), have indicated that CRH hypersecretion likely underlies the pathogenesis of HPA axis derangements in depression. More illuminating is the finding that CRH neurons reside in extrahypothalamic regions of the central nervous system (CNS) and may mediate the neurovegetative symptoms of depression via limbic projections (60). Though the neurocircuitry of central CRH alterations during depression remains obscure, it can now be stated with confidence that depression is commonly associated with both CRH hypersecretion and hypercortisolemia, with the added aberration of dysfunctional HPA axis negative feedback (36, 60–63).
Monoamine neurotransmitters, particularly norepinephrine and serotonin (5-HT), have likewise garnered considerable scrutiny in studies of the neurobiology of depression. Noradrenergic systems, once postulated to be central to the pathophysiology of depression (64), have produced largely inconsistent results in the intervening years. Long-standing psychophysiological research has nonetheless repeatedly demonstrated physical responses to stress suggestive of sympathetic activation, including tachycardia, hypertension, diaphoresis, dizziness, heightened skin temperature and skin conductance, and increased electromyographic activity. It now appears unlikely that alterations in central norepinephrine activity are necessary to the pathophysiology of depression; however, there remains substantial evidence that dysregulation of locus ceruleus norepinephrine systems contributes to the elaboration of numerous depressive symptoms (65). Some theories have implicated a central role for 5-HT dysfunction in depression (66). The widespread utility of serotonergic antidepressants lends considerable credence to such hypotheses. The identification of numerous anatomical and functional interfaces between CRH and 5-HT systems in the CNS (36, 67) has provided a theoretical framework for understanding the neurobiology of depression that is only now beginning to be explored.
It must be acknowledged that the psychophysiological and peripheral neuroendocrine correlates of depression provide no direct explanation of disease etiopathogenesis. Such measures have nonetheless been useful because they use minimally invasive procedures to obtain a peripheral window into higher cortical functioning (63, 68). Consequently, studies of HPA axis dysfunction do not directly explicate the pathophysiology of depression but nevertheless offer important insights leading the elucidation of comprehensive theories regarding the primary role of the dysfunction of cortical CRH systems (60, 69, 70).
Comparing the biobehavioral similarities of animal studies to parallel indices in human depression research thus becomes the basis for assessing the valid application of animal investigations to the consequences of parental depression on child development. Our focus is therefore on identifying animal behaviors and peripheral neurobiological alterations that resemble the current clinical findings in depression and the children of depressed parents.
Homologous Models of Parental Depression and Child Development
Neonatal Stress Models
Noxious stimuli
One approach to studying early life stress by using animal models has been to implement stressors akin to those experienced by adult animals. In so doing, researchers expose young animals to noxious stimuli, including thermal extremes, pinprick, foot shock, or surgical procedures.
Despite clear evidence of the presence of CRH-containing neurons in the fetal rat (71), noxious stimuli such as these evoke only a subnormal HPA axis response during the first 2 weeks of life (72, 73). During this so-called stress hyporesponsive period, baseline plasma corticosterone concentrations are lower than normal (74) and are only minimally increased by exposure to a noxious stressor (74–76). Stress-induced CRH and ACTH responses are also attenuated during the stress hyporesponsive period (77–79). The HPA axis is not a static but a dynamic system that at least in the rat continues to undergo maturational adaptations postnatally. The failure to mount a full stress response during early development may increase the young rat’s vulnerability, but this may serve as an adaptive evolutionary trade-off, protecting the growing animal from the catabolic effects of glucocorticoid hypersecretion. There is no evidence that a similar stress hyporesponsive period exists in the human neonate. This may, of course, be related to the fact that the CNS of the newborn rat pup is developmentally similar to that of a 24-week human fetus (80). Thus, if a stress hyporesponsive period exists in humans, it may occur prenatally.
Although studies utilizing the exposure of neonates to noxious stimuli have limited face validity in addressing the issue of parenting, and in fact even fail to provide predictive validity for the expected neurobiological stress responses, these studies do illustrate the dynamic nature of the stress response. If HPA axis responsivity during childhood is modulated by programmed maturational processes, then this stress-responsive biological system may serve as a template for the environmental contribution of early life stress on the diathesis for subsequent illness.
Brief neonatal handling in rodents
Another applicable line of animal research has used brief neonatal handling of rodents as the initial stressor. In this paradigm, rodent pups are removed daily from their cages and handled briefly by laboratory personnel. The biobehavioral responses of handled and nonhandled pups to postweaning stress are then examined to determine the persistent effects of the handling exposure.
The expectation that neonatal handling would increase anxiety and biological measures of stress responsivity in future novel situations has not been realized. In fact, neonatal handling has been found paradoxically to decrease anxiety-like behaviors and biological stress responsivity. Handled pups are resistant to stress and aging decrements in learning ability (81, 82), exhibit higher levels of exploratory behavior in novel environments (83, 84), and demonstrate an attenuated activation of neuronal fear circuitry in response to stress during adulthood (85).
Neonatal handling produces transient norepinephrine circuit hyperactivity (86) but surprisingly leads to a decreased norepinephrine response to stress during adulthood that appears to be a consequence of an up-regulation of α2 autoreceptors in the locus ceruleus (87). This paradigm also produces persistent increases in the CNS activity of γ-aminobutyric acid (GABA) (88), 5-HT (89, 90), and endogenous opioids (91–94). Undoubtedly, however, the most intriguing findings are decreases in HPA axis activity induced by neonatal handling. Early studies demonstrated lower ACTH (95) and corticosterone (96, 97) responses to subsequent stressors. Neonatal handling also produces reductions in basal circulating levels of ACTH and corticosterone (98). These diminished peripheral measures of HPA axis activity are, at least in part, a consequence of decreased CRH secretion as evidenced by lower median eminence concentrations of CRH, decreased expression of hypothalamic CRH mRNA (95, 99), and diminished CRH release in response to subsequent stress (99, 100).
Two lines of evidence indicate that brief neonatal handling may also induce an increased sensitivity to HPA axis negative feedback. Specifically, handled pups demonstrated enhanced dexamethasone suppression of corticosterone and a more rapid return of corticosterone to baseline measures after stress exposure (101). This enhanced sensitivity to negative feedback is likely a consequence of glucocorticoid receptor up-regulation at the hippocampus and other sites (101–105).
Neonatal handling studies have indicated that subtle environmental variations can affect the ontogeny of biological systems but on first review may not be directly applicable to a study of the impact of stress on parenting. How are we to understand the effects of neonatal handling? In fact, neonatal handling so improves the rat’s adaptability to subsequent stress that one study implemented “nonhandling” as an early life stressor (106). One potential explanation is that rodent pups do not experience handling as a particularly unpleasant stimulus. Although handling introduces an environmental change, it is not empirically noxious. Early exposure to novel nonnoxious environmental events such as handling may instead improve the animal’s adaptability to subsequent environmental changes. These studies may simply represent a variant of other early life stress paradigms and are otherwise irrelevant to our consideration of parental depression.
However, it has been observed that neonatal handling of rodent pups stimulates maternal grooming of pups (97, 107–110). The biobehavioral changes induced by neonatal handling may thus be mediated indirectly through changes in maternal care. On closer inspection, neonatal handling studies may in fact be relevant to the study of parenting in humans. This hypothesis has been supported by work that has documented that increased maternal grooming (apart from a handling paradigm) produces similar down-regulation of HPA axis responsivity to stress (111) and, in fact, increases hippocampal synaptogenesis (112). However, although increased maternal care may be one mechanism by which handling reduces HPA axis responsivity, it may not be the only or even a necessary mechanism for the oft-reported handling effect (113). In summary, with respect to parenting, the rodent neonatal handling paradigm bears face and predictive validity not for parental depression but for the beneficial effects of attentive parenting.
Maternal deprivation in rodents
In contrast to the previous paradigms, maternal deprivation protocols represent overt models of disrupted parenting. In these models, rat pups are separated from their mother for prescribed interval(s) berfore weaning. The pups are not only deprived of maternal care during the separation, but maternal behavior typically remains aberrant even after reunion (114), thereby exhibiting greater face validity in relation to the behavior of depressed parents than the earlier paradigms. This parallel to the human condition is underscored by Bowlby’s early application of the term “maternal deprivation” to the impact of mental illness on maternal behavior (115).
Maternal separation in the rat potentiated behavioral changes resembling depression and anxiety (116, 117). Maternally separated female offspring were also more likely to display aberrant maternal care with their own litters (118, 119), although not all studies have demonstrated this finding (120). Furthermore, maternal separation induced acute changes in HPA axis activity, as indicated by increases in serum corticosterone (121, 122) and ACTH concentrations (79). CRH secretion was also altered by maternal deprivation, but the pattern of alteration depended to some extent on the timing of the separation. For example, maternal separation at postnatal day 10 resulted in decreased CRH concentrations in the median eminence but no change in the density of pituitary CRH receptors (123). The decrease in median eminence CRH was presumably due to depletion of CRH stores from nerve terminals at that site, and the absence of any alteration in pituitary CRH receptors could be attributable to the immaturity of that system. By postnatal day 18, maternal separation produced a down-regulation of pituitary CRH receptors (presumably a consequence of CRH hypersecretion) associated with no changes in CRH concentrations in the median eminence, presumably a consequence of the further maturation of the system resulting in an increased rate of CRH synthesis (123).
The neurobiological changes induced by maternal deprivation are also long lasting, but the timing of the separation appears to modify the specific biological sequelae. For example, repeated separation of 4–6 hours per day on postnatal days 6–20 produced increased ACTH responses but normal corticosterone responses to subsequent stress in adulthood (124). By contrast, a single 24-hour separation beginning on postnatal day 3 produced a normal ACTH response but an increased corticosterone response to subsequent stress. Separation beginning on postnatal day 11 induced a normal corticosterone but a blunted ACTH response to stress (125, 126). Taken in isolation, the specific significance of these findings may be unclear, but overall they demonstrate that interruption in maternal care during a period of rapid CNS development may produce lasting neurobiological alterations.
CRH hypersecretion likely underlies the persistent HPA axis alterations associated with early maternal deprivation. Maternally deprived adult rats exhibited increases in hypothalamic expression of CRH mRNA in the paraventricular nucleus, increased CRH concentrations in the median eminence, and dexamethasone nonsuppression (124–127). Diminished elaboration of glucocorticoid receptors in the CNS of maternally deprived rats may underlie alterations not only within the HPA axis but also within noradrenergic systems emanating from the locus ceruleus (128).
One rat study compared the impact of maternal deprivation on two rat strains: one bred for susceptibility to the inescapable shock (“learned helplessness”) paradigm and another bred for resistance to inescapable shock (129). When the two rat strains were exposed to maternal deprivation and a subsequent stressor, HPA axis stress responses in the offspring were markedly dissimilar. The stress-susceptible group exhibited an increased ACTH response, but a normal cortisol response, to stress at postnatal day 21, a pattern similar to the HPA axis changes associated with posttraumatic stress disorder (PTSD) (130–132). The stress-resistant line did not demonstrate the exaggerated ACTH response to stress. This study provided an excellent example of the potential relative genetic and acquired contributions to the vulnerability for illness.
The genesis of the effects of maternal deprivation on HPA axis activity has been the subject of some debate. The stress experienced by the maternally deprived rat pup may result from either the absence of maternal tactile stimulation or secondary food deprivation (133). A study designed to address this issue isolated the effects of food intake and tactile stimulation (134). Providing surrogate maternal care (e.g., stroking the anogenital region with a warm brush) reversed the effects of maternal separation on CRH type 2 receptor mRNA expression in the hypothalamus. However, neither food supplementation nor contact with a sedated or nonlactating mother reversed this effect, suggesting that alterations in maternal care underlie the biological changes induced by maternal separation in the neonate.
Maternally separated rat pups also demonstrated alterations in the function of endogenous opioids. This is likely a byproduct of hypothalamic CRH hypersecretion that induces pituitary secretion of proopiomelanocortin, a prohormone that is not only a precursor of ACTH but also of melatonin and certain endogenous opioids. In studies that used a hotplate for nociception testing, maternally separated rat pups demonstrated increased response latencies believed to be a consequence of the analgesic effects of elevated concentrations of endogenous opioids (135). This effect was potentiated by morphine and blocked by pretreatment with the opioid antagonist naloxone (135). A recent study indicated that adult male rats subjected to repeated maternal separation during the first 2 weeks of life exhibit diminished morphine antinociception during testing with a hotplate (136). Since the effect was not witnessed in adult female rats, this study raises interesting questions regarding the gender-specific effects of diminished maternal care on the maturation of opioid systems.
A limited subset of rodent studies has investigated the effects of maternal deprivation on other neurotransmitter systems. Although there is some inconsistency in the data, numerous studies have suggested that the activity of the principal inhibitory CNS neurotransmitters is diminished in adult rats previously exposed to maternal separation. For example, maternally deprived adult rats in novel situations exhibited heightened anxiety-like behaviors and showed evidence of reduced GABA activity, including decreased GABAA binding, and decreased levels of CNS benzodiazepine type I receptors (137). These findings contrast with earlier findings that the offspring of attentive female rats who more frequently groomed their young showed less fearfulness in novel situations and increased CNS levels of benzodiazepine type 1 receptors (138). Surprisingly little attention has been devoted to the effect of rodent maternal deprivation on the ontogeny of central 5-HT systems. Maternal deprivation has been associated with decreased 5-HT concentrations in the dorsal hippocampus and prefrontal cortex of offspring (139), but this finding is in contrast to other results demonstrating no alterations in the density of 5-HT fibers in the prelimbic, anterior cingulate, and precentral medial cortices of maternally deprived rats (140). Although only tangentially relevant to animal modeling of parenting, postweaning social isolation of young rats has been shown to decrease the serotonergic innervation of the hippocampus (141) but to have no impact on 5-HT concentrations in the hedonic circuitry innervating the nucleus accumbens (142). To our knowledge, only one rodent maternal deprivation study has examined the function of neuropeptide Y, another transmitter with putative inhibitory activity at the locus ceruleus (143). In that study, pups demonstrated increased expression of preproneuropeptide Y mRNA immediately after a 24-hour separation. Because the pups were also deprived of food and water during the 24-hour separation, it is difficult to ascertain whether the result was primarily an effect of nutritional deprivation or of disrupted maternal care.
Social and maternal deprivation in primates
Scrutiny of the primate social deprivation model has been quite extensive. Initial reports accentuated its value as a homologous model for depression (144). Experimental refinements in which the monkey’s mother is replaced with a series of incrementally more realistic maternal surrogates (145) have lent the model utility for investigating the consequences of disrupted parenting.
Most neurobiological research using the social deprivation model has focused on catecholamine systems. Monkeys reared with no attachment object or an inanimate surrogate exhibit depressive-like symptoms during later separations and markedly lower CSF norepinephrine concentrations (146). In most studies, although not all (147), peer-raised monkeys have exhibited less pronounced despair on separation and have shown baseline CSF norepinephrine concentrations that are intermediate between those of mother-reared monkeys and total isolates (148–150). These findings suggest an inverse relationship between maternal contact, norepinephrine neuronal activity, and later socialization. It is noteworthy that desipramine administration produced elevations in CSF norepinephrine concentrations largely consistent with its known pharmacology in both peer-raised and mother-raised monkeys (148). Differential rearing may (147) or may not (148) produce alterations in CSF concentrations of 5-HT metabolites. One intriguing study did indicate that maternally deprived monkeys with the short rhesus macaque serotonin transporter allele (rh5-HTTLPR) exhibit an exaggerated expression of the attentional and emotional alterations commonly associated with this genotype (151).
HPA axis function has also been studied in socially deprived primates. A study comparing socially isolated to peer-reared rhesus monkeys demonstrated higher basal cortisol levels in the isolates but no differences between groups in cortisol responses to a novel situation (152). Another study comparing peer-reared to mother-reared rhesus monkeys surprisingly reported higher basal concentrations of ACTH and more pronounced stress-induced increases in both ACTH and cortisol in the mother-reared offspring (153) that was not appreciably altered by antidepressant administration (154). These surprising results contrast with more recent findings of higher cortisol increases during subsequent social separation in peer-raised than in mother-raised monkeys that were correlated with higher self-administration of alcohol under typical living conditions (155).
The magnitude of social deprivation in these models extends beyond the loss of parent-child interaction. In some models, animals are deprived of an entire range of social interaction when they are removed not only from their mothers but from the social group as a whole. Therefore, findings from such research bear limited face validity to the study of parental depression (156). In designs that recognize this limitation, other studies have evaluated the acute sequelae of brief maternal separation. For example, one study examined effects of 24-hour separation by comparing four conditions: total isolation, remaining with the mother, being placed with a peer of the same group, or being placed with a peer from another group (157). Thirty minutes after separation, the isolated infants and the infants placed with a strange peer had markedly elevated cortisol concentrations; 24 hours later only the isolated infants exhibited hypercortisolemia. Thus, the presence of peer-aged conspecifics modulated the acute biological stress response.
Other studies have modified the manner of separation from the infant’s mother. A study of 24-hour separation placed infants in one of three conditions: total isolation, remaining with the mother, or separated from the mother but able to see her (158). In this study, the totally isolated infants were the least likely to vocalize but had the highest plasma cortisol concentrations; there were no detectable differences in the behavioral measures or cortisol concentrations of the other two groups. In a similar study in which infants were totally isolated, left with the mother, or placed in an adjacent cage with the mother in full view, elevations in plasma cortisol and CSF catecholamine metabolites (i.e., 3-methoxy-4-hydroxyphenylglycol, homovanillic acid) were greatest in the total isolates and at an intermediate level in the infants that were separated from but able to see the mother (159). In yet another primate study, monkeys were subjected to a 2-hour separation from their social group at the ages of 6 months and 1 year to investigate the impact of aging on the HPA axis response to separation (160). In this investigation, there were considerable individual differences in both the behavioral and cortisol responses to separation at both intervals, although the cortisol response was attenuated at 1 year of age. A recent study suggested that these alterations in HPA axis responsivity may be a consequence of changes in negative feedback mediated by the glucocorticoid receptor (161).
Variable foraging in primates
Although separation paradigms that interfere with the quality of parenting reliably induce neurobiological and behavioral alterations in both rodents and primates, these strategies depend on an experimental intervention that is disparate from normal human experience. The face validity of the research protocol may be improved by instead altering a naturalistic stressor that indirectly stresses the infant by contesting the parent’s ability to provide adequate care.
All mammalian species face the challenge of feeding their offspring. The difficulty of this task depends both on the availability of a food source and the proficiency of the parent in procuring food from that source. During periods of nutritional stress, mammalian parents may struggle heroically to feed their young (162), but they may also abandon their young (163). Incorporating this reality of survival into an experimental paradigm provides a technique for modulating parental stress and thereby investigating the adequacy of parental care.
The objective in these studies is not to subject the animals to malnutrition but to increase the effort of parenting while permitting adequate nutrition. If the model achieves this goal, then the effects of parental stress on offspring development can be ascertained without the confounding effects of aberrant nutrition. A suitable primate model has been implemented by using three foraging conditions: low foraging demand, high foraging demand, and variable foraging demand. In the low foraging demand condition, food is readily available and requires no maternal effort for procurement. By contrast, in the high foraging demand condition, mothers are required to perform a task such as digging through wood chip bedding to obtain food. In the variable foraging demand condition, the requirements for food procurement are unpredictable. In this paradigm, the high foraging demand condition serves both as a comparator state and a nutritional control.
Infants raised in the variable foraging demand condition exhibited behavioral alterations suggestive of insecure attachment that resemble anxiety in humans (164–168). As juveniles and adults, primates raised under variable foraging demand conditions exhibited persistently elevated CSF CRH (169, 170). The CRH elevations, however, were not accompanied as anticipated by elevations in cortisol. Instead, these offspring demonstrated lower CSF, and presumably plasma, cortisol concentrations than the comparator groups (169). This finding is strikingly similar to the profile of HPA axis activity reported in patients with PTSD (130–132). A follow-up study demonstrated that CSF concentrations of somatostatin, a peptide whose release has been reported to be potentiated by CRH but that itself suppresses HPA axis activity, was also elevated in adult primates raised under variable foraging demand conditions (171). Thus, hypersecretion of somatostatin is one potential explanation for the conundrum of decreased cortisol release in the face of CRH hypersecretion. In primates raised in variable foraging demand conditions, the growth hormone response to clonidine was inversely correlated with CSF CRH concentrations (172), suggesting a potential mechanism for this often reported finding in depressed patients.
The impact of variable foraging demand on biogenic amine systems has had limited investigation. CSF concentrations of 5-HT and dopamine, but not norepinephrine, metabolites were elevated in adults reared in conditions of variable foraging demand (171). The 5-HT results are consistent with previous findings of hyposensitivity to a pharmacological challenge with the 5-HT agonist m-chlorophenylpiperazine in subjects reared in variable foraging demand conditions; however, the norepinephrine findings are surprising given the behavioral sensitivity to the α2 antagonist yohimbine exhibited by variable foraging demand subjects (173).
Prenatal Stress Models
It is not surprising that many view parenting as beginning not only before birth but even before conception. For example, avian species diligently build nests before laying their eggs. Likewise, agencies such as Planned Parenthood encourage prospective parents to prepare for parenting before conception. This is an important concept because there is growing evidence that the quality of prenatal parenting has a significant impact on the well-being of the offspring.
Mental illness during pregnancy may well represent the first adverse life event for the developing child (174). For example, maternal depression during pregnancy may slow fetal growth (175, 176), increase the risk of obstetrical and postnatal complications (177–180), and precipitate long-term behavioral changes in the offspring (181, 182). These observations have tremendous implications for perinatal psychiatric practice.
A long line of animal research has investigated the effects of prenatal stress on the developing offspring. Consistent with the limited human data, data from animal studies have suggested that stress during pregnancy can adversely affect offspring growth (183–187), learning ability (188–191), and attainment of developmental milestones (192). Prenatal stress may also induce persistent behavioral aberrations. In particular, adult rats exposed to prenatal stress continued to exhibit anxiety-like behaviors in novel situations (193–198), depression-like behaviors (199), and exaggerated behavioral responses to stress (200–202). Primate studies have also demonstrated decreased exploratory behavior in prenatally stressed offspring (203, 204).
As expected, these behavioral changes have been associated with long-term neurobiological changes. Pregnant rats exposed to an uncontrollable stressor exhibited increases in basal corticosterone concentrations and decreases in corticosteroid-binding globulin (further elevating the free fraction of corticosterone) (205). Fetuses of these stressed pregnant dams also demonstrated increased concentrations of plasma corticosterone (205). Initial evidence suggested that fetal hypersecretion of CRH may underlie the increased HPA axis activity. For example, repeated but brief exposure to prenatal stress increased the expression of CRH mRNA in the paraventricular nucleus of the fetal hypothalamus; however, prolonged stress exposure induced neuronal apoptosis (206).
Prenatally induced alterations in HPA axis activity may persist into adulthood. For example, prenatally stressed adult rats demonstrated exaggerated corticosterone responses to subsequent mild stressors (202, 207–212) and showed a phase advance in the circadian rhythm of corticosterone secretion (213). Basal concentrations and stress-induced elevations of ACTH have been reported to be higher in prenatally stressed rats than in comparison animals (208, 214). These changes are accompanied by a down-regulation of glucocorticoid receptors at the hippocampus (209). The elevations in ACTH concentrations are paralleled by heightened concentrations of β-endorphin in prenatally stressed rats at 10 days of age (215). The effects of prenatal stress on the ontogeny of opioid systems may have consequences both for the behavioral responses to novelty (216) and the differentiation of sexually dimorphic behaviors (217). However, measures of CRH secretion have been inconsistent. One study of prenatally stressed adult rats did not detect any alterations in median eminence CRH concentrations (100), but another reported increased concentrations of CRH in the amygdala (218).
The mechanism by which maternal stress during pregnancy modulates the fetal HPA axis is unclear. Placental passage of maternal corticosterone during prenatal stress exposure may be one mechanism by which stress exposure during pregnancy affects the offspring. For example, eradicating maternal corticosterone via bilateral adrenalectomy before stress exposure obviated the impact of prenatal stress on neonatal hippocampal glucocorticoid receptors (219). However, the finding that neonatal adoption reversed the impact of prenatal stress on hippocampal glucocorticoid receptors suggests that the effect may be mediated through changes in postnatal maternal care (209). Consistent with the latter hypothesis is evidence that stress during pregnancy adversely affected postnatal efforts at maternal care (220, 221).
There has been relatively little study of the impact of prenatal stress on primate HPA axis activity. In one study, prenatally stressed juvenile rhesus monkeys exhibited increases in basal and stress-responsive ACTH and cortisol, compared with juveniles whose mothers were not stressed (222). Another study in which the administration of ACTH to pregnant rhesus monkeys produced developmental changes similar to other stress paradigms during pregnancy lends additional credence to the theory that HPA axis hyperactivation mediates the long-term sequelae of prenatal stress (223).
Preclinical studies have also indicated that prenatal stress affects biogenic amine systems. For example, prenatally stressed animals have exhibited alterations in the plasma concentrations of norepinephrine, dopamine, and their metabolites (212, 224–229) that were consistent with increases in the turnover of these catecholamines. Furthermore, prenatal stress has been associated with alterations in both norepinephrine (230) and dopamine receptors (231). Changes in the asymmetric patterns of dopamine neural circuitry in the CNS of prenatally stressed adult rats have also been reported (232, 233).
Prenatal stress has also been associated with long-term alterations in serotonergic activity. Decreased plasma and CNS concentrations of 5-HT and its metabolites in prenatally stressed animals have been reported (212, 234, 235). 5-HT receptor profiles in the hippocampus (234, 236) and behavioral responses to 5-HT challenge (237, 238) were also changed in adult rats subjected to prenatal stress. These changes in serotonergic function may in part be programmed by increased glucocorticoid exposure during pregnancy (239). Moreover, the effect of prenatal stress on cholinergic neurotransmission appears to be mediated by CRH activity at the hippocampus (240).
Maternal stress during pregnancy is associated with persistent behavioral and cognitive changes in both rodents and nonhuman primates, paralleling concerns arising from clinical psychiatric experience. The long-term neurobiological effects may be mediated in large part through organizational effects of maternal HPA axis activation on fetal CNS maturation (241). Although it remains unclear whether the placental passage of maternal glucocorticoids or the impact of inadequate postnatal care mediates the alterations in offspring HPA axis function, the ultimate clinical importance of these data is the same. It would seem prudent to treat perinatal maternal mental illness aggressively to circumvent the potential adverse impact of maternal illness on the developing child.
Two-Parent Models
Despite the considerable breadth of animal research investigating parental care, the study of paternal care is woefully inadequate. Throughout most modern cultures, humans are monogamous (albeit in many cases serially so) and provide biparental care (although mothers continue to provide a disproportionate share of child care). However, single-parent households are increasingly prevalent, and we have made little progress in understanding the behavioral and neurobiological consequences of single parenting. Without this knowledge, our efforts to educate and guide single parents are not evidence-based and may therefore be seriously deficient.
Two-parent animal models that incorporate paternal care and consider the impact of its absence are sorely needed. Unfortunately, nonhuman primate research has provided little insight. Group-living primates typically are not monogamous; therefore, male primates have little assurance of paternity and from an evolutionary standpoint little incentive to provide parental care. In fact, adult male primates of several species interact no more often with their own offspring than with other infants (242–244). Adult male primates appear to initiate interaction with infants, not in an effort to provide care for their own offspring or even to curry favor with potential mates, but instead as part of a triadic interaction to buffer tensions with other adult males (243). Numerous avian species mate for life and provide biparental care, but the complex neurobiological and behavioral issues requiring study are not likely to be addressed adequately in a nonmammalian species.
Fortunately, a small number of monogamous mammalian species that provide biparental care are being studied. In particular, rodent species such as the prairie and meadow voles, Siberian and Djungarian dwarf hamsters, and California mice have been studied. Most of this research has scrutinized the neurobiology underlying the affiliative behaviors of these species. There is clear evidence that oxytocin stimulates maternal behavior, mating activity in males, and infant attachment behaviors in these highly social creatures (245, 246). A limited subset of these investigations have studied the determinants of paternal care and the impact on the offspring when paternal care is unavailable.
Research with voles has demonstrated that the father and the mother demonstrate distinct patterns of interaction with their young that suggest unique parental roles (247). Furthermore, the presence of the father during infancy has a positive effect on the subsequent paternal behavior of male offspring. Juvenile male voles allowed to remain in the nest when a subsequent litter is delivered were more likely to provide alloparental care if their fathers were present during upbringing (248). Similarly, paternal care was more readily initiated by adult male voles reared as neonates with their fathers present rather than with unfamiliar males (249). However, exposure to pheromones of the mate also facilitated the initiation of paternal care (250). In addition, continued exposure to pups eventually induced paternal care even in voles raised without a father (249, 250).
The success of another species, the Djungarian dwarf hamster, appears to be particularly dependent on the participation of both parents in rearing the offspring. Pup survival was compromised by the removal of the father (251). The introduction of a second unmated female to the nest did not compensate for the father’s absence (252).
The biological substrate for paternal behavior in these species remains unclear. It is unlikely to be solely predicated on oxytocin. In males of numerous species including humans, oxytocin has been shown to potentiate sexual behavior and to facilitate the ejaculatory response (245). A study with the monogamous, biparental California mouse, which provides biparental care, has shown that plasma oxytocin remains elevated in the male after copulation and during early gestation but declines before delivery and remains low throughout lactation (253). Preliminary evidence indicated instead that vasopressin may potentiate the effects of androgens to induce paternal behavior in these rodent species (254, 255).
Although the data produced to date are limited, two-parent, monogamous animal models offer numerous advantages. They provide greater face validity for human parenting by affording an opportunity to investigate paternal care. They also furnish a means to study the impact of parenting without a father. Future studies should investigate the neurobiological effects of paternal separation not only on the developing offspring but also on the mother who remains behind to care for her litter. Unfortunately, no two-parent primate models have been identified.
Conclusions
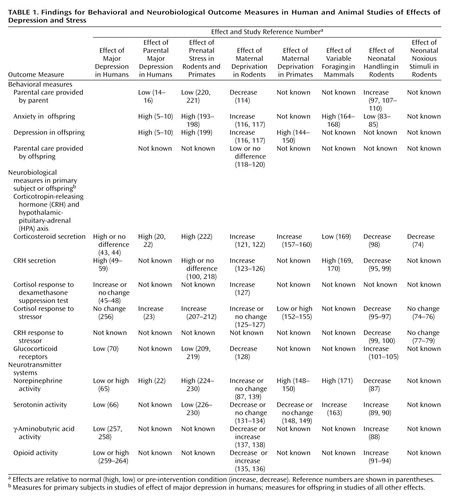
An impressive array of evidence indicates that stressful events in early life contribute significantly to the vulnerability for adulthood psychopathology (Table 1). Parental nurture helps to foster adaptive responses to early life stressors, thereby protecting the child from this vulnerability to later illness; however, parental depression may undermine this protection and occasionally is itself the source of early life stress. Increasing evidence substantiates the observation that parental depression contributes to the child’s diathesis for subsequent stress-related illness.
Although ideal parenting may be poorly defined, the animal literature indicates that deficits in parental care may lead to persistent adverse sequelae for the offspring. As animal modeling of human parental behavior is further refined, we will gain additional insights into the qualitative impact on psychiatric vulnerability in the offspring of events such as depression that interfere with parental nurture. Animal models already are an important component of psychiatric research offering several distinct advantages over human research. First, some species such as rodents have brief gestational and developmental periods, enabling researchers to study outcomes in an expeditious manner. Second, animal models permit researchers to invoke particular environmental manipulations (e.g., maternal deprivation, food supply limitation, pharmaceutical challenge) that would be impractical in naturalistic human studies. Third, invasive measurement of CNS activity is only feasible in an animal model. Despite these advantages, an animal model with poor homology to the human experience is not particularly useful. Defining and validating homologous animal models is in fact often the most challenging aspect of preclinical psychiatric research.
Homologous studies relevant to our consideration of parental depression have used both rodent and primate models. Among the existing animal paradigms, the variable foraging model demonstrates the greatest face validity for parental depression in humans. This model avoids direct interaction between the researchers and the animal offspring. Furthermore, the variable foraging model does not abolish parental care (even transiently) but introduces instead an environmental stressor that challenges the maternal capacity for caregiving. Although this more closely parallels some of the difficulties encountered by depressed parents, it must be acknowledged that the stress of a variable environmental perturbation is not fully homologous (i.e., does not bear absolute construct validity) to the presence of a stress-related illness (i.e., depression).
Both prenatal and postnatal experimental laboratory stressors that either directly or indirectly impair maternal care produce a persistent deleterious impact on offspring. By contrast, the only early life stressor studied to date that tends to bolster maternal care, i.e., neonatal rat handling, typically produces adaptive behavioral effects and reduces neurobiological sensitivity to subsequent stress in the offspring. Consequently, the neonatal stress studies exhibit predictive validity with regard to the clinical sequelae of parental depression in that they demonstrate that deficits in maternal care are associated with anxiety-like and depression-like behaviors in the offspring that commonly persist into adulthood. The predictive validity is further underscored by the fact that the stress paradigms also uniformly produce acute hyperactivity and long-term hyperresponsivity of HPA axis function. The key event in these HPA axis changes appears to be CRH hypersecretion. This pattern bears striking parallels to findings in human studies of acute stress responses and may also be germane to the pathophysiology of stress-related illnesses, including depression. In addition, CRH not only drives the endocrine stress response but also integrates immunological, autonomic, and behavioral responses. Manageable designs for multisystem stress studies are only now being contemplated.
Once appropriate homologous animal models of parental depression are identified, they should dramatically hasten the progress of this important line of research, which is currently hampered by the inherent limitations of conducting neurobiological research with children at risk. We anticipate that this research will ultimately help clarify the alterations in central CRH function that are believed to exist in those vulnerable to psychiatric illness by virtue of early adverse life events such as parental depression. Moreover, appropriate animal models will ease the implementation of multisystem research. For example, because most norepinephrine-secreting neurons emanate from a single brain stem region, the locus ceruleus, interaction between the norepinephrine system and other neurobiological systems is robust. Future research will undoubtedly investigate the homeostatic interactions between norepinephrine activity and other biological systems. The well-documented stimulatory effects of CRH (265, 266) and glutamate (267, 268), in addition to the inhibitor effects of 5-HT (269–271), GABA (272), endogenous opioids (265, 273), and neuropeptide Y (274), on locus ceruleus activity provide one potential framework for future cross-system research.
The preclinical findings regarding parenting carry profound clinical implications. First, it is imperative that we better understand the mechanisms whereby parental depression contributes to the developing child’s subsequent psychiatric vulnerability. These animal data may direct the development of pharmacological interventions for a child once an insult from parental depression has occurred. Such treatments may find utility both for children in the acute aftermath of a childhood stressor and for adult offspring with a persistent vulnerability to illness that is, at least in part, a consequence of the persistent effects of depressive illness suffered by their parents.
Second, these animal data pose a seminal clinical question relevant to the psychiatric care of parents with young children. Does parental mental illness pose the first adverse life event for a child (174)? The preclinical data have, for example, been absent in many of the proposed treatment guidelines for perinatal psychiatric illness (275–279). We contend that the clinician should consider the preclinical data regarding the risk to offspring posed by untreated parental mental illness during the child’s formative years when planning treatment for both men and women with children (280, 281). Data from animal parenting research, for reasons we have described, have helped to delineate the potential adverse consequences of parental depression and must be incorporated into rational clinical decision making in the context of parental mental illness.
 |
Received March 19, 2001; revision received Jan. 9, 2002; accepted Jan. 25, 2002. From the Department of Psychiatry and Behavioral Sciences and the Department of Gynecology and Obstetrics, Emory University School of Medicine. Address reprint requests to Dr. Newport, Women’s Mental Health Program, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 1365 Clifton Rd., Suite B6100, Atlanta, GA 30322; [email protected] (e-mail). Supported by an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr. Nemeroff) and by NIMH grants MH-42088, MH-39415, MH-58922, and MH-63507.
1. Freud S: The Complete Introductory Lectures on Psychoanalysis. Translated and edited by Strachey J. New York, WW Norton, 1966Google Scholar
2. Bowlby J: Attachment and Loss. New York, Basic Books, 1969Google Scholar
3. Klein M: Notes on some schizoid mechanisms, in Developments in Psycho-Analysis. Edited by Klein M, Heimann P, Isaacs S, Riviere J. London, Hogarth, 1952, pp 292-320Google Scholar
4. Klein M: The Psychoanalysis of Children. Translated by Strachey A. New York, Grove Press, 1960Google Scholar
5. Weissman MM, Prusoff BA, Gammon GD, Merikangas KR, Leckman JF, Kidd KK: Psychopathology in the children (ages 6-18) of depressed and normal parents. J Am Acad Child Psychiatry 1984; 23:78-84Crossref, Medline, Google Scholar
6. Anderson C, Hammen C: Psychosocial outcomes of unipolar depressed, bipolar, medically ill, and normal women: a longitudinal study. J Consult Clin Psychol 1993; 61:448-454Crossref, Medline, Google Scholar
7. Billings A, Moos R: Comparisons of children of depressed and nondepressed parents: a social-environmental perspective. J Abnorm Child Psychol 1983; 11:483-486Crossref, Google Scholar
8. Goodman S, Brogan D, Lynch M, Fielding B: Social and emotional competence in children of depressed mothers. Child Dev 1993; 64:516-531Crossref, Medline, Google Scholar
9. Hammen C, Gordon D, Burge D, Adrian C, Jaenicke C, Hiroto D: Maternal affective disorders, illness, and stress: risk for children’s psychopathology. Am J Psychiatry 1987; 144:736-741Link, Google Scholar
10. Shiner R, Marmorstein N: Family environments of adolescents with lifetime depression: associations with maternal depression history. J Am Acad Child Adolesc Psychiatry 1998; 37:1152-1160Crossref, Medline, Google Scholar
11. Beardslee W, Keller M, Klerman G: Children of parents with affective disorder. Intl J Fam Psychiatry 1985; 6:283-299Google Scholar
12. Downey G, Coyne J: Children of depressed parents: an integrative review. Psychol Bull 1990; 108:50-76Crossref, Medline, Google Scholar
13. Goodman SH, Gotlib IH: Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev 1999; 106:458-490Crossref, Medline, Google Scholar
14. Lovejoy C, Graczyk P, O’Hare E, Neuman G: Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev 2000; 20:561-592Crossref, Medline, Google Scholar
15. Jacob T, Johnson S: Sequential interactions in the parent-child communications of depressed fathers and depressed mothers. J Family Psychol 2001; 15:38-52Crossref, Medline, Google Scholar
16. Field T, Hossain Z, Malphurs J: “Depressed” fathers’ interactions with their infants. Infant Ment Health J 1999; 20:322-332Crossref, Google Scholar
17. Atkinson L, Paglia A, Coolbear J, Niccols A, Parker K, Guger S: Attachment security: a meta-analysis of maternal mental health correlates. Clin Psychol Rev 2000; 20:1019-1040Crossref, Medline, Google Scholar
18. Jones N, Field T, Davalus M: Right frontal EEG asymmetry and lack of empathy in preschool children of depressed mothers. Child Psychiatry Hum Dev 2000; 30:189-204Crossref, Medline, Google Scholar
19. Embry L, Dawson G: Disruptions in parenting behavior related to maternal depression: influences on children’s behavioral and psychological development, in Parenting and the Child’s World: Influences on Academic, Intellectual, and Social-Emotional Development. Edited by Borkowski JG, Ramey SL. Mahwah, NJ, Lawrence Erlbaum Associates, 2001, pp 203-213Google Scholar
20. Lupien S, King S, Meaney M, McEwen B: Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry 2000; 48:976-980Crossref, Medline, Google Scholar
21. Lyons-Ruth K, Wolfe R, Lyubchik A: Depression and the parenting of young children: making the case for early preventive mental health services. Harv Rev Psychiatry 2000; 8:148-153Crossref, Medline, Google Scholar
22. Lundy B, Jones N, Field T, Nearing G, Davalos M, Pietro P, Schanberg S, Kuhn C: Prenatal depression effects on neonates. Infant Behav Dev 1999; 22:119-129Crossref, Google Scholar
23. Calhoun KA, Brennan P, Walker EF, Fisher AD, Stowe ZN: Modulation of infant salivary cortisol by maternal depression, in 2001 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 2001, number 122Google Scholar
24. Weiss J, Kilts C: Animal models of depression and schizophrenia, in The American Psychiatric Press Textbook of Psychopharmacology, 2nd ed. Edited by Schatzberg A, Nemeroff C. Washington, DC, American Psychiatric Press, 1998, pp 89-132Google Scholar
25. Geyer M, Markou A: Animal models of psychiatric disorders, in Psychopharmacology: The Fourth Generation of Progress. Edited by Bloom F, Kupfer D. New York, Raven Press, 1995, pp 787-798Google Scholar
26. McKinney W, Bunney W: Animal model of depression. Arch Gen Psychiatry 1969; 21:240-248Crossref, Medline, Google Scholar
27. Willner P: The validity of the animal models of depression. Psychopharmacology (Berl) 1984; 83:1-16Crossref, Medline, Google Scholar
28. Kendler KS, Karkowski LM, Prescott CA: Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156:837-841Link, Google Scholar
29. Adler C, Hillhouse J: Stress, health, and immunity: a review of the literature, in Theory and Assessment of Stressful Life Events. Edited by Miller TW. Madison, Conn, International Universities Press, 1996, pp 109-138Google Scholar
30. Levenson J, Bemis C: The role of psychological factors in cancer onset and progression. Psychosomatics 1991; 32:124-132Crossref, Medline, Google Scholar
31. Elliott G: Stress and illness, in Psychosomatic Medicine: Theory, Physiology, and Practice. Edited by Cheren S. Madison, Conn, International Universities Press, 1989, pp 45-90Google Scholar
32. Hurst MW, Jenkins CD, Rose RM: The relation of psychological stress to onset of medical illness. Annu Rev Med 1976; 27:301-312Crossref, Medline, Google Scholar
33. Goldsmith H, Gottesman I, Lemery K: Epigenetic approaches to developmental psychopathology. Dev Psychopathol 1997; 9:365-387Crossref, Medline, Google Scholar
34. Monroe S, Simons A: Diathesis/stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull 1991; 110:406-425Crossref, Medline, Google Scholar
35. Coyne JC, Whiffen VE: Issues in personality as diathesis for depression: the case of sociotropy-dependency and autonomy-self-criticism. Psychol Bull 1995; 118:358-378Crossref, Medline, Google Scholar
36. Nemeroff C: The neurobiology of depression. Sci Am 1998; 278:28-35Crossref, Medline, Google Scholar
37. Paris J: Nature and Nurture in Psychiatry: A Predisposition-Stress Model of Mental Disorders. Washington, DC, American Psychiatric Press, 1999Google Scholar
38. Kendler KS: Parenting: a genetic-epidemiologic perspective. Am J Psychiatry 1996; 153:11-20Link, Google Scholar
39. Bolton F: “Normal” violence in the adult-child relationship: a diathesis-stress approach to child maltreatment within the family, in Family Abuse and Its Consequences: New Directions in Research. Edited by Hotaling G, Finkelhor D. Newbury Park, Calif, Sage Publications, 1988, pp 61-76Google Scholar
40. Adamec R, Shallow T: Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav 1993; 54:101-109Crossref, Medline, Google Scholar
41. Adamec R, Burton P, Shallow T, Budgell J: NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure: implications for anxiety associated with posttraumatic stress disorder. Physiol Behav 1999; 65:723-737Crossref, Medline, Google Scholar
42. Pynoos RS, Ritzmann R, Steinberg A, Goenjian A, Prisecaru I: A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 1996; 39:129-134Crossref, Medline, Google Scholar
43. Board F, Persky H, Hamburg D: Psychological stress and endocrine functions: blood levels of adrenocortical and thyroid hormones in acutely disturbed patients. Psychosom Med 1956; 18:324-333Crossref, Medline, Google Scholar
44. Carpenter WT Jr, Bunney WE Jr: Adrenal cortical activity in depressive illness. Am J Psychiatry 1971; 128:31-40Link, Google Scholar
45. Arana G, Baldesarrini R, Ornsteen M: The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Arch Gen Psychiatry 1985; 42:1192-1204Crossref, Google Scholar
46. Evans DL, Nemeroff CB: Use of dexamethasone suppression test using DSM-III criteria on an inpatient psychiatric unit. Biol Psychiatry 1983; 18:505-511Medline, Google Scholar
47. Krishnan K, France P, Pelton S, McCann U, Manepalli A, Davidson J: What does the dexamethasone suppression test identify? Biol Psychiatry 1985; 20:957-964Crossref, Medline, Google Scholar
48. Schatzberg A, Rothschild A, Bond T, Cole J: The DST in psychotic depression: diagnostic and pathophysiologic implications. Psychopharmacol Bull 1984; 20:362-364Medline, Google Scholar
49. Arato M, Banki C, Nemeroff C, Bissett G: Hypothalamic-pituitary-adrenal axis and suicide. Ann NY Acad Sci 1986; 487:263-270Crossref, Medline, Google Scholar
50. Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB: CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 1987; 144:873-877Link, Google Scholar
51. France R, Urban B, Krishnan K, Bissette G, Banki C, Nemeroff C, Balleyer J: CSF corticotropin-releasing factor-like immunoreactivity in chronic pain patients with and without major depression. Biol Psychiatry 1988; 23:86-88Crossref, Medline, Google Scholar
52. Nemeroff C, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts C, Loosen P, Vale V: Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 1984; 226:1342-1344Crossref, Medline, Google Scholar
53. Risch S, Lewine R, Kalin N, Jewart R, Risby E, Caudle J, Stipetic M, Turner J, Eccard M, Pollard W: Limbic-hypothalamic-pituitary-adrenal axis activity and ventricular-to-brain ratio studies in affective illness and schizophrenia. Neuropsychopharmacology 1992; 6:95-100Medline, Google Scholar
54. Amsterdam JD, Maislin G, Winokur A, Berwish N, Kling M, Gold P: The oCRH stimulation test before and after clinical recovery from depression. J Affect Disord 1988; 14:213-222Crossref, Medline, Google Scholar
55. Gold PW, Chrousos G, Kellner C, Post R, Roy A, Augerinos P, Schulte H, Oldfield E, Loriaux DL: Psychiatric implications of basic and clinical studies with corticotropin-releasing factor. Am J Psychiatry 1984; 141:619-627Link, Google Scholar
56. Holsboer F, Von Bardeleben U, Gerken A, Stalla GK, Muller OA: Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression (letter). N Engl J Med 1984; 311:1127Medline, Google Scholar
57. Kathol RG, Jaeckle RS, Lopez JF, Meller WH: Consistent reduction of ACTH responses to stimulation with CRH, vasopressin and hypoglycaemia in patients with depression. Br J Psychiatry 1989; 155:468-478Crossref, Medline, Google Scholar
58. Young EA, Watson SJ, Kotun J, Haskett RF, Grunhaus L, Murphy-Weinberg V, Vale W, Rivier J, Akil H: Beta-lipotropin-beta-endorphin response to low-dose ovine corticotropin releasing factor in endogenous depression. Arch Gen Psychiatry 1990; 47:449-457Crossref, Medline, Google Scholar
59. Raadsheer F, Hoogendijk W, Stam F, Tilders F, Swaab D: Increased number of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 1994; 60:436-444Crossref, Medline, Google Scholar
60. Nemeroff CB: The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry 1996; 1:336-342Medline, Google Scholar
61. Heit S, Owens M, Plotsky P, Nemeroff C: Corticotropin-releasing factor, stress and depression. Neuroscientist 1997; 3:186-194Crossref, Google Scholar
62. Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB: The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 1999; 160:1-12Crossref, Medline, Google Scholar
63. Plotsky PM, Owens MJ, Nemeroff CB: Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 1998; 21:293-307Crossref, Medline, Google Scholar
64. Schildkraut JJ: The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 1965; 122:509-522Link, Google Scholar
65. Ressler KJ, Nemeroff CB: Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry 1999; 46:1219-1233Crossref, Medline, Google Scholar
66. Stockmeier C: Neurobiology of serotonin in depression and suicide. Ann NY Acad Sci 1997; 836:220-232Crossref, Medline, Google Scholar
67. Lopez J, Akil H, Watson S: Neural circuits mediating stress. Biol Psychiatry 1999; 46:1461-1471Crossref, Medline, Google Scholar
68. Joyce P: Neuroendocrine changes in depression. Aust N Z J Psychiatry 1985; 19:120-127Crossref, Medline, Google Scholar
69. Keck M, Holsboer F: Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides 2001; 22:835-844Crossref, Medline, Google Scholar
70. Holsboer F: The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000; 23:477-501Crossref, Medline, Google Scholar
71. Insel T, Battaglia G, Fairbanks D, DeSouza E: The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. Neuroscience 1988; 8:4151-4158Crossref, Medline, Google Scholar
72. De Kloet E, Rosenfeld P, Van Eekelen A, Sutanto W, Levine S: Stress, glucocorticoids and development. Prog Brain Res 1988; 73:101-120Crossref, Medline, Google Scholar
73. Shapiro AP: Maturation of the neuroendocrine response to stress in the rat, in Early Experience and Behavior. Edited by Newton G, Levine S. Springfield, Ill, Charles C. Thomas, 1968, pp 198-257Google Scholar
74. Sapolsky RM, Meaney MJ: Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 1986; 396:64-76Crossref, Medline, Google Scholar
75. Walker C, Perrin M, Vale W, Rivier C: Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology 1986; 118:1445-1451Crossref, Medline, Google Scholar
76. Walker C, Akana S, Cascio C, Dallman M: Adrenalectomy in the neonate: adult-like adrenocortical system responses to both removal and replacement of corticosterone. Endocrinology 1990; 127:832-842Crossref, Medline, Google Scholar
77. Grino M, Burgunder J, Eskay R: Onset of glucocorticoid responsiveness of anterior pituitary corticotrophs during development is scheduled by corticotropin-releasing factor. J Endocrinol 1989; 124:2686-2692Crossref, Google Scholar
78. Baram T, Yi S, Arishi-Eliver S, Schultz L: Developmental neurobiology of the stress response: multi-level regulation of corticotropin-releasing hormone function. Ann NY Acad Sci 1997; 814:252-265Crossref, Medline, Google Scholar
79. Walker C, Scribner K, Cascio C, Dallman M: The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 1991; 128:1385-1395Crossref, Medline, Google Scholar
80. Anand K, Aynsley-Green A: Measuring the severity of surgical stress in newborn infants. J Pediatr Surg 1988; 23:297-305Crossref, Medline, Google Scholar
81. Zaharia MD, Kulczycki J, Shanks N, Meaney MJ, Anisman H: The effects of early postnatal stimulation on Morris water-maze acquisition in adult mice: genetic and maternal factors. Psychopharmacology (Berl) 1996; 128:227-239Crossref, Medline, Google Scholar
82. Meaney MJ, Aitken DH, Van Berkel C, Bhatnagar S, Sapolsky RM: Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 1988; 239(4841 Part 1):766-768Google Scholar
83. Weinberg J, Smotherman W, Levine S: Early handling effects on neophobia and conditioned taste aversion. Physiol Behav 1978; 20:589-596Crossref, Medline, Google Scholar
84. Weinberg J, Krahn E, Levine S: Differential effects of handling on exploration in male and female rats. Dev Psychobiol 1978; 11:251-259Crossref, Medline, Google Scholar
85. Abraham I, Kovacs K: Postnatal handling alters the activation of stress-related neuronal circuitries. European J Neurosci 2000; 12:3003-3014Crossref, Medline, Google Scholar
86. McCarty R, Horbaly W, Brown M, Baucom K: Effects of handling during infancy on the sympathetic-adrenal medullary system of rats. Dev Psychobiol 1981; 14:533-539Crossref, Medline, Google Scholar
87. Liu D, Caldji S, Sharma S, Plotsky P, Meaney M: Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinephrine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol 2000; 12:5-12Crossref, Medline, Google Scholar
88. Bolden S, Hambley J, Johnston G, Rogers L: Neonatal stress and long-term modulation of GABA receptors in rat brain. Neurosci Lett 1990; 111:258-262Crossref, Medline, Google Scholar
89. Smythe JW, Rowe WB, Meaney MJ: Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Brain Res Dev Brain Res 1994; 80:183-189Crossref, Medline, Google Scholar
90. Mitchell JB, Iny LJ, Meaney MJ: The role of serotonin in the development and environmental regulation of type II corticosteroid receptor binding in rat hippocampus. Brain Res Dev Brain Res 1990; 55:231-235Crossref, Medline, Google Scholar
91. D’Amore A, Marano G, Loizzo A: Reduced antinociceptive response to beta-endorphin in adult mice after chronic neonatal handling. Physiol Behav 1993; 53:1025-1027Crossref, Medline, Google Scholar
92. Irazusta J, Tejedor-Real P, Varona A, Costela C, Gilbert-Rahola J, Casis L: Effect of neonatal handling on brain enkephalin-degrading peptidase activities. Neurochem Int 1999; 35:357-361Crossref, Medline, Google Scholar
93. Ploj K, Pham T, Bergstrom L, Mohammed A, Henriksson B, Nylander I: Neonatal handling in rats induces long-term effects on dynorphin peptides. Neuropeptides 1999; 33:468-474Crossref, Medline, Google Scholar
94. Ploj K, Roman E, Bergstrom L, Nylander I: Effects of neonatal handling on nociceptin/orphanin FQ and opioid peptide levels in female rats. Pharmacol Biochem Behav 2001; 69:173-179Crossref, Medline, Google Scholar
95. Viau V, Sharma S, Plotsky P, Meaney M: Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J Neurosci 1993; 13:1097-1105Crossref, Medline, Google Scholar
96. Hess J, Denenberg V, Zarrow M, Pfeifer W: Modification of the corticosterone response curve as a function of handling in infancy. Physiol Behav 1969; 4:109-111Crossref, Google Scholar
97. Levine S: Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 1967; 156:258-260Crossref, Medline, Google Scholar
98. Meaney M, Aitken D, Sharma S, Viau V: Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology 1992; 55:204-213Crossref, Medline, Google Scholar
99. Plotsky PM, Meaney MJ: Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) in adult rats. Brain Res Mol Brain Res 1993; 18:195-200Crossref, Medline, Google Scholar
100. Smythe J, McCormick C, Meaney M: Median eminence corticotrophin-releasing hormone content following prenatal stress and neonatal handling. Brain Res Bull 1996; 40:195-199Crossref, Medline, Google Scholar
101. Meaney M, Aitken D, Viau V, Sharma S, Sarrieau A: Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology 1989; 50:597-604Crossref, Medline, Google Scholar
102. Meaney M, Aitken D: The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res 1985; 354:301-304Crossref, Medline, Google Scholar
103. Meaney M, Aitken D, Bodnoff S, Iny L, Tatarewicz J, Sapolsky R: Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci 1985; 99:765-770Crossref, Medline, Google Scholar
104. Meaney M, Aitken D, Bodnoff S, Iny L, Sapolsky R: The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Prog Neuropsychopharmacol Biol Psychiatry 1985; 9:731-734Crossref, Medline, Google Scholar
105. O’Donnell D, Larocque S, Seckl J, Meaney M: Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Brain Res Mol Brain Res 1994; 26:242-248Crossref, Medline, Google Scholar
106. Vaidl R, Yee B, Shalev U, Rawlins J, Weiner I, Feldon J, Totterdell S: Neonatal nonhandling and in utero prenatal stress reduce the density of NADPH-diaphorase-reactive neurons in the fascia dentata and Ammon’s horn of rats. J Neurosci 1997; 17:5599-5609Crossref, Medline, Google Scholar
107. Schreiber H: Early handling and maternal behavior: effect on d-amphetamine responsiveness in rats. Pharmacol Biochem Behav 1978; 9:785-789Crossref, Medline, Google Scholar
108. Hennessy M, Li J, Lowe E, Levine S: Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol 1980; 13:573-584Crossref, Medline, Google Scholar
109. Hennessy M, Vogt J, Levine S: Strain of foster mother determines long-term effects of early handling: evidence for maternal mediation. Physiol Psychology 1982; 10:153-157Crossref, Google Scholar
110. Smotherman WP: Mother-infant interaction and the modulation of pituitary-adrenal activity in rat pups after early stimulation. Dev Psychobiol 1983; 16:169-176Crossref, Medline, Google Scholar
111. Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P, Meaney M: Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997; 277:1659-1662Crossref, Medline, Google Scholar
112. Liu D, Diorio J, Day J, Francis D, Meaney M: Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience 2000; 3:799-806Crossref, Medline, Google Scholar
113. Denenberg V: Commentary: is maternal stimulation the mediator of the handling effect in infancy? Dev Psychobiol 1999; 34:1-3Crossref, Medline, Google Scholar
114. Huot R, Smith M, Plotsky P: Alterations of maternal-infant interaction as a result of maternal separation in Long Evans rats and its behavioral and neuroendocrine consequences (abstract). Psychoneuroendocrinology 1997; 22(suppl 2):S173Google Scholar
115. Bowlby J: Maternal Care and Mental Health. Geneva, World Health Organization, 1952Google Scholar
116. Levine S, Haltmeyer G, Karas G: Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2:55-63Crossref, Google Scholar
117. Finamore T, Port R: Developmental stress disrupts habituation but spares prepulse inhibition in young rats. Physiol Behav 2000; 69:527-530Crossref, Medline, Google Scholar
118. Gonzalez A, Lovic V, Ward G, Wainwright P, Fleming A: Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol 2001; 38:11-32Crossref, Medline, Google Scholar
119. Lovic V, Gonzalez A, Fleming A: Maternally separated rats show deficits in maternal care in adulthood. Dev Psychobiol 2001; 39:19-33Crossref, Medline, Google Scholar
120. Pryce C, Bettschen D, Feldon J: Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol 2001; 38:239-251Crossref, Medline, Google Scholar
121. Schanberg S, Kuhn C: The biochemical effects of tactile deprivation in neonatal rats. Perspec Behav Med 1985; 2:133-148Google Scholar
122. Pauk J, Kuhn C, Field T, Schanberg S: Positive effects of tactile vs kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally deprived rat pups. Life Sci 1986; 39:2081-2087Crossref, Medline, Google Scholar
123. Pihoker C, Owens M, Kuhn C, Schanberg S, Nemeroff C: Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology 1993; 7:485-493Crossref, Google Scholar
124. Ladd C, Owens M, Nemeroff C: Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 1996; 137:1212-1218Crossref, Medline, Google Scholar
125. Workel J, Oitzl M, Ledeboer A, DeKloet E: The Brown Norway rat displays enhanced stress-induced ACTH reactivity at day 18 after 24 h maternal deprivation at day 3. Brain Res 1997; 103:199-203Crossref, Google Scholar
126. van Oers H, DeKloet E, Levine S: Early versus late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res 1998; 111:245-252Crossref, Google Scholar
127. Plotsky P, Thrivikraman K, Meaney M: Central and feedback regulation of hypothalamic corticotropin-releasing factor secretion, in Symposium on Corticotropin-Releasing Factor. Edited by Chadwick D, Marsh J, Ackrill K. London, John Wiley & Sons, 1993, pp 59-84Google Scholar
128. Dent G, Smith M, Levine S: Stress-induced alterations in locus coeruleus gene expression during ontogeny. Brain Res Dev Brain Res 2001; 127:23-30Crossref, Medline, Google Scholar
129. King J, Edwards E: Early stress and genetic influences on hypothalamic-pituitary-adrenal axis functioning in adulthood. Horm Behav 1999; 36:79-85Crossref, Medline, Google Scholar
130. Bremner J, Southwick S, Charney D: The neurobiology of posttraumatic stress disorder: an integration of animal and human research, in Posttraumatic Stress Disorder: A Comprehensive Text. Edited by Saigh P, Bremner J. Boston, Allyn & Bacon, 1999, pp 103-143Google Scholar
131. Yehuda R: Psychoneuroendocrinology of post-traumatic stress disorder. Psychiatr Clin North Am 1998; 21:359-379Crossref, Medline, Google Scholar
132. Yehuda R, Giller E, Levengood R, Southwick S, Siever L: Hypothalamic-pituitary-adrenal functioning in post-traumatic stress disorder, in Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to Post-Traumatic Stress Disorder. Edited by Friedman M, Charney D, Deutch A. Philadelphia, Lippincott-Raven, 1995, pp 351-365Google Scholar
133. Rosenfeld P, Ekstrand J, Olson E, Suchecki D: Maternal regulation of adrenocortical activity in the infant rat: effects of feeding. Dev Psychobiol 1993; 26:261-277Crossref, Medline, Google Scholar
134. Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski C, Baram T: Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. J Neurosci 1999; 19:3982-3991Crossref, Medline, Google Scholar
135. Kehoe P, Blass EM: Behaviorally functional opioid system in infant rats: II. evidence for pharmacological, physiological, and psychological mediation of pain and stress. Behav Neurosci 1986; 100:624-630Crossref, Medline, Google Scholar
136. Kalinichev M, Easterling K, Holtzman S: Repeated neonatal maternal separation alters morphine-induced antinociception in male rats. Brain Res Bull 2001; 54:649-654Crossref, Medline, Google Scholar
137. Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ: The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 2000; 22:219-229Crossref, Medline, Google Scholar
138. Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky P Meaney M: Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 1998; 95:5335-5340Crossref, Medline, Google Scholar
139. Matthews K, Dalley J, Matthews C, Tsai T, Robbins T: Periodic maternal separation of neonatal rats produces region- and gender- specific effects on biogenic amine content in postmortem adult brain. Synapse 2001; 40:1-10Crossref, Medline, Google Scholar
140. Braun K, Lange E, Metzger M, Poeggel G: Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience 2000; 95:309-318Crossref, Medline, Google Scholar
141. Whitaker-Azmitia P, Zhou F, Hobin J, Borella A: Isolation-rearing of rats produces deficits as adults in the serotonergic innervation of hippocampus. Peptides 2000; 1755-1759Google Scholar
142. Howes S, Dalley J, Morrison C, Robbins T, Everitt B: Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 2000; 151:55-63Crossref, Medline, Google Scholar
143. Kowalski T, Houpt T, Jahng J, Okada N, Chua S, Smith G: Ontogeny of neuropeptide Y expression in response to deprivation in lean Zucker rat pups. Am J Physiol 1998; 275(2 pt 2):R466-470Google Scholar
144. Harlow H, Harlow M: The effect of rearing conditions on behavior. Bull Menninger Clin 1962; 26:213-224Medline, Google Scholar
145. Harlow H, Harlow M, Suomi S: From thought to therapy: lessons from a primate laboratory. Am Scientist 1971; 59:538-549Medline, Google Scholar
146. Kraemer G, Ebert M, Schmidt D, McKinney W: Strangers in a strange land: a psychobiological study of infant monkeys before and after separation from real or inanimate mothers. Child Dev 1991; 62:548-566Crossref, Medline, Google Scholar
147. Clarke A, Hedeker D, Ebert M, Schmidt D, McKinney W, Kraemer G: Rearing experience and biogenic amine activity in infant rhesus monkeys. Biol Psychiatry 1996; 40:338-352Crossref, Medline, Google Scholar
148. Clarke A, Ebert M, Schmidt D, McKinney W, Kraemer G: Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry 1999; 46:221-228Crossref, Medline, Google Scholar
149. Kraemer G, Ebert M, Lake C, McKinney W: Cerebrospinal fluid changes associated with pharmacological alteration of the despair response to social separation in rhesus monkeys. J Psychiatry Res 1984; 11:303-315Crossref, Google Scholar
150. Kraemer GW, McKinney WT: Interactions of pharmacological agents which alter biogenic amine metabolism and depression: an analysis of contributing factors within a primate model of depression. J Affect Disord 1979; 1:33-54Crossref, Medline, Google Scholar
151. Higley J, Bennett A, Heils A, Long J, Lorenz J, Champoux M, Suomi S, Lesch K: Early rearing and genotypic influences on CNS serotonin and behavior in nonhuman primates (abstract). Biol Psychiatry 2000; 47:10SCrossref, Google Scholar
152. Sackett G: Adrenocortical and behavioral reactions by differentially raised rhesus monkeys. Physiol Psychology 1973; 1:209-212Crossref, Google Scholar
153. Clarke A: Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev Psychobiol 1993; 26:433-446Crossref, Medline, Google Scholar
154. Clarke A, Kraemer G, Kupfer D: Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res 1998; 79:91-104Crossref, Medline, Google Scholar
155. Fahlke C, Lorenz J, Long J, Champoux M, Suomi S, Higley J: Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res 2000; 24:644-650Crossref, Medline, Google Scholar
156. Maestripieri D, Carroll K: Child abuse and neglect: usefulness of the animal data. Psychol Bull 1998; 123:211-223Crossref, Medline, Google Scholar
157. Gunnar M, Gonzalez C, Levine S: The role of peers in modifying behavioral distress and pituitary-adrenal response to a novel environment in year-old rhesus monkeys. Physiol Behav 1980; 25:795-798Crossref, Medline, Google Scholar
158. Levine S, Johnson D, Gonzalez C: Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behav Neurosci 1985; 99:399-410Crossref, Medline, Google Scholar
159. Wiener S, Bayart F, Faull K, Levine S: Behavioral and physiological responses to maternal separation in squirrel monkeys (Saimiri sciureus). Behav Neurosci 1990; 104:108-115Crossref, Medline, Google Scholar
160. Byrne G, Suomi SJ: Social separation in infant Cebus appella: patterns of behavioral and cortisol response. Int J Dev Neurosci 1999; 17:265-274Crossref, Medline, Google Scholar
161. Lyons D, Yang C, Mobley B, Nickerson J, Schatzberg A: Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. J Neuroendocrinol 2000; 12:723-728Crossref, Medline, Google Scholar
162. Sikes R: Maternal response to resource limitations in eastern woodrats. Animal Behav 1995; 49:1551-1558Crossref, Google Scholar
163. Langenau E, Lerg J: The effects of winter nutritional stress on maternal and neonatal behavior in penned white-tailed deer. Appl Animal Ethology 1976; 2:207-223Crossref, Google Scholar
164. Rosenblum L, Paully G: The effects of varying environmental demands on maternal and infant behavior. Child Dev 1984; 55:305-314Crossref, Medline, Google Scholar
165. Andrews M, Rosenblum L: Developmental consequences of altered dyadic coping patterns in bonnet macaques, in Current Primatology: Social Development, Learning and Behavior. Edited by Roeder J, Thierry B, Anderson J, Herrenschmidt N. Strasbourg, France, Université Louis Pasteur, 1994, pp 265-271Google Scholar
166. Andrews M, Rosenblum L: Attachment in monkey infants raised in variable and low-demand environments. Child Dev 1991; 62:686-693Crossref, Medline, Google Scholar
167. Andrews M, Rosenblum L: Relationship between foraging and affiliative social referencing in primates, in Ecology and Behavior of Food-Enhanced Primate Groups. Edited by Fa J, Southwick C. New York, Alan R Liss, 1988, pp 247-268Google Scholar
168. Rosenblum L, Forger C, Noland S, Trost R, Coplan J. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Dev Psychobiol 2001; 39:40-45Crossref, Medline, Google Scholar
169. Coplan J, Andrews M, Rosenblum L, Owens M, Friedman S, Gorman J, Nemeroff C: Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA 1996; 93:1619-1623Crossref, Medline, Google Scholar
170. Coplan J, Smith E, Altemus M, Scharf B, Owens M, Nemeroff C, Gorman J, Rosenblum L. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry 2001; 50:200-204Crossref, Medline, Google Scholar
171. Coplan J, Trost R, Owens M, Cooper T, Gorman J, Nemeroff C, Rosenblum L: Cerebrospinal fluid concentrations of somatostatin and biogenic amines in grown primates reared by mothers exposed to manipulated foraging conditions. Arch Gen Psychiatry 1998; 55:473-477Crossref, Medline, Google Scholar
172. Coplan J, Smith E, Trost R, Scharf B, Altemus M, Bjornson L, Owens M, Gorman J, Nemeroff C, Rosenblum L: Growth hormone response to clonidine in adversely reared young adult primates: relationship to serial cerebrospinal fluid corticotropin-releasing factor concentrations. Psychiatry Res 2000; 95:93-102Crossref, Medline, Google Scholar
173. Rosenblum L, Coplan J, Friedman S, Bassoff T, Gorman J: Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biol Psychiatry 1994; 35:221-227Crossref, Medline, Google Scholar
174. Newport DJ, Wilcox MM, Stowe ZN: Maternal depression: a child’s first adverse life event. Semin Clin Neuropsychiatry 2002; 7:113-119Crossref, Medline, Google Scholar
175. Shell L: Environmental noise and human prenatal growth. Am J Physiol Anthropol 1981; 56:63-70Crossref, Medline, Google Scholar
176. Hedegaard M, Henriksen T, Sabroe S, Secher N: The relationship between psychological distress during pregnancy and birth weight for gestational age. Acta Obstet Gynecol Scand 1996; 75:32-39Crossref, Medline, Google Scholar
177. Korebrits C, Ramirez M, Watson L, Brinkman E, Bocking A, Challis J: Maternal corticotropin-releasing hormone is increased with impending preterm birth. J Clin Endocrinol Metab 1998; 83:1585-1591Crossref, Medline, Google Scholar
178. Orr S, Miller C: Maternal depressive symptoms and the risk of poor pregnancy outcome. Epidemiology Review 1995; 17:165-170Crossref, Medline, Google Scholar
179. Steer R, Scholl T, Hediger M, Fischer R: Self-reported depression and negative pregnancy outcomes. Epidemiology 1992; 45:1093-1099Google Scholar
180. Perkins R, Bland J, Peacock J, Anderson R: The effect of anxiety and depression during pregnancy on obstetric complications. Br J Obstet Gynaecol 1993; 100:629-634Crossref, Medline, Google Scholar
181. Meijer A: Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand 1985; 72:505-511Crossref, Medline, Google Scholar
182. Stott D: Follow-up study from birth of the effects of prenatal stresses. Dev Med Child Neurol 1973; 15:770-787Crossref, Medline, Google Scholar
183. Herrenkohl L, Gala R: Serum prolactin levels and maintenance of progeny by prenatally-stressed female offspring. Experientia 1979; 35:702-704Crossref, Medline, Google Scholar
184. Drago F, Di Leo F, Giardina L: Prenatal stress induces body weight deficit and behavioral alterations in rats: the effect of diazepam. Eur Neuropsychopharmacol 1999; 9:239-245Crossref, Medline, Google Scholar
185. Mooney MP, Siegel MI, Gest TR: Prenatal stress and increased fluctuating asymmetry in the parietal bones of neonatal rats. Am J Phys Anthropol 1985; 68:131-134Crossref, Medline, Google Scholar
186. Pollard I: Prenatal stress effects over two generations in rats. J Endocrinol 1986; 109:239-244Crossref, Medline, Google Scholar
187. Schneider M, Roughton E, Koehler A, Luback G: Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev 1999; 70:263-274Crossref, Medline, Google Scholar
188. Smith BL, Wills G, Naylor D: The effects of prenatal stress on rat offsprings’ learning ability. J Psychol 1981; 107:45-51Crossref, Medline, Google Scholar
189. Weller A, Glaubman H, Yehuda S, Caspy T, Ben-Uria Y: Acute and repeated gestational stress affect offspring learning and activity in rats. Physiol Behav 1988; 43:139-143Crossref, Medline, Google Scholar
190. Jaiswal A, Bhattacharya S: Effects of gestational undernutrition, stress and diazepam treatment on spatial discrimination learning and retention in young rats. Indian J Exp Biol 1993; 31:353-359Medline, Google Scholar
191. Lehmann J, Stohr T, Feldon J: Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res 2000; 107:133-144Crossref, Medline, Google Scholar
192. Fride E, Weinstock M: The effects of prenatal exposure to predictable or unpredictable stress on early development in the rat. Dev Psychobiol 1984; 17:651-660Crossref, Medline, Google Scholar
193. Takahashi L, Haglin C, Kalin N: Prenatal stress potentiates stress-induced behavior and reduces the propensity to play in juvenile rats. Physiol Behav 1992; 51:319-323Crossref, Medline, Google Scholar
194. Weinstock M, Fride E, Hertzberg R: Prenatal stress effects on functional development of the offspring. Prog Brain Res 1988; 73:319-331Crossref, Medline, Google Scholar
195. Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S: Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci 1997; 17:2626-2636Crossref, Medline, Google Scholar
196. Szuran T, Zimmerman E, Pliska V, Pfister H, Welzl H: Prenatal stress effects on exploratory activity and stress-induced analgesia in rats. Dev Psychobiol 1991; 24:361-372Crossref, Medline, Google Scholar
197. Poltyrev T, Keshet G, Kay G, Weinstock M: Role of experimental conditions in determining differences in exploratory behavior of prenatally stressed rats. Dev Psychobiol 1996; 29:453-462Crossref, Medline, Google Scholar
198. Fride E, Weinstock M: Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 1988; 42:1059-1065Crossref, Medline, Google Scholar
199. Secoli S, Teixeira N: Chronic prenatal stress affects development and behavioral depression in rats. Stress 1998; 2:273-280Crossref, Medline, Google Scholar
200. Wakshlak A, Weinstock M: Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav 1990; 48:289-292Crossref, Medline, Google Scholar
201. Pfister H, Muir J: Prenatal exposure to predictable and unpredictable novelty stress and oxytocin treatment affects offspring development and behavior in rats. Int J Neurosci 1992; 62:227-241Crossref, Medline, Google Scholar
202. Fride E, Dan Y, Feldon J, Halevy G, Weinstock M: Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav 1986; 37:681-687Crossref, Medline, Google Scholar
203. Schneider M: Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev Psychobiol 1992; 25:529-540Crossref, Medline, Google Scholar
204. Clarke A, Schneider M: Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol 1993; 26:293-304Crossref, Medline, Google Scholar
205. Takahashi L: Prenatal stress: consequences of glucocorticoids on hippocampal development and function. Int J Dev Neurosci 1998; 16:199-207Crossref, Medline, Google Scholar
206. Fujioka T, Sakata Y, Yamaguchi K, Shibasaki T, Kato H, Nakamura S: The effects of prenatal stress on the development of hypothalamic paraventricular neurons in fetal rats. Neuroscience 1999; 92:1079-1088Crossref, Medline, Google Scholar
207. Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S: Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 1994; 6:341-345Crossref, Medline, Google Scholar
208. McCormick C, Smythe J, Sharma S, Meaney M: Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res 1995; 84:55-61Crossref, Google Scholar
209. Maccari S, Piazza P, Kabbaj M, Barbazanges A, Simon H, Le Moal M: Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 1995; 15(1 pt 1):110-116Google Scholar
210. Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R: Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav 1998; 64:439-444Crossref, Medline, Google Scholar
211. Weinstock M, Matlina E, Maor G, Rosen H, McEwen B: Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary-adrenal system in female rats. Brain Res 1992; 595:195-198Crossref, Medline, Google Scholar
212. Peters D: Prenatal stress: effects on brain biogenic amine and plasma corticosterone levels. Pharmacol Biochem Behav 1982; 17:721-725Crossref, Medline, Google Scholar
213. Koehl M, Barbazanges A, Le Moal M, Maccari S: Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res 1997; 759:317-320Crossref, Medline, Google Scholar
214. Takahashi LK, Kalin NH: Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res 1991; 558:75-78Crossref, Medline, Google Scholar
215. Sanchez M, Milanes M, Fuente T, Laorden M. The beta-endorphin response to prenatal stress during postnatal development in the rat. Brain Res 1993; 73:142-145Crossref, Google Scholar
216. Poltyrev T, Weinstock M. Effect of prenatal stress on opioid component of exploration in different experimental situations. Pharmacol Biochem Behav 1997; 58:387-393Crossref, Medline, Google Scholar
217. Ward I, Monaghan E, Ward I: Naltrexone blocks the effects of prenatal stress on sexual behavior differentiation in male rats. Pharmacol Biochem Behav 1986; 25:573-576Crossref, Medline, Google Scholar
218. Cratty M, Ward H, Johnson E, Azzaro A, Birkle D: Prenatal stress increases corticotropin releasing factor (CRF) content and release in rat amygdala minces. Brain Res 1995; 675:297-302Crossref, Medline, Google Scholar
219. Barbazanges A, Piazza PV, Le Moal M, Maccari S: Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci 1996; 16:3943-3949Crossref, Medline, Google Scholar
220. Moore C, Power K: Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol 1986; 19:235-245Crossref, Medline, Google Scholar
221. Power K, Moore C: Prenatal stress eliminates differential maternal attention to male offspring in Norway rats. Physiol Behav 1986; 38:667-671Crossref, Medline, Google Scholar
222. Clarke A, Wittwer D, Abbott D, Schneider M: Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol 1994; 27:257-269Crossref, Medline, Google Scholar
223. Schneider M, Coe C, Luback G: Endocrine activation mimics the adverse effects of prenatal stress on the neuromotor development of the infant primate. Dev Psychobiol 1992; 25:427-439Crossref, Medline, Google Scholar
224. Alonso S, Navarro E, Rodriguez M: Permanent dopaminergic alterations in the nucleus accumbens after prenatal stress. Pharmacol Biochem Behav 1994; 49:353-358Crossref, Medline, Google Scholar
225. Alonso S, Navarro E, Santana C, Rodriguez M: Motor lateralization, behavioral despair and dopaminergic brain asymmetry after prenatal stress. Pharmacol Biochem Behav 1997; 58:443-448Crossref, Medline, Google Scholar
226. Fride E, Dan Y, Gavish M, Weinstock M: Prenatal stress impairs maternal behavior in a conflict situation and reduces hippocampal benzodiazepine receptors. Life Sci 1985; 36:2103-2109Crossref, Medline, Google Scholar
227. Reznikov A, Nosenko N: Early postnatal changes in sexual dimorphism of catecholamine and indoleamine content in the brain of prenatally stressed rats. Neuroscience 1996; 70:547-551Crossref, Medline, Google Scholar
228. Schneider ML, Clarke AS, Kraemer GW, Roughton EC, Lubach G, Rimm-Kaufman S, Schmidt D, Ebert M: Prenatal stress alters brain biogenic amine levels in primates. Dev Psychopathol 1998; 10:427-440Crossref, Medline, Google Scholar
229. Takahashi L, Turner J, Kalin N: Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res 1992; 574:131-137Crossref, Medline, Google Scholar
230. Peters D: Prenatal stress: effect on development of rat brain adrenergic receptors. Pharmacol Biochem Behav 1984; 21:417-422Crossref, Medline, Google Scholar
231. Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, Demotes-Mainard J: Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res 1995; 685:179-186Crossref, Medline, Google Scholar
232. Fride E, Weinstock M: Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacol Biochem Behav 1989; 32:425-430Crossref, Medline, Google Scholar
233. Fride E, Weinstock M: Increased interhemispheric coupling of the dopamine systems induced by prenatal stress. Brain Res Bulletin 1987; 18:457-461Crossref, Medline, Google Scholar
234. Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N: Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci 1998; 16:209-216Crossref, Medline, Google Scholar
235. Peters D: Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacol Biochem Behav 1990; 35:943-947Crossref, Medline, Google Scholar
236. Peters D: Prenatal stress: effect on development of rat brain serotonergic neurons. Pharmacol Biochem Behav 1986; 24:1377-1382Crossref, Medline, Google Scholar
237. Peters D: Prenatal stress increases the behavioral response to serotonin agonists and alters open field behavior in the rat. Pharmacol Biochem Behav 1986; 25:873-877Crossref, Medline, Google Scholar
238. Peters D: Effects of maternal stress during different gestational periods on the serotonergic system in adult rat offspring. Pharmacol Biochem Behav 1988; 31:839-843Crossref, Medline, Google Scholar
239. Slotkin T, Barnes G, McCook E, Seidler F: Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Brain Res 1996; 93:155-161Crossref, Google Scholar
240. Day JC, Koehl M, Deroche V, Le Moal M, Maccari S: Prenatal stress enhances stress and corticotropin-releasing factor-induced stimulation of hippocampal acetylcholine release in adult rats. J Neurosci 1998; 18:1886-1892Crossref, Medline, Google Scholar
241. Sapolsky R, Romero L, Munck A: How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21:55-89Medline, Google Scholar
242. Gust D, Gordon T, Gergits W, Casna N: Male dominance rank and offspring-initiated affiliative behaviors were not predictors of paternity in a captive group of pigtail macaques (Macaca nemestrina). Primates 1996; 37:271-278Crossref, Google Scholar
243. Paul A, Kuester J, Arnemann J: The sociobiology of male-infant interactions in Barbary macaques, Macaca sylvanu. Anim Behav 1996; 51:155-170Crossref, Google Scholar
244. Pruetz J, Bloomsmith M: The effect of paternity on interactions between adult male and immature chimpanzees in captivity. Folia Primatol 1995; 65:174-180Crossref, Medline, Google Scholar
245. Insel T: Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 1992; 17:3-35Crossref, Medline, Google Scholar
246. Wang Z, Liu Y, Young L, Insel T: Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol 2000; 12:111-120Crossref, Medline, Google Scholar
247. Lonstein J, De Vries G: Comparison of the parental behavior of pair-bonded female and male prairie voles (Microtus ochrogaster). Physiol Behav 1999; 66:33-40Crossref, Medline, Google Scholar
248. Wang Z, Novak M: Parental care and litter development in primiparous and multiparous prairie voles (Microtus ochrogaster). J Mammal 1994; 75:18-23Crossref, Google Scholar
249. Storey A, Joyce T: Pup contact promotes paternal responsiveness in male meadow voles. Animal Behav 1995; 49:1-10Crossref, Google Scholar
250. Storey A, Walsh C: Are chemical cues as effective as pup contact for inducing paternal behaviour in meadow voles? Behav 1994; 131:139-151Crossref, Google Scholar
251. Wynne-Edwards K, Lisk R: Differential effects of paternal presence on pup survival in two species of dwarf hamster (Phodopus sungorus and Phodopus campbelli ). Physiol Behav 1989; 45:465-469Crossref, Medline, Google Scholar
252. Wynne-Edwards K: Evidence for obligate monogamy in the Djungarian hamster, Phodopus campbelli: pup survival under different parenting conditions. Behav Ecol Sociobiol 1987; 20:427-437Crossref, Google Scholar
253. Gubernick D, Winslow J, Jensen P, Jeanotte L: Oxytocin changes in males over the reproductive cycle in the monogamous, biparental California mouse, Peromyscus californicus. Horm Behav 1995; 29:59-73Crossref, Medline, Google Scholar
254. Wang Z, De Vries G: Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster). Brain Res 1993; 631:156-160Crossref, Medline, Google Scholar
255. Bester-Meredith J, Young L, Marler C: Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav 1999; 36:25-38Crossref, Medline, Google Scholar
256. Heim C, Newport D, Heit S, Graham Y, Wilcox M, Bonsall R, Miller A, Nemeroff C: Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284:592-597Crossref, Medline, Google Scholar
257. Gold B, Bowers M, Roth R: GABA levels in cerebrospinal fluid of patients with psychiatric disorders. Am J Psychiatry 1980; 137:362-364Link, Google Scholar
258. Honig A, Bartlett J, Bouras N: Amino acid levels in depression: a preliminary investigation. J Psychiatr Res 1989; 22:150-164Google Scholar
259. Goodwin G, Austin M, Curran S, Ross M: The elevation of plasma beta-endorphin levels in major depression. J Affect Disord 1993; 29:281-289Crossref, Medline, Google Scholar
260. Maes M, Meltzer H, Cosyns P, Calabrese J, D’Hondt P, Blockx P: Adrenocorticotropic hormone, beta-endorphin and cortisol responses to oCRH in unipolar depressed patients pretreated with dexamethasone. Prog Neuropsychopharmacol Biol Psychiatry 1994; 18:1273-1292Crossref, Medline, Google Scholar
261. Morphy M, Fava G, Pedersen R, Zielezny M: Beta-endorphin responses to metyrapone and dexamethasone in depressed patients. Eur Neuropsychopharmacol 1992; 2:421-424Crossref, Medline, Google Scholar
262. Darko D, Irwin M, Risch S, Gillin J: Plasma beta-endorphin and natural killer cell activity in major depression: a preliminary study. Psychiatry Res 1992; 43:111-119Crossref, Medline, Google Scholar
263. Maes M, Jacobs M, Suy E, Leclercq C, Christiaens F, Raus J: An augmented escape of beta-endorphins to suppression by dexamethasone in severely depressed patients. J Affect Disord 1990; 18:149-156Crossref, Medline, Google Scholar
264. Galard R, Gallart J, Arguello J, Schwartz S, Castellanos JM, Catalan R: Plasma levels of beta-endorphin, cortisol, prolactin and growth hormone in depressed patients. Acta Psychiatr Scand 1988; 78:230-233Crossref, Medline, Google Scholar
265. Van Bockstaele E, Bajic D, Proudfit H, Valentino R: Topographical architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav 2001; 73:273-283Crossref, Medline, Google Scholar
266. Curtis AL, Valentino RJ: Corticotropin-releasing factor neurotransmission in locus coeruleus: a possible site of antidepressant action. Brain Res Bull 1994; 35:581-587Crossref, Medline, Google Scholar
267. Liu N, Ho I, Rockhold R: Contribution of glutamatergic systems in locus coeruleus to nucleus paragigantocellularis stimulation-evoked behavior. Pharmacol Biochem Behav 1999; 63:555-567Crossref, Medline, Google Scholar
268. Clayton E, Williams C: Glutamatergic influences on the nucleus paragigantocellularis: contribution to performance in avoidance and spatial memory tasks. Behav Neurosci 2000; 114:707-712Crossref, Medline, Google Scholar
269. Kaehler ST, Singewald N, Sinner C, Thurnher C, Philippu A: Conditioned fear and inescapable shock modify the release of serotonin in the locus coeruleus. Brain Res 2000; 859:249-254Crossref, Medline, Google Scholar
270. Otellin V, Gilerovich E, Mikhailova N: Target cells of serotoninergic innervation in the locus coeruleus. Neurosci Behav Physiol 1994; 24:457-461Crossref, Medline, Google Scholar
271. Shiekhattar R, Aston-Jones G: Sensory responsiveness of brain noradrenergic neurons is modulated by endogenous brain serotonin. Brain Res 1993; 623:72-76Crossref, Medline, Google Scholar
272. Mileykovskiy B, Kiyashchenko L, Kodama T, Lai Y, Siegel J: Activation of pontine and medullary motor inhibitory regions reduces discharge in neurons located in the locus coeruleus and the anatomical equivalent of the midbrain locomotor region. J Neurosci 2000; 20:8551-8558Medline, Google Scholar
273. Arnsten A, Segal D, Loughlin S, Roberts D: Evidence for an interaction of opioid and noradrenergic locus coeruleus systems in the regulation of environmental stimulus-directed behavior. Brain Res 1981; 222:351-363Crossref, Medline, Google Scholar
274. Kask A, Raego L, Harro J: Anxiolytic-like effect of neuropeptide Y (NPY) and NPY13-36 microinjected into vicinity of locus coeruleus in rats. Brain Res 1998; 788:345-348Crossref, Medline, Google Scholar
275. Altshuler LL, Cohen L, Szuba MP, Burt VK, Gitlin M, Mintz J: Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry 1996; 153:592-606Link, Google Scholar
276. Cohen L, Rosenbaum J: Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998; 59(suppl 2):18-28Google Scholar
277. Cohen L, Altshuler L: Pharmacologic management of psychiatric illness during pregnancy and the postpartum period. Psychiatr Clin North Am 1997; 4:21-59Google Scholar
278. Wisner KL, Zarin DA, Holmboe ES, Appelbaum PS, Gelenberg AJ, Leonard HL, Frank E: Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry 2000; 157:1933-1940Link, Google Scholar
279. Wisner K, Perel J: Psychopharmacological treatment during pregnancy and lactation, in Psychopharmacology and Women: Sex, Gender, and Hormones. Edited by Jensvold MF, Halbreich U. Washington, DC, American Psychiatric Press, 1996, pp 191-224Google Scholar
280. Stowe Z, Calhoun K, Ramsey C, Sadek N, Newport D: Mood disorders during pregnancy and lactation: defining issues of exposure and treatment. CNS Spectrums 2001; 6:150-166Crossref, Google Scholar
281. Newport D, Wilcox M, Stowe Z: Antidepressants during pregnancy and lactation: defining exposure and treatment issues. Sem Perinatology 2001; 25:177-190Crossref, Medline, Google Scholar



