Neurocognitive Effects of Clozapine, Olanzapine, Risperidone, and Haloperidol in Patients With Chronic Schizophrenia or Schizoaffective Disorder
Abstract
OBJECTIVE: Newer antipsychotic drugs have shown promise in ameliorating neurocognitive deficits in patients with schizophrenia, but few studies have compared newer antipsychotic drugs with both clozapine and conventional agents, particularly in patients who have had suboptimal response to prior treatments. METHOD: The authors examined the effects of clozapine, olanzapine, risperidone, and haloperidol on 16 measures of neurocognitive functioning in a double-blind, 14-week trial involving 101 patients. A global score was computed along with scores in four neurocognitive domains: memory, attention, motor function, and general executive and perceptual organization. RESULTS: Global neurocognitive function improved with olanzapine and risperidone treatment, and these improvements were superior to those seen with haloperidol. Patients treated with olanzapine exhibited improvement in the general and attention domains but not more than that observed with other treatments. Patients treated with risperidone exhibited improvement in memory that was superior to that of both clozapine and haloperidol. Clozapine yielded improvement in motor function but not more than in other groups. Average effect sizes for change were in the small to medium range. More than half of the patients treated with olanzapine and risperidone experienced “clinically significant” improvement (changes in score of at least one-half standard deviation relative to baseline). These findings did not appear to be mediated by changes in symptoms, side effects, or blood levels of medications. CONCLUSIONS: Patients with a history of suboptimal response to conventional treatments may show cognitive benefits from newer antipsychotic drugs, and there may be differences between atypical antipsychotic drugs in their patterns of cognitive effects.
Neurocognitive deficits are now recognized as an important dimension of schizophrenia that may be more closely linked to functional outcome than are symptoms (1). Until recently a spirit of nihilism characterized most cognition treatment studies of patients with schizophrenia, since conventional antipsychotic drugs yielded at best limited normalization of selected deficits (2). More recent studies that have used the atypical antipsychotic drug clozapine and other newer antipsychotic drugs such as risperidone and olanzapine have shown greater promise in treating neurocognitive deficits (3, 4). There is more information about clozapine’s effects on cognition than there is for other atypical antipsychotic drugs, which reflects clozapine’s earlier availability and use (5). So far only a few studies have reported head-to-head comparisons of clozapine and other newer antipsychotic drugs on cognitive functions, and none has used a double-blind design (6–8).
Since clozapine has remained the “gold standard” for patients with a history of suboptimal clinical response to treatment, it is important to determine specifically how its cognitive effects compare with those of newer agents and conventional treatment in this population. Published trials involving newer antipsychotic drugs have also been limited by a range of methodological issues, including small patient group sizes and the lack of random assignment to treatments (reviewed in reference 3). There is so far little evidence suggesting differences among atypical antipsychotic drugs in their cognitive effects, despite putative differences in mechanisms of action (5, 9, 10). One recent comparison of atypical agents showed a cognitive advantage for olanzapine over risperidone (11), but a larger trial revealed no clear differences (12). If there are differences between the neurocognitive effects of these agents, it could be of both clinical and theoretical importance.
We aimed to assess neurocognitive effects of four treatments—clozapine, haloperidol, olanzapine, and risperidone—in a group of patients with schizophrenia or schizoaffective disorder who had not responded fully to previous treatments; a 14-week, double-blind design was used. The primary clinical findings of this study have been previously published (13). In brief, clozapine, olanzapine, and risperidone (but not haloperidol) yielded significant improvements in symptoms. All three atypical antipsychotic drugs were superior to haloperidol in ameliorating negative symptoms, and this was not attributable to differences in extrapyramidal side effects. Clozapine was superior to risperidone in its effects on negative symptoms. We report here the results of neurocognitive assessments conducted at baseline (before random assignment) and at endpoint. We hypothesized that the atypical antipsychotic drugs would yield greater cognitive benefits than would haloperidol and aimed to determine whether there were any differences in neurocognitive effects among the atypical agents.
Method
Participants
The participants were 18- to 60-year-old inpatients at four state psychiatric hospitals (two in New York and two in North Carolina). After complete description of the study to the participants, written informed consent was obtained in line with each institution’s review board guidelines.
For inclusion in the study, patients were required to have a diagnosis of treatment-resistant DSM-IV schizophrenia or schizoaffective disorder. Treatment resistance was defined by two criteria, both of which had to be present. The first criterion was persistent positive symptoms (hallucinations, delusions, or marked thought disorder) after at least 6 contiguous weeks of treatment, presently or documented in the past, with one or more typical antipsychotics at doses ≥600 mg/day of chlorpromazine. The second criterion of treatment resistance was a poor level of functioning over the past 2 years, defined by the lack of competitive employment or enrollment in an academic or vocational program and not having age-expected interpersonal relations with someone outside the biological family of origin with whom ongoing regular contacts were maintained. In addition, patients were required to have a baseline total score ≥60 on the Positive and Negative Syndrome Scale (14).
Patients were excluded from the study if they had a history of nonresponse to clozapine, risperidone, or olanzapine, defined as an unambiguous lack of improvement despite a contiguous adequate trial of at least 6 weeks (for risperidone or olanzapine) or 14 weeks (for clozapine). Patients with a history of clozapine, olanzapine, risperidone, or haloperidol intolerance as well as those who received a depot antipsychotic within 30 days before random assignment were also excluded.
Treatments
During a baseline period of 1 to 2 weeks, patients’ prestudy antipsychotic medications were adjusted so that daily dose did not exceed 750 mg in chlorpromazine equivalents. Other concomitant medications such as mood stabilizers and antidepressants were gradually tapered and discontinued before patients received study medication. After baseline assessments, patients were randomly assigned to one of four treatment arms: clozapine, olanzapine, risperidone, or haloperidol. Trial antipsychotics were administered double-blind; all patients had weekly blood tests. Psychiatrists blind to treatment group assignment changed doses by prescribing “levels” of medication (detailed explanation of the procedures available on request). The 14-week trial consisted of an 8-week escalation and fixed-dose period and a 6-week variable-dose period.
During the first 8 weeks of the study, the prestudy antipsychotic was gradually discontinued while the doses of olanzapine, risperidone, and haloperidol were escalated to their target levels (20, 8, and 20 mg/day, respectively) at which they remained fixed until the end of the first study period. We aimed to reach the target level of clozapine (500 mg/day) on day 24; the dose remained fixed at that level until the end of the first study period. Dosing schedules were adjusted depending on the patient’s clinical status, including side effects. Mean dose levels (mg/day) achieved during this first period of the study (last observation carried forward) for patients included in the neurocognitive analysis were 452 (SD=121) for clozapine, 20.2 (SD=1.0) for olanzapine, 8.3 (SD=2.2) for risperidone, and 19.6 (SD=2.0) for haloperidol.
During the last 6 weeks of the study, antipsychotic dose was allowed to vary within the following ranges: clozapine, 200–800 mg/day; olanzapine, 10–40 mg/day; risperidone, 4–16 mg/day; and haloperidol, 10–30 mg/day. In general, doses were gradually increased if adequate improvement was not achieved. Side effects could preclude dose escalation and could lead to dose reductions. Psychiatrists who were blind to antipsychotic assignment prescribed all dose changes.
Throughout the study, all patients were receiving (double-blind) either benztropine or benztropine placebo or a combination of both. Benztropine (4 mg/day) was administered prophylactically to all patients receiving haloperidol. Patients assigned to atypical antipsychotic drugs were initially receiving only benztropine placebo. If the treating psychiatrist (who was unaware of antipsychotic assignments) determined clinically that a patient should be treated for extrapyramidal side effects, the psychiatrist could write prescriptions for “benztropine supplements” that would result in real benztropine gradually replacing benztropine placebo (up to 6 mg/day). An analogous arrangement for “supplements” was available to raise the dose of benztropine from 4 to 6 mg/day in patients assigned to haloperidol for emerging extrapyramidal symptoms. Propranolol was allowed for the treatment of akathisia.
Lorazepam, diphenhydramine hydrochloride, or chloral hydrate were prescribed open-label (by psychiatrists who were blind to antipsychotic treatment assignment) as needed for the treatment of agitation and insomnia in the dose ranges recommended by the manufacturers. No other adjunctive psychotropic medications (e.g., mood stabilizers and antidepressants) were allowed.
Clinical Assessments
Blind raters performed all clinical research assessments. The clinical symptom ratings included primarily the Positive and Negative Syndrome Scale (14) as a measure of efficacy, as reported separately (13). It was administered at baseline and then weekly during the first month of the study and every other week thereafter. Extrapyramidal side effects were assessed by trained raters using the Extrapyramidal Symptom Rating Scale (15). The time schedule for administration of the paired and single-rater Extrapyramidal Symptom Rating Scale was the same as for the Positive and Negative Syndrome Scale. Nursing staff rated sedation with the Nurses’ Observation Scale for Inpatient Evaluation (NOSIE) (16). Trained raters also used the Quality of Life Scale (17) to evaluate patients at baseline and endpoint.
Blood samples for the determination of the antipsychotic plasma levels were drawn at baseline and at weeks 5, 8, and 10–14. Samples were drawn approximately 12 hours after the last dose of medication. Clozapine assay used a modification of a published method (18). Olanzapine assay was a modified validated liquid chromatographic procedure with electrochemical detection (19). Risperidone and 9-hydroxyrisperidone assays used a modified liquid chromatographic method (20). Haloperidol assay was based on a published method (21).
Neurocognitive Assessments
The neurocognitive battery was designed to examine functional domains previously considered important by virtue of their demonstrated impairment in people with schizophrenia, their relations to functional outcomes, or their demonstrated change in prior studies of antipsychotic treatment. These assessments focused on measures of general ability, learning and memory, attention, executive functions, and motor skills. The battery included 15 tests that assessed these domains. The specific tests used have been described extensively in prior research by us and others (1, 22, 23). Patients were assessed at the end of the lead-in period before random assignment and at the end of the 14-week trial. In case of premature termination between weeks 4 and 14, endpoint neuropsychological assessments were performed. To minimize sedation effects, assessments were postponed until at least 24 hours after the last dose of any treatment for agitation.
Data Analysis
Before analysis of neurocognitive data, we examined major demographic variables, treatment, and clinical outcomes in the patients that completed neurocognitive testing compared with those who did not. All neurocognitive variables were examined in terms of their distribution properties to determine whether baseline differences in neurocognitive scores existed between treatment groups. Sixteen variables were selected from 12 tests for analysis of treatment effects on neurocognitive domains (the three tests not considered for scale construction were the Mini-Mental State Examination, which is a general screening instrument, and the WAIS-R vocabulary and information subtests, which were only administered at baseline to help estimate general cognitive ability and index “premorbid” cognitive capacity). Principal-components analysis on all nonmissing baseline data was used to determine whether the 16 neurocognitive test variables could be reduced to a smaller number of interpretable domain scores. For each test variable, z scores (with mean=0 and SD=1) were computed by using the baseline means and standard deviations from patients who completed that test at both baseline and endpoint. One “global” score (reflecting the equally weighted mean of all nonmissing scores on the 16 test variables) and domain scores were then computed by averaging the z scores on contributing variables; all z scores were computed so that positive scores indicate better performance. Analysis of treatment effects in each domain used the mixed-models approach to repeated-measures analysis of variance (SAS Institute, Cary, N.C.) with baseline and endpoint scores as dependent variables, time as a within-subject repeated measure, and treatment group (clozapine, haloperidol, olanzapine, risperidone) as a between-subjects fixed factor. To control for type I error given the multiplicity of statistical tests, we used a step-down Bonferroni procedure that considers the fact that effects of interest may be observed for both the main effect of time (since there are four treatment groups, any one of which can show change) and the interaction effect of treatment group with time (yielding six pairwise comparisons between the treatment groups) (24). For analysis of the global scale the family-wise alpha was set at 0.05, and for the tests of the four domain scores we divided alpha by the number of scales to set the critical alpha level (0.05/4=0.0125). Individual test scores were not analyzed separately, since with 16 tests we felt the analysis would suffer either from capitalization on chance (given the number of tests) or from overly conservative alpha levels that would be needed to control for test multiplicity.
There is no widely accepted method for deciding what are clinically significant (as opposed to statistically significant) changes in neurocognitive performance. Some investigators have suggested that a change of 0.5 standard deviation units (with respect to baseline score and standard deviation) is “clinically meaningful” (12). To determine whether treatment groups might differ in clinical improvement, we examined the proportions of patients within each treatment group that showed at least a 0.5 standard deviation improvement on the global scale and tested the significance of differences among the treatments by using chi-square analysis.
Results
Demographic and clinical characteristics of the 101 patients who contributed neurocognitive data are shown in Table 1. There were no significant differences among treatment groups in race, sex, or diagnostic composition, nor were there differences in age, duration of illness or age at onset, number of previous hospitalizations, or duration of time spent in the study. There were no differences in baseline psychopathology scores, except that patients in the clozapine group had higher baseline scores on the general psychopathology subscale of the Positive and Negative Syndrome Scale.
Comparisons of the 101 patients who participated in the neurocognitive assessments to the 56 patients who did not revealed no significant differences on any of the demographic or clinical variables, including baseline scores on the Positive and Negative Syndrome Scale (for total score or positive symptom, negative symptom, or general psychopathology subscale scores, all p>0.15).
Neurocognitive Test Results
Principal-components analysis on the 16 baseline scores yielded four factors with eigenvalues greater than one that were retained and subjected to varimax rotation (Table 2). While labeling of factors derived from principal-components analysis is a subjective process, we believe the assignment of tests to each domain (indicated by bold in Table 2) was interpretable on the basis of existing theory. We also conducted a principal-components analysis that included the baseline WAIS-R Information and Vocabulary scores to determine whether the factor structure might be influenced by these measures often considered indices of “premorbid” intellectual ability. That analysis yielded the same four factors as the original principal-components analysis plus a fifth factor that contained only these two WAIS-R variables, which supported the original principal-components analysis and the four-factor solution. Thus, four domain scores were constructed on the basis of the four-factor principal-components analysis, as the mean of z scores on contributing variables, with each variable weighted equally (i.e., each variable z score was weighted 0 or 1, rather than using the rotated factor scores). These z scores were based on the mean and standard deviation of each test at baseline and used the group of patients who completed each test at both baseline and follow-up. Signs were adjusted so that higher positive values indicated better performance, while more negative values indicated worse performance. The psychometric properties of the resulting scales were evaluated by computing coefficient alpha and test-retest stability coefficients (global scale: 0.89 and 0.73, respectively; general executive and perceptual organization: 0.81 and 0.73; declarative verbal learning and memory: 0.88 and 0.63; processing speed and attention: 0.81 and 0.66; simple motor: 0.89 and 0.62).
Baseline and endpoint scores for the individual tests and the neurocognitive domains within each treatment group are shown in Table 3. While there was an overall tendency for scores to be higher at endpoint, performance on individual neurocognitive tests remained at impaired levels with respect to healthy normative standards.
Treatment Effects
The main analyses of treatment effects are presented in Table 4.
For each score we examined the main effect of time (baseline, endpoint) and the group-by-time interaction effect. Table 4> shows that there was a significant main effect of time on the global score and on three of the four domain scores, suggesting that some groups showed significant changes from baseline to endpoint. Post hoc tests revealed which groups changed: 1) in global score, patients treated with olanzapine and risperidone improved; 2) in general executive and perceptual organization, patients treated with olanzapine improved; 3) in processing speed and attention, patients treated with olanzapine improved; and 4) in simple motor functioning, patients treated with clozapine improved. The group-by-time interaction effects were also significant for the global score and for the declarative verbal learning and memory domain. For the global score, post hoc tests revealed that both olanzapine and risperidone treatment resulted in greater improvement over time than did haloperidol treatment. For the declarative verbal learning and memory domain, treatment with risperidone resulted in greater improvement over time than did treatment with either clozapine or haloperidol. Inspection of the least squares means in Table 3 reveals that the changes from baseline to endpoint ranged up to 0.68 scale score points. The actual effect sizes (i.e., using Cohen’s d) tended to be slightly larger, since the standard deviations of the composite scores tended to be less than 1.0. The highest effect size (d=0.74) was seen with olanzapine treatment for change in processing speed and attention. Medium effect sizes for change on the global scale were seen for olanzapine (d=0.59) and risperidone (d=0.55), whereas the effect sizes were small for clozapine (d=0.21) and haloperidol (d=–0.08).
Relation of Neurocognitive Change to Clinical and Treatment Variables
A series of additional analyses examined the degree to which changes in neurocognitive scores might relate to clinical rating scale assessments, side effects, and treatments received.
To assess relations to clinical change, the clinical variables were used as time-varying covariates in the mixed-model repeated-measures analyses of variance. Clinical variables included the total score and positive symptom, negative symptom, and general psychopathology subscale scores from the Positive and Negative Syndrome Scale and the Quality of Life scale scores. These analyses were designed both to assess the degree of association between the clinical variables and the neurocognitive effects and the degree to which the neurocognitive effects might remain significant even after accounting for the variance explained by clinical change.
We used the step-down Bonferroni procedure to establish the same thresholds for assessing statistical significance as we did in the main analyses (for the global scale, family-wise alpha=0.05; for the four neurocognitive domain scores: family-wise alpha=0.0125). The Positive and Negative Syndrome Scale total score was significantly related to the global score (F=6.86, df=1, 95, p<0.01) and the domains of general executive and perceptual organization (F=6.53, df=1, 94, p<0.01) and processing speed and attention (F=6.86, df=1, 90, p<0.01). The positive symptom subscale score was not significantly related to any of the neurocognitive measures. The negative symptom subscale score was most robustly associated with the neurocognitive scores and significantly associated with the global score (F=15.27, df=1, 95, p<0.0002) and the domains of general executive and perceptual organization (F=11.31, df=1, 94, p<0.001) and processing speed and attention (F=6.79, df=1, 90, p<0.01). The general psychopathology subscale score was significantly related only to the global neurocognitive score (F=4.68, df=1, 95, p<0.03). There were no significant associations of Quality of Life ratings with any of the neurocognitive scores. Among adverse effects, we found no significant relations with ratings of sedation from the NOSIE, but we did find significant effects of Extrapyramidal Symptom Rating Scale ratings on the global scale (F=4.78, df=1, 95, p<0.03), which appeared to be due to a relatively strong effect on the simple motor functioning domain (F=14.18, df=1, 74, p<0.0003).
Despite the relations of the covariates with neurocognitive variables, the Positive and Negative Syndrome Scale scores had relatively little influence on the significance of the main effects of time, the group-by-time interaction effects, or the post hoc comparisons among treatment groups that were reported in the main analyses. For the global score, none of these covariates modified the pattern of significant findings as reported in Table 4. Among the other neurocognitive domain scores, the pattern of significant findings remained, with one exception. There was no longer a main effect of time at the 0.0125 alpha level for the general executive and perceptual organization domain after the Positive and Negative Syndrome Scale total score (F=3.96, df=1, 94, p<0.05) and the positive symptom (F=5.75, df=1, 94, p<0.019), negative symptom (F=6.02, df=1, 94, p=0.016), and general psychopathology (F=5.15, df=1, 94, p<0.03) subscale scores were entered as covariates; all other effects and post hoc comparisons remained significant. Analyses that used benztropine dose as a covariate also rendered nonsignificant the main effect of time on the general executive and perceptual organization domain (F=2.48, df=1, 54, p<0.12), but other significant effects noted in Table 4 remained. Quality of Life scale, sedation ratings, and Extrapyramidal Symptom Rating Scale scores did not alter the pattern of effects in Table 4.
To examine possible effects of medication blood levels, we used the mixed-model approach separately within each treatment group (since the values were highly unbalanced across the groups) with endpoint dose as a time-invariant covariate. Here again we used family-wise alpha=0.05 for global score and family-wise alpha=0.0125 for the four neurocognitive domains. There were no significant interactions of blood level with time for any scale within any treatment group. Within the haloperidol group, higher blood levels were associated with less improvement on the motor functioning domain, but the finding did not achieve significance (F=4.45, df=1, 24, p<0.05).
“Clinically Significant” Improvement in Neurocognitive Function
Finally, we examined the proportions of patients improving by at least 0.5 standard deviation on the global scale and found significant differences between treatment groups in the proportions improved (χ2=17.5, df=3, p<0.0006) (Figure 1). Pairwise comparisons revealed that more patients were classified as having improved in the olanzapine group than in either the haloperidol group (χ2=14.3, df=1, p<0.0002) or the clozapine group (χ2=9.6, df=1, p<0.002); the percentage of patients experiencing clinically significant improvement did not significantly differ between those given olanzapine and those given risperidone. Significantly more patients treated with risperidone than with haloperidol met the clinically significant improvement criteria (χ2=6.0, df=1, p<0.02).
Discussion
This study of patients randomly assigned to treatments with the atypical antipsychotic agents clozapine, olanzapine, and risperidone or the conventional neuroleptic agent haloperidol showed an overall superiority of olanzapine and risperidone on a range of neurocognitive functions. When we compared the gains in global cognitive performance, both olanzapine and risperidone showed statistically significant changes with treatment, and both olanzapine and risperidone were superior to haloperidol, but their effects did not differ significantly from each other or from clozapine.
The change in global neurocognitive performance (using standard deviations of the baseline scores as an index of effect size) was of medium magnitude for treatment with olanzapine (0.59) and risperidone (0.55). For comparison to a widely used metric, this would be approximately eight to nine “IQ-equivalent” points. While our patients continued to have test scores reflecting significant neurocognitive impairment, more than half of the patients who received olanzapine or risperidone showed cognitive gains greater than 0.5 standard deviation (i.e., 7.5 IQ-equivalent points). These gains were large enough to be considered clinically significant and were larger than would be expected from prior test exposure effects. Overall the findings suggest that significant cognitive improvements may be possible even among patients who have shown limited response to conventional treatment.
One potentially surprising aspect of this study was the relatively modest neurocognitive benefit of clozapine treatment, despite the finding that clozapine had robust effects on clinical symptoms in these patients (13). It is possible that the failure to demonstrate neurocognitive benefits of clozapine reflects our failure to test relevant constructs. It was commented previously that clozapine’s unique mechanism of action may preferentially involve “paleocortical” systems, particularly those of the ventral and orbital frontal regions, that have been linked to social cognition and response-inhibition functions (5). Assessment of these functions has often been incomplete in conventional neurocognitive batteries (25, 26).
Clozapine only had a significant beneficial impact on motor performance. This might be explained by clozapine’s relative low affinity for the dopamine-2 (D2) receptor or its unique regional distribution of effects within the basal ganglia. The lack of differences between treatments on motor functioning should be considered in the context of the treatment algorithm, which was designed to minimize adverse motor side effects through prescription (by psychiatrists blind to treatment assignment) of adjunctive antiparkinsonian treatments where indicated. These results might be considered support for the idea that clozapine exerts its most prominent beneficial cognitive effects by means of reduction in motor and cognitive slowing associated with D2 antagonism in the frontostriatal system (5).
Olanzapine and risperidone had a similar magnitude of effect on global neurocognitive function, but inspection of results from individual neurocognitive domains suggests that there may be more specific effects of these drugs that could be pursued in future investigations. Treatment with olanzapine resulted in significant improvement in the domains of processing speed and attention and general executive and perceptual organization. Various explanations for olanzapine-associated benefits have usually centered on less activity at the D2 receptor, along with more pronounced serotonergic, adrenergic, histaminic, or effects at non-D2 dopamine receptors (10). In contrast, risperidone appeared to show more robust effects in the domain of declarative verbal learning and memory, for which it was superior to both haloperidol and clozapine treatment.
These differences might be explained in part by anticholinergic mechanisms that are known to affect memory. Anticholinergic effects could be associated with the intrinsic properties of the antipsychotics or be due to co-administered benztropine (1, 27, 28). Risperidone might be superior to clozapine because clozapine treatment involves high levels of intrinsic anticholinergic activity and has been associated with memory deficit (29). The observation that olanzapine was not superior to either haloperidol or clozapine in its effect on memory might also be explained by its high levels of intrinsic anticholinergic activity, which may be higher than those observed with clozapine treatment (30). Anticholinergic effects may also have led the haloperidol patients to perform worse, since they received obligatory benztropine. Although we found no statistical association of anticholinergic blood levels with memory change, paralleling the findings of Green and colleagues (31), it is possible that more sensitive measures of anticholinergic activity might detect such effects.
Our findings might be considered at odds with some conclusions presented elsewhere in the literature. For example, one review suggested different profiles of selective cognitive efficacy for different atypical antipsychotic drugs (10). On the basis of a survey of 18 publications and their own open trial of 20 patients switched from typical antipsychotics to olanzapine, Meltzer and McGurk suggested that effects were most robust with clozapine for attention and verbal fluency; risperidone for working memory, executive functioning, and attention; and olanzapine for verbal learning/memory, fluency, and executive function. While efforts to summarize these findings are laudable, it should be noted that the conclusions were based on early studies with relatively small study group sizes, only two of which used double-blind designs, and none of which provided head-to-head comparisons among all three agents, as our study did. One recent double-blind study did include head-to-head comparisons of patients randomly assigned to treatment with risperidone, haloperidol, or olanzapine and concluded that olanzapine had superior cognitive benefits than both risperidone and haloperidol (11). Some interpretive challenges for that study are posed by the relatively small study group sizes (among 65 patients completing baseline assessments, 10 terminated before completing a second assessment, and 27 more failed to complete the study). The authors of that study highlighted the possible problem of selective attrition in both the haloperidol group (which had a disproportionate number of early dropouts) and the risperidone group (for which there were different results from the intent-to-treat last-observation-carried-forward analyses, compared with other analyses). Overall, however, we believe that the findings published so far are relatively consistent in showing cognitive benefits of the newer antipsychotics compared with conventional treatments. We further believe efforts to discern differences in the neurocognitive profiles of different agents are likely to require substantial further study with designs that specifically target this goal, with perhaps meta-analysis of such studies to help overcome the necessary limitations of any individual project.
There were generally modest associations of neurocognitive change with symptom ratings. Paralleling prior research, negative symptoms showed a more consistent pattern of association with neurocognitive deficits. Of possible interest are the observations that these symptoms were associated with general, executive, processing speed, and attentional measures but not with verbal learning/memory or motor functions, suggesting that these might constitute unique syndromal elements. The treatment effects on neurocognitive function did not appear to be substantially modulated by symptoms. These findings support the contention that treatment-related modulation of cognition and symptoms may proceed with considerable independence. Neurocognitive change also was not clearly related to a range of other possible mediating factors, including extrapyramidal symptoms, sedation, or adjunctive medication use (which did not differ among treatment groups [13]).
The use of cognitive domain scores likely increased the sensitivity of this study compared with those that used individual neurocognitive test scores. First, composite scores have higher reliability and stability than do individual test scores. Second, reducing the number of variables examined (in this case from 16 to four) reduces the stringency of corrections for multiple comparisons. The assignment of variables to domains, which in this study was informed by factor analysis, would likely differ in other samples or if different tests were used. For this patient group and this set of variables, however, these domain scores appeared to provide an intuitively appealing and psychometrically reasonable summary of neurocognitive performance.
Finally, there are limitations of this study that should be acknowledged. First, it is not clear how well the results may generalize to patients with a more favorable response to treatment. The findings appear consistent, however, with other studies of the neurocognitive effects of atypical antipsychotics in treatment-responsive and first-episode patient groups (reviewed in references 3 and 4; see also recent presentations [12, 32]). Second, this study used relatively high doses of all medications, particularly risperidone. This might be expected to yield higher levels of extrapyramidal side effects and higher doses of adjunctive anticholinergic treatments and thus either more motor slowing or more memory impairment than might be the case with lower-dose treatment. It should be noted, however, that all treatments were adjusted by clinicians who were blind to treatment assignment, and extrapyramidal symptoms actually decreased with all treatments. These patients had not shown good clinical responses to a broad range of prior treatments, usually reflecting attempts with multiple conventional antipsychotic drugs and adjunctive agents. Third, it should be noted that the study inclusion/exclusion criteria permitted patients who had limited prior response to haloperidol, but not patients who had demonstrated lack of response to the other treatments. This might have biased the findings toward showing less improvement in the haloperidol group. Fourth, while the double-blind, random assignment design of this trial had many advantages, we cannot rule out the possibility that apparent improvements attributable to some treatments (i.e., the newer agents) may instead reflect the effects of prior exposure or experience with the tests, while other treatments (i.e., haloperidol) might have prevented these effects. Future trials might incorporate multiple baseline assessments, additional control groups, or other strategies to address the possibility that some treatments permit benefit from prior test exposure whereas others do not. In the meantime we believe the findings reported here reflect cognitive changes that may be clinically meaningful, regardless of whether these are due to direct effects of the newer agents or whether the observed changes reflect differences in the capacity to benefit from experience. It remains to be demonstrated whether cognitive changes of this magnitude, in this group of patients, make a substantial difference in patients’ social and vocational functioning or other indices of outcome that were not assessed in our study. It must be remembered that despite cognitive gains, our patients continued to suffer from significant impairments of cognitive ability and social/vocational functioning. Overall, however, we believe that these patients represent a large and important population of individuals who may show meaningful neurocognitive benefits from treatment with newer antipsychotic agents. We hope that future research will examine further domains of neurocognitive and social/emotional functions that may be modulated by these treatments and delineate the relations of these effects to both underlying mechanisms and functional outcomes.
 |
 |
 |
 |
Presented in part at the eighth International Congress on Schizophrenia Research, Whistler, B.C., Canada, April 28–May 2, 2001. Received June 18, 2001; revision received Dec. 10, 2001; accepted Dec. 27, 2001. From the Nathan S. Kline Institute for Psychiatric Research; Dorothea Dix Hospital, Raleigh, N.C.; Manhattan Psychiatric Center, New York; and John Umstead Hospital, Butner, N.C. Address reprint requests to Dr. Bilder, Nathan S. Kline Institute for Psychiatric Research, 140 Old Orangeburg Rd., Orangeburg, NY 10962; [email protected] (e-mail). NIMH grant MH-53550 provided the principal support for this project, with additional support provided by the University of North Carolina Mental Health and Neuroscience Clinical Research Center (MH-33127) and the Foundation of Hope, Raleigh, N.C. Janssen Pharmaceutica Research Foundation, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, and Merck and Co., Inc., provided the medications. Eli Lilly and Company contributed supplemental funding. However, overall experimental design, data acquisition, statistical analyses, and interpretation of the results were implemented with no input from any of the pharmaceutical companies.
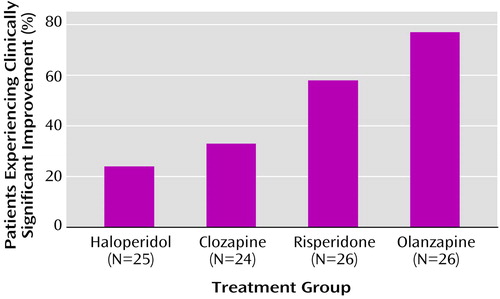
Figure 1. Percentages of Subjects Experiencing Significant Clinical Improvementa Among Patients With Treatment-Resistant Schizophrenia or Schizoaffective Disorder Randomly Assigned to Receive Clozapine, Haloperidol, Olanzapine, or Risperidone
aChange of at least one-half standard deviation in global neurocognitive score.
1. Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA: Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry 2000; 157:549-559Link, Google Scholar
2. Spohn HE, Strauss ME: Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J Abnorm Psychol 1989; 98:367-380Crossref, Medline, Google Scholar
3. Keefe RS, Silva SG, Perkins DO, Lieberman JA: The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 1999; 25:201-222Crossref, Medline, Google Scholar
4. Harvey PD, Keefe RSE: Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 2001; 158:176-184Link, Google Scholar
5. Bilder RM: Neurocognitive impairment in schizophrenia and how it affects treatment options. Can J Psychiatry 1997; 42:255-264Crossref, Medline, Google Scholar
6. Daniel DG, Goldberg TE, Weinberger DR, Kleinman JE, Pickar D, Lubick LJ, Williams TS: Different side effect profiles of risperidone and clozapine in 20 outpatients with schizophrenia or schizoaffective disorder: a pilot study. Am J Psychiatry 1996; 153:417-419Link, Google Scholar
7. Meyer-Lindenberg A, Gruppe H, Bauer U, Lis S, Krieger S, Gallhofer B: Improvement of cognitive function in schizophrenic patients receiving clozapine or zotepine: results from a double-blind study. Pharmacopsychiatry 1997; 30:35-42Crossref, Medline, Google Scholar
8. Lindenmayer JP, Iskander A, Park M, Apergi FS, Czobor P, Smith R, Allen D: Clinical and neurocognitive effects of clozapine and risperidone in treatment-refractory schizophrenics: a prospective study. J Clin Psychiatry 1998; 59:521-527Crossref, Medline, Google Scholar
9. Meltzer HY: The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 1999; 21:106S-115SCrossref, Medline, Google Scholar
10. Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 1999; 25:233-255Crossref, Medline, Google Scholar
11. Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD (Canadian Collaborative Group for Research in Schizophrenia): Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Arch Gen Psychiatry 2000; 57:249-258Crossref, Medline, Google Scholar
12. Harvey PD: Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder, in 2000 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 2000, p 149Google Scholar
13. Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002; 159:255-262Link, Google Scholar
14. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
15. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
16. Honigfeld G, Klett CJ: The Nurses’ Observation Scale for Inpatient Evaluation: a new scale for measuring improvement in chronic schizophrenia. J Clin Psychol 1965; 21:65-71Crossref, Medline, Google Scholar
17. Heinrichs DW, Hanlon TE, Carpenter WT Jr: The Quality of Life Scale: an instrument for rating the schizophrenic deficit synrome. Schizophr Bull 1984; 10:388-398Crossref, Medline, Google Scholar
18. Simpson GM, Cooper TA: Clozapine plasma levels and convulsions. Am J Psychiatry 1978; 135:99-100Link, Google Scholar
19. Catlow JT, Barton RD, Clemens M, Gillespie TA, Goodwin M, Swanson SP: Analysis of olanzapine in human plasma utilizing reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl 1995; 668:85-90Crossref, Medline, Google Scholar
20. LeMoing JP, Edouard S, Levron JC: Determination of risperidone and 9-hydroxyrisperidone in human plasma by high-performance liquid chromatography with electrochemical detection. J Chromatogr 1993; 614:333-339Crossref, Medline, Google Scholar
21. Bianchetti G, Morselli PL: Rapid and sensitive method for determination of haloperidol in human samples using nitrogen-phosphorus selective detection. J Chromatogr 1978; 153:203-209Crossref, Medline, Google Scholar
22. Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA: Anterior hippocampal volume reductions predict “frontal lobe” dysfunction in first episode schizophrenia. Schizophr Res 1995; 17:47-58Crossref, Medline, Google Scholar
23. Brandt J: The Hopkins Verbal Learning Test: development of a memory test with six equivalent forms. Clin Neuropsychol 1991; 5:125-142Crossref, Google Scholar
24. Westfall PH: Multiple testing of general contrasts using logical constraints and correlations. J Am Stat Assoc 1997; 92:299-306Crossref, Google Scholar
25. Lencz T, Cornblatt B, Bilder RM: Neurodevelopmental models of schizophrenia: pathophysiologic synthesis and directions for intervention research. Psychopharmacol Bull 2001; 35:95-125Medline, Google Scholar
26. Christensen BK, Bilder RM: Dual cytoarchitectonic trends: an evolutionary model of frontal lobe functioning and its application to psychopathology. Can J Psychiatry 2000; 45:247-256Crossref, Medline, Google Scholar
27. Strauss ME, Reynolds KS, Jayaram G, Tune LE: Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res 1990; 3:127-129Crossref, Medline, Google Scholar
28. Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT: Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry 1982; 139:1460-1462Link, Google Scholar
29. Goldberg TE, Weinberger DR: The effects of clozapine on neurocognition: an overview. J Clin Psychiatry 1994; 55(Sept suppl B):88-90Google Scholar
30. Chengappa KNR, Pollock BG, Parepally H, Levine J, Kirshner MA, Brar JS: Anticholinergic differences among patients receiving standard clinical doses of olanzapine or clozapine. J Clin Psychopharmacol 2000; 20:311-316Crossref, Medline, Google Scholar
31. Green MF, Marshall BD Jr, Wirshing WC, Ames D, Marder SR, McGurk S, Kern RS, Mintz J: Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry 1997; 154:799-804Link, Google Scholar
32. Keefe RSE, Seidman LJ, Christensen BK, Yurgelun-Todd DA, Lewine RJ, Sitskoorn M, Lieberman JA: Treatment of neurocognitive deficits with olanzapine or low-dose haloperidol in first episode psychosis (abstract). Schizophr Res 2001; 49(suppl 1):234Google Scholar



