The Functional Neuroanatomy of the Placebo Effect
Abstract
OBJECTIVE: Administration of placebo can result in a clinical response indistinguishable from that seen with active antidepressant treatment. Functional brain correlates of this phenomenon have not been fully characterized. METHOD: Changes in brain glucose metabolism were measured by using positron emission tomography in hospitalized men with unipolar depression who were administered placebo as part of an inpatient imaging study of fluoxetine. Common and unique response effects to administration of placebo or fluoxetine were assessed after a 6-week, double-blind trial. RESULTS: Placebo response was associated with regional metabolic increases involving the prefrontal, anterior cingulate, premotor, parietal, posterior insula, and posterior cingulate and metabolic decreases involving the subgenual cingulate, parahippocampus, and thalamus. Regions of change overlapped those seen in responders administered active fluoxetine. Fluoxetine response, however, was associated with additional subcortical and limbic changes in the brainstem, striatum, anterior insula, and hippocampus, sources of efferent input to the response-specific regions identified with both agents. CONCLUSIONS: The common pattern of cortical glucose metabolism increases and limbic-paralimbic metabolism decreases in placebo and fluoxetine responders suggests that facilitation of these changes may be necessary for depression remission, regardless of treatment modality. Clinical improvement in the group receiving placebo as part of an inpatient study is consistent with the well-recognized effect that altering the therapeutic environment may significantly contribute to reducing clinical symptoms. The additional subcortical and limbic metabolism decreases seen uniquely in fluoxetine responders may convey additional advantage in maintaining long-term clinical response and in relapse prevention.
There is little debate as to the power of the placebo effect in controlled short-term clinical trials of antidepressants, as well as in other medical and surgical treatments (1–3). Placebo response in the acute phase of antidepressant trials has often been seen as an unavoidable and distracting consequence inherent in the assessment of any given treatment intervention—whether cognitive, pharmacological, or surgical (4–11). While continuation studies (12–16) have repeatedly demonstrated an advantage of maintenance medication over continued placebo administration in preventing relapse and recurrence, the presence of a significant placebo effect with short-term administration provides a unique opportunity to examine brain mechanisms mediating clinical antidepressant response unencumbered by nonspecific drug, lesion, or learning effects evoked by medication, surgery, or cognitive therapy.
Positron emission tomography (PET) measures of regional glucose metabolism have proven to be sensitive indices of brain function in patients both in the untreated depressed state (17–24) and after disparate treatments (24–38). Functional changes in cortical (dorsal and ventral prefrontal, anterior temporal, inferior parietal), limbic-paralimbic (anterior, subgenual, and posterior cingulate; hippocampus; amygdala; anterior and posterior insula) and subcortical (basal ganglia, thalamus, brainstem) regions have also been described after various types of treatments, including medication, sleep deprivation, ECT, repetitive transcranial magnetic stimulation, and ablative surgery. Normalization of frontal hypometabolism is the best-replicated finding, seen mainly with medication treatment (25–31). Decreases in glucose metabolism in limbic and paralimbic regions, varying with drug treatment, are more common in studies of sleep deprivation, ECT, and surgery (29–38). There are surprisingly few data available on functional brain changes associated with cognitive interventions (39, 40), despite the repeated evidence that these strategies are of equal efficacy to drugs in alleviating core features in depressed patients with mild to moderately severe illness (41–44). Studies characterizing consistent changes common to treatment with different modes of action (39, 40, 45) are also sparse, as is the literature concerning the variability of regional effects associated with response to different treatments (46). We know of no PET studies of brain changes associated with placebo administration.
This study examined changes in regional brain glucose metabolism associated with placebo response in patients with major depression who were participating in a double-blind, placebo-controlled PET imaging study of the effects of the antidepressant fluoxetine (28, 29). We hypothesized the existence of a common pattern of regional metabolic changes with clinical response, independent of whether a patient was given active or inactive medication, with response to active fluoxetine showing additional regional changes reflective of drug-specific effects.
Method
Treatment Protocol
Seventeen unmedicated depressed men (age: mean=49 years, SD=9; current episode duration: mean=18 weeks, SD=2; score on 17-item Hamilton Depression Rating Scale: mean=22, SD=5, score on Mini-Mental State Examination: mean=29, SD=2) with symptoms requiring treatment were recruited from the inpatient psychiatry unit at the Audie L. Murphy Memorial Veterans Administration Hospital in San Antonio, Tex. The clinical diagnosis of a major depressive episode, unipolar type, was confirmed by two independent psychiatrists using DSM-IV criteria and a structured psychiatric interview (47). None of the enrolled subjects was considered treatment resistant by history. Exclusion criteria included a history of neurological disease, head trauma, or other axis I psychiatric diagnoses, as well as having current psychotic symptoms, substance abuse, antidepressant treatment within the preceding month, previous nonresponse to fluoxetine, or previous ECT. Written informed consent was obtained from all subjects, and the study was conducted as approved by the institutional review board of the University of Texas Health Science Center.
The patients were treated on an inpatient research unit for 6 weeks. The patients were randomly assigned, with use of a double-blind study design, to either fluoxetine, 20 mg/day, fixed dose, or placebo. Additionally, all patients received the therapeutic benefits of the standard ward milieu, which included daily individual meetings with the treating physician, group therapy, and various ward activities. The patients did not receive interpersonal psychotherapy or cognitive behavior therapy during the 6 weeks. The patients, treating physicians, ward personnel, and PET imaging team remained blind to the specific substance received during the full 6-week study.
Imaging Studies
Regional cerebral glucose metabolism was measured with standard methods (48) in all patients by using [18F]fluorodeoxyglucose (FDG) and PET before and after 1 and 6 weeks of administration of fluoxetine or placebo. For each scan, a 5-mCi dose of FDG was injected intravenously, with image acquisition beginning after 40 minutes (scan duration: 20 minutes; GE/Scanditronix 4096 [General Electric, Milwaukee]; 15 parallel slices; 6.5-mm, center-to-center interslice distance; measured attenuation correction with transmission scans; reconstructed with a Hann filter; final in-plane resolution: 7.0 mm, full width at half maximum). The absolute glucose utilization rate was not calculated.
All scans were acquired with the patients in the supine position, awake, in the resting state, with eyes closed and ears uncovered. The patients were checked every 10 minutes to ensure they were not asleep. The subjects were not explicitly instructed to monitor their mood state or to perform any specific cognitive task. This approach was aimed at examining regional effects associated with illness remission without the potential interpretive confounds introduced by explicit manipulation of affective or cognitive states (29, 49). A debriefing session after the uptake period documented compliance with the instructions in all subjects. The patients did not smoke in the 60 minutes before FDG injection. An anatomical magnetic resonance imaging scan was also acquired in each subject for spatial transformation of the PET data, region-of-interest analysis, and parametric image display (Elscint Gyrex 2T-DLX [Haifa, Israel]; three-dimensional gradient/recall acquisition in the steady state; TR=33 msec; TE=12 msec; flip angle=60°; volume=256×256×127; spatial resolution=1 mm3).
Data Analysis
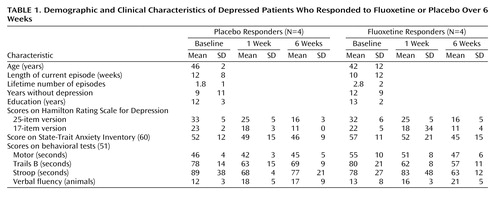
Overall clinical improvement was quantified at the 1-week and 6-week PET sessions by using the Hamilton Depression Rating Scale (50) and a computerized battery of standardized behavioral tests targeting mood, motor performance, and cognition, with special emphasis on motor performance, information-processing speed, and executive functioning (51). Included were tests of simple and choice reaction times, versions of the Stroop and Trails tests, tests of verbal fluency, and tests of fine and gross motor coordination. Responders were defined as having a minimum of 50% decrease in Hamilton depression scale scores relative to those at baseline (50, 52).
State-related changes in regional glucose metabolism (after treatment versus baseline) were detected by using change distribution analysis (53) and interpreted by using coordinates and Brodmann’s areas from the Talairach and Tournoux atlas of the brain (54). Value and spatially normalized images were first trilinearly interpolated, resampled (60 slices, 8-mm3 voxels), and Gaussian-filtered to a final resolution of 9.9 mm (full width at half maximum) (55). A voxel-by-voxel subtraction using the pairs for the targeted condition was next performed for each individual. Within-subject differences were then averaged across subjects, creating a grand-mean-difference image for each contrast. A beta-2 statistic measuring kurtosis of the histogram of the difference image (change distribution curve) was used as an omnibus test to assess overall significance (56). The beta-2 test was implemented with MIPS software (Research Imaging Center, San Antonio, Tex.) in a manner similar to that of the gamma-2 statistic (53). The beta-2 improves on the gamma-2 by using a better estimate of the degrees of freedom, i.e., the number of “resyls” in the PET images (57). The omnibus test was followed by a maxima and minima search to identify local extrema within a search volume measuring 125 mm3(58).
To facilitate reporting and visualization of these maxima and minima, group-mean-subtraction images were converted post hoc to statistical parametric images of z scores on the basis of the variance of all local changes within the subtraction images (Figure 1). Locations of focal maxima and minima exceeding a z score of 2.6 (p<0.01) were determined, with the peak voxel of each area described in x, y, and z coordinates as millimeters relative to the anterior commissure.
Six-week versus baseline and 1-week versus baseline comparisons were computed separately for placebo-responder and drug-responder groups. The 6-week response effect with placebo administration was the primary focus of this study. Anatomical concordance (and discordance) of focal maxima and minima across contrasts (response effect: changes in placebo versus fluoxetine responders) was defined as common (or different) Brodmann’s area identifiers for the coordinate locations (59), as well as evidence of anatomical overlap in the extent of significant activations or deactivations seen with the use of a logical contrast of two z score maps (Figure 2) (28). Comparisons of 1-week versus 6-week regional metabolic changes in fluoxetine-treated patients (both responders and nonresponders) were the subject of a previous report (29).
Results
Symptom remission was seen in eight of the 15 study completers. Breaking of the blind revealed that four of the eight responders had been treated with placebo and four with active drug. Mechanical problems with the PET camera precluded the timely scanning of two of the original 17 subjects, both of whom remained clinically symptomatic at 6 weeks and later proved to have been given placebo.
Clinical improvement was comparable for the placebo- and fluoxetine-responder groups, and there were no differences in pretrial demographic or illness characteristics or in cognitive performance variables (Table 1). There were no significant differences after 1 week of the trial on any clinical variable that predicted 6-week clinical outcome in either group or distinguished patients given placebo from patients given drug. Factor subscores on the Hamilton depression rating scale (61) evaluating mood, sleep, and somatic symptoms also did not distinguish the two groups.
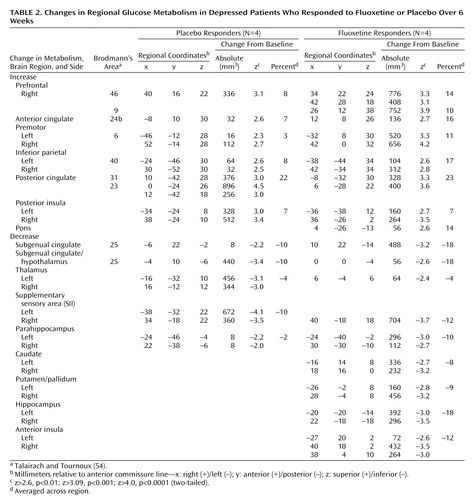
Placebo response at 6 weeks was associated with significant regional metabolic changes (scan 3 versus baseline metabolism: beta-2=3.97, df=1972, p<0.0001). Both increases and decreases in glucose metabolism that involved both neocortical and limbic-paralimbic regions were identified (Figure 1, top). Areas of significant increases in metabolism were seen in the prefrontal cortex (Brodmann’s area 9/46), premotor cortex (Brodmann’s area 6), inferior parietal cortex (Brodmann’s area 40), posterior insula, and posterior cingulate (Brodmann’s area 23/31). Decreases in metabolism were localized to the subgenual cingulate (Brodmann’s area 25), hypothalamus, thalamus, supplementary sensory area insula, and parahippocampus (Table 2, left).
One-week metabolic changes in placebo responders did not meet the omnibus threshold for significance. Placebo nonresponders were not evaluated because of an inadequate group size (N=3; no 6-week scan for two of the three nonresponders).
Response to active fluoxetine treatment was also associated with significant regional metabolic changes (scan 3 minus baseline metabolism: beta-2=3.44, df=1972, p<0.0006). The pattern of metabolic change closely matched that seen with response to placebo (Table 2, right). Drug responders, however, showed additional changes in metabolism in subcortical and limbic regions (Figure 1, bottom). Specifically, fluoxetine response was associated with unique increases in brainstem metabolism and metabolic decreases in the striatum, hippocampus, and anterior insula. There were no regional changes that were unique to placebo responders at 6 weeks.
While the locations of regional changes showed remarkable concordance across groups, the magnitude of changes with fluoxetine treatment was generally greater and the number of pixels demonstrating significant changes for a given region generally involved a greater volume than that seen with placebo response (Table 2, Figure 2).
Discussion
There are two key findings from this small but unique study of brain changes in glucose metabolism associated with placebo response. First, placebo response was associated with regionally specific changes in brain function. Second, while comparable brain changes were seen with both drug and placebo administration, drug response was not merely the same as the placebo effect, as active fluoxetine treatment was associated with additional and unique changes in the brainstem, striatum, and hippocampus. Despite these differences, clinical response to treatment, independent of whether the substance administered was active fluoxetine or placebo, was associated with a common pattern of reciprocal changes in specific cortical and paralimbic regions.
Response Mechanisms
Mechanisms of antidepressant medication response have generally focused on adaptive neurochemical changes, including long-term aminergic reuptake inhibition and associated presynaptic autoregulatory desensitization, up- and down-regulation of multiple postsynaptic receptor sites, and receptor-mediated second messengers and neurotrophic effects (62–64). Pharmacological studies have emphasized a bottom-up cascade; brainstem, limbic, and subcortical sites are generally viewed as the primary sites of drug action (65, 66), with secondary cortical changes seen as secondary effects of long-term treatment (67–71).
Nonpharmacological antidepressant treatments, on the other hand, emphasize the alteration of relevant cognitions, affects, and maladaptive information processing caused by the clinical depression (72–75). Attention to development of new cognitive strategies that enhance awareness of self-defeating thinking styles and behavioral patterns that contribute to feelings of depression is a primary goal (76–80). These approaches can also be conceptualized as affecting changes in specific neural pathways by means of top-down or cortical mechanisms.
Placebo administration, while inarguably a nonpharmacological intervention, has certain distinctions from more explicit cognitive or psychotherapeutic strategies. From a cognitive perspective, placebo administration, unlike formal cognitive therapies, is an implicit form of antidepressant treatment (1–3). Also, there is an expectation that improvement will take place, but unlike cognitive or other psychotherapeutic approaches, there is no specific or explicit process or procedure involved. Moreover, placebo administration is also not a totally passive process. Patients in a placebo-controlled trial enter into a formal research relationship with a well-defined beginning and end, with the hope and expectation that their symptoms will improve over the course of the study (81, 82). The therapeutic milieu of an inpatient psychiatric ward and the associated support system may be an additional factor (83, 84). A “resetting” of normal social and physical rhythms disrupted by psychosocial stressors that contribute to the initiation and maintenance of a depressive state may be indirectly facilitated by an inpatient treatment trial that removes a patient from the often maladaptive environment in which his or her illness is perpetuated (85, 86). It is therefore emphasized that administration of placebo is not absence of treatment, just an absence of active medication. These findings have important implications for more specific types of nonpharmacological therapies that explicitly target maladaptive cognitive processing. Clearly, this psychosocial and physical re-setting facilitated by the hospitalization itself was not adequate to initiate or maintain a clinical response in all patients in our study.
Fluoxetine-Specific Metabolic Effects
The unique brain changes in the brainstem and hippocampus seen in the group receiving active fluoxetine might be interpreted as medication effects responsible for side effects rather than as clinical response. Said another way, one might view the findings as supporting the premise that both groups showed a placebo response, with no added value of active drug. However, this does not appear to be the case, because responders and nonresponders receiving the active drug did not show comparable brain changes (29). To the contrary, modification of hippocampal and brainstem metabolic changes seen early (1 week) in the course of treatment in all patients receiving active drug did not occur in the patients who showed no clinical improvement at 6 weeks. A drug-response interaction effect appeared necessary for treatment response, with the brainstem and hippocampus playing a fundamental role in mediating cortical and limbic effects (63, 64). These time-course effects were discussed in detail in a previous article on this patient group (29).
Time Course of Regional Changes
Also unanswered is whether the additional hippocampal, brainstem, striatal, and insula changes seen uniquely in drug-treated responders facilitates and maintains clinical response in the long term. It might be speculated that modulation of these regions results in the observed changes of greater magnitude in response-specific cortical and paralimbic regions, thus strengthening or stabilizing the newly established state of equilibrium over time (64, 67–70). It is additionally hypothesized that absence of these changes in placebo responders may put this group at greater risk for relapse and recurrence. Clinical experience and research studies of relapse suggest that long-term response to therapy is better maintained with active drug than with placebo (13). Mechanisms mediating chronic fluctuations in the disease state with anticipated recurrences, however, remain an open issue (14–16). Since the placebo responders, like the drug responders, did not have a complete remission by the end of the 6-week trial, all of the patients were offered active drug and individualization of treatment at the completion of the PET study. It is, therefore, not possible to determine if the brain metabolism changes were adequate to prevent relapse in those receiving placebo over time.
In this study, there were no significant demographic, clinical, or neuropsychological markers indicating propensity for placebo response, although the group was clearly very small (87–90). It was noted, however, that placebo responders, unlike drug responders, showed a common 6-week, response-specific increase in posterior cingulate metabolism at 1 week. While the overall 1-week metabolic pattern did not reach omnibus significance, this early change is noteworthy since with active fluoxetine, metabolism in this region decreases at 1 week and then increases by 6 weeks of treatment in drug responders. The switch at 1 and 6 weeks does not occur in drug nonresponders (29). Presence of this early response-specific change in placebo responders suggests an important early effect critical to facilitating more widespread changes seen with long-term administration.
The ability to up-regulate activity in the posterior cingulate (i.e., increase metabolism) may be an early indicator of the brain’s compensatory capacity. This speculation is supported by a recent report (40) demonstrating comparable increases in metabolism in the posterior cingulate midway through a 12-week course of interpersonal psychotherapy. Known anatomical connections from the posterior cingulate to the identified cortical, limbic, and brainstem regions that are necessary for clinical response are one possible mechanism mediating these effects (91–93). Early increases in metabolism in the posterior cingulate may also prove to be a marker of drug responsivity, identifiable during the placebo wash-in phase of a clinical trial (94–96). Evidence that placebo nonresponders fail to show this 1-week posterior cingulate change would strongly support this hypothesis. There was inadequate power to test this hypothesis in the current study.
Other Considerations
The possibility of spontaneous remission as part of the natural course of a major depressive episode is also an issue in the evaluation of clinical response to any treatment intervention. In the present study, there was no way to determine whether spontaneous remission might have contributed to the effect for either the patients administered placebo or drug. The ideal control group to address this question—patients actively prescribed no treatment—were not studied (97), as we did not consider this situation to be ethical. There were, however, no significant differences between groups with respect to episode duration before hospitalization (Table 1). Statistical power was inadequate to examine time course differences in clinical response between the 1-week and 6-week PET scans. Nonetheless, even if some patients did have spontaneous recovery rather than a placebo or drug response, the common changes across groups would still support our hypothesis that symptom improvement requires changes in specific brain regions.
In conclusion, the combination of dorsal-cortical increases and limbic-paralimbic decreases in glucose metabolism, with response to both drug and placebo intervention, suggests that therapy for depression targeting either subcortical (brainstem) or cortical (frontal-posterior cingulate) sites should be equally effective if there is a preserved compensatory capacity in the obligatory circuit overall (Figure 3). Progressively more aggressive treatments needed to ameliorate symptoms in some patients may reflect the poor adaptive capacity of this network. Similarly, the functional integrity of these pathways may explain the comparable clinical efficacy of pharmacological and cognitive treatments in randomized controlled trials conducted in patients with nonrefractory depression. The results of this study therefore suggest that facilitation of specific adaptive reciprocal limbic-cortical changes is necessary for depression remission, regardless of the mode of treatment (98).
 |
 |
Presented in part at the 56th annual meeting of the Society of Biological Psychiatry, New Orleans, May 3–5, 2001. Received July 30, 2001, revision received Oct. 23, 2001; accepted Oct. 24, 2001. From the Research Imaging Center, the Departments of Psychiatry, Radiology, and Medicine (Neurology), and the Health Science Center, University of Texas at San Antonio. Address reprint requests to Dr. Mayberg, Rotman Research Institute, Baycrest Centre, University of Toronto, 3560 Bathurst St., Toronto, Ontario M6A 2E1, Canada; [email protected] (e-mail). Supported by NIMH grant MH-49553 and a physician-initiated grant from Eli Lilly and Company. The authors thank Betty Heyl, Ralph Evans, and Sergio Leal for technical assistance and Stanley R. Mayberg, M.D., for comments and criticisms.
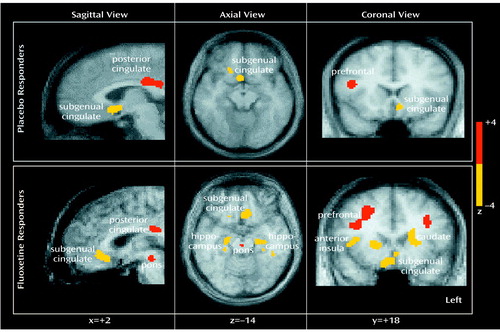
Figure 1. Changes in Regional Glucose Metabolism in Eight Depressed Patients Who Responded to Fluoxetine or Placebo Over 6 Weeksa
aSlice location is in millimeters relative to the anterior commissure line. Increases in metabolism are in red; decreases are in yellow. Cortical increases and limbic-paralimbic decreases were seen under both conditions. Fluoxetine response was additionally associated with brainstem increases and hippocampal and striatal decreases.
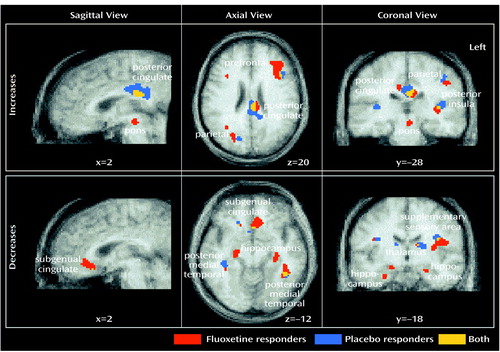
Figure 2. Anatomical Concordance of Changes in Regional Glucose Metabolism in Eight Depressed Patients Who Responded to Fluoxetine or Placebo Over 6 Weeks
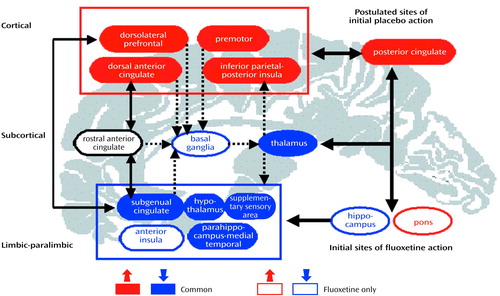
Figure 3. Relationships Among Brain Regions Mediating Response in Eight Depressed Patients Who Responded to Fluoxetine or Placebo Over 6 Weeksa
aRegions with known anatomical and functional connections that also show significant metabolic changes after 6 weeks of successful treatment are grouped into three compartments. Solid blue regions signify areas with a net metabolic decrease with treatment; solid red areas are those with a net increase. Open black areas signify areas with hypermetabolism before treatment that were unchanged by treatment. Open red (increases) and blue (decreases) areas showed unique changes after active fluoxetine treatment. Solid arrows identify known reciprocal cortical-limbic, limbic-paralimbic, and cingulate-cingulate connections. Dotted arrows indicate known cortical-striatal-thalamic pathways. The model proposes that illness remission occurs when there is inhibition of paralimbic and subcortical regions and activation of previously hypofunctioning dorsal areas, an effect facilitated by the bottom-up (brainstem/hippocampus) actions of fluoxetine and the top-down (posterior cingulate/cortical) effects of placebo.
1. Shapiro AK: A historic and heuristic definition of the placebo. Psychiatry 1964; 27:52-58Medline, Google Scholar
2. Oh VMS: The placebo effect: can we use it better? Br Med J 1994; 309:69-70Crossref, Medline, Google Scholar
3. Shapiro AK, Shapiro E: The placebo: is it much ado about nothing? in The Placebo Effect: An Interdisciplinary Exploration. Edited by Harrington A. Cambridge, Mass, Harvard University Press, 1997, pp 12-36Google Scholar
4. Kirsch I, Sapirstein G: Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prevention and Treatment 1998; I:article 0002a. http://journals.apa.org/prevention/volume1/pre0010002a.htmlGoogle Scholar
5. Khan A, Warner HA, Brown WA: Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the FDA database. Arch Gen Psychiatry 2000; 57:311-317Crossref, Medline, Google Scholar
6. Quitkin FM, Rabkin JG, Gerald J, Davis JM, Klein DF: Validity of clinical trials of antidepressants. Am J Psychiatry 2000; 157:327-337Link, Google Scholar
7. Enserink M: Can the placebo be the cure? Science 1999; 284:238-240Crossref, Medline, Google Scholar
8. Talbot M: The placebo prescription. New York Times Magazine, Jan 9, 2000, p 35Google Scholar
9. Thompson WG: Placebos: a review of the placebo response. Am J Gastroenterol 2000; 95:1637-1643Crossref, Medline, Google Scholar
10. Quitkin FM, Klein DF: What conditions are necessary to assess antidepressant efficacy? Arch Gen Psychiatry 2000; 57:323-324Crossref, Medline, Google Scholar
11. Andrews G: Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry 2001; 178:192-194Crossref, Medline, Google Scholar
12. Montgomery SA, Dufour H, Brion S, Gailledreau J, Laqueille X, Ferrey G, Moron P, Parant-Lucena N, Singer L, Danion JM: The prophylactic efficacy of fluoxetine in unipolar depression. Br J Psychiatry 1988; 153(suppl 3):69-76Google Scholar
13. Montgomery SA: Efficacy in long-term treatment of depression. J Clin Psychiatry 1996; 57(suppl 2):24-30Google Scholar
14. Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ: Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093-1099Crossref, Medline, Google Scholar
15. Stewart JW, Quitkin FM, McGrath PJ, Amsterdam J, Fava M, Fawcett J, Reimherr F, Rosenbaum J, Beasley C, Roback P: Use of pattern analysis to predict differential relapse on fluoxetine and placebo during continuation/maintenance treatment. Arch Gen Psychiatry 1998; 55:334-343Crossref, Medline, Google Scholar
16. McGrath PJ, Stewart JW, Petkova E, Quitkin FM, Amsterdam JD, Fawcett J, Reimherr FW, Rosenbaum JF, Beasley CM: Predictors of relapse during fluoxetine continuation or maintenance treatment of major depression. J Clin Psychiatry 2000; 61:518-524Crossref, Medline, Google Scholar
17. Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM: Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243-250Crossref, Medline, Google Scholar
18. Post RM, DeLisi LE, Holcomb HH, Uhde TW, Cohen R, Buchsbaum MS: Glucose utilization in the temporal cortex of affectively ill patients: positron emission tomography. Biol Psychiatry 1987; 22:545-553Crossref, Medline, Google Scholar
19. Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ: The anatomy of melancholia—focal abnormalities of cerebral blood flow in major depression. Psychol Med 1992; 22:607-615Crossref, Medline, Google Scholar
20. Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME: A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628-3641Crossref, Medline, Google Scholar
21. Mayberg HS: Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci 1994; 6:428-442Crossref, Medline, Google Scholar
22. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT: Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8:1057-1061Crossref, Medline, Google Scholar
23. Ketter TA, George MS, Kimbrell TA, Benson BE, Post RM: Functional brain imaging, limbic function, and affective disorders. Neuroscientist 1996; 2:55-65Google Scholar
24. Martinot JL, Hardy P, Feline A, Huret JD, Mazoyer B, Attar-Levy D, Pappata S, Syrota A: Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry 1990; 147:1313-1317Link, Google Scholar
25. Bench CJ, Frackowiak RSJ, Dolan RJ: Changes in regional cerebral blood flow on recovery from depression. Psychol Med 1995; 25:247-251Crossref, Medline, Google Scholar
26. Passero S, Nardini M, Battistini N: Regional cerebral blood flow changes following chronic administration of antidepressant drugs. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19:627-636Crossref, Medline, Google Scholar
27. Buchsbaum MS, Wu J, Siegel BV, Hackett E, Trenary M, Abel L, Reynolds C: Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry 1997; 41:15-22Crossref, Medline, Google Scholar
28. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675-682Abstract, Google Scholar
29. Mayberg HS, Brannan SK, Mahurin RK, McGinnin S, Silva JA, Tekell JL, Jerabek PA, Martin CC, Fox PT: Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000; 48:830-843Crossref, Medline, Google Scholar
30. Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR Jr: Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res 1999; 91:127-139Crossref, Medline, Google Scholar
31. Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ: Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158:899-905Link, Google Scholar
32. Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Keator D, Fallon JH, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE Jr: Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 1999; 156:1149-1158; correction, 156:1666Abstract, Google Scholar
33. Smith GS, Reynolds CF III, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ: Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry 1999; 156:683-689Abstract, Google Scholar
34. Henry ME, Schmidt ME, Matochik JA, Stoddard EP, Potter WZ: The effects of ECT on brain glucose metabolism: a pilot FDG PET study. J ECT 2000; 17:33-40Crossref, Google Scholar
35. Yatham LN, Clark CC, Zis AP: A preliminary study of the effects of electroconvulsive therapy on regional brain glucose metabolism in patients with major depression. J ECT 2000; 16:171-176Crossref, Medline, Google Scholar
36. Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell C, Sackeim HA, Mann JJ: Decreased regional brain metabolism after ECT. Am J Psychiatry 2001; 158:305-308Link, Google Scholar
37. Malizia AL: The frontal lobes and neurosurgery for psychiatric disorders. J Psychopharmacol 1997; 11:179-187Crossref, Medline, Google Scholar
38. Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, Risch SC, George MS: Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci 1999; 11:426-435Medline, Google Scholar
39. Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbank LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR: Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy. Arch Gen Psychiatry 2001; 58:631-640Crossref, Medline, Google Scholar
40. Martin SD, Martin E, Rai SS, Richards MA, Royal R, Eng C: Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride. Arch Gen Psychiatry 2001; 58:641-664Crossref, Medline, Google Scholar
41. Elkin I, Shea MT, Watkins JT, Imber SD, Sotsky SM, Collins JF, Glass DR, Pilkonis PA, Leber WR, Docherty JP, Fiester SJ, Parloff MB (National Institute of Mental Health Treatment of Depression Collaborative Research Program): General effectiveness of treatments. Arch Gen Psychiatry 1989; 46:971-982Crossref, Medline, Google Scholar
42. Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, Grove WM, Tuason VB: Cognitive therapy and pharmacotherapy for depression: singly and in combination. Arch Gen Psychiatry 1992; 49:774-781Crossref, Medline, Google Scholar
43. DeRubeis RJ, Galfand LA, Tang TZ, Simons AD: Medications versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry 1999; 156:1007-1013Abstract, Google Scholar
44. American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder (Revision). Am J Psychiatry 2000; 157(April suppl)Google Scholar
45. Little JT, Ketter TA, Kimbrell TA, Danielson A, Benson B, Willis MW, Post RM: Venlafaxine or bupropion responders but not nonresponders show baseline prefrontal and paralimbic hypometabolism compared with controls. Psychopharmacol Bull 1996; 32:629-635Medline, Google Scholar
46. Ketter TA, Kimbrell TA, George MS, Willis MW, Benson BE, Danielson A, Frye MA, Herscovitch P, Post RM: Baseline cerebral hypermetabolism associated with carbamazepine response, and hypometabolism with nimodipine response in mood disorders. Biol Psychiatry 1999; 46:1364-1374Crossref, Medline, Google Scholar
47. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1988Google Scholar
48. Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE: Tomographic measurement of local cerebral glucose metabolic rate in humans with (F18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 1979; 6:371-388Crossref, Medline, Google Scholar
49. Raichle ME, MacLeon AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL: A default mode of brain function. Proc Natl Acad Sci USA 2001; 98:676-682Crossref, Medline, Google Scholar
50. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
51. Mahurin RK: Neurocog: Administration Manual. Seattle, Neurocog Assessment Systems, 1995Google Scholar
52. Stassen HH, Delini-Stula A, Angst J: Time course of improvement under antidepressant treatment: a survival-analytical approach. Eur Neuropsychopharmacol 1993; 3:127-135Crossref, Medline, Google Scholar
53. Fox PT, Mintun MA: Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med 1989; 30:141-149Medline, Google Scholar
54. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
55. Lancaster JL, Glass TH, Lankipalli BR, Downs H, Mayberg HS, Fox PT: A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp 1995; 3:209-223Crossref, Google Scholar
56. Agostino RB, Belanger A, D’Agostino RB Jr: A suggestion for using powerful and informative tests of normality. Am Statistician 1990; 44:316-321Google Scholar
57. Worsley KJ, Evans AC, Marrett S, Neelin A: Three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900-918Crossref, Medline, Google Scholar
58. Mintun MA, Fox PT, Raichle ME: A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab 1989; 9:96-103Crossref, Medline, Google Scholar
59. Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC: Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp 1997; 5:238-242Crossref, Medline, Google Scholar
60. Spielberger CD: State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1985Google Scholar
61. Ramos-Brieva JA, Villafafila AC: A new validation of the Hamilton Rating Scale for Depression. J Psychiatr Res 1988; 22:21-28Crossref, Medline, Google Scholar
62. Hyman SE, Nestler EJ: Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry 1996; 153:151-162Link, Google Scholar
63. Blier P, de Montigny C: Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology 1999; 21(2 suppl):91S-98SGoogle Scholar
64. Duman RS, Malberg J, Thome J: Neural plasticity to stress and antidepressant treatment. Biol Psychiatry 1999; 46:1181-1191Crossref, Medline, Google Scholar
65. Gurevich EV, Joyce JN: Comparison of [3H]paroxetine and [3H]cyanoimipramine for quantitative measurement of serotonin transporter sites in human brain. Neuropsychopharmacology 1996; 14:309-323Crossref, Medline, Google Scholar
66. Bergstrom KA, Halldin C, Hall H, Lundkwvist C, Ginovart N, Swahn CG, Farde L: In vitro and in vivo characterisation of nor-beta-CIT: a potential radioligand for visualisation of the serotonin transporter in the brain. Eur J Nucl Med 1997; 24:596-601Crossref, Medline, Google Scholar
67. Chaput Y, deMontigny C, Blier P: Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments: an in vitro electrophysiologic study in the rat. Neuropsychopharmacology 1991; 5:219-229Medline, Google Scholar
68. Haddjeri N, Blier P, de Montigny C: Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci 1998; 18:10150-10156Medline, Google Scholar
69. Frechilla D, Otano A, Del Rio J: Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 1998; 22:787-802Crossref, Medline, Google Scholar
70. Freo U, Ori C, Dam M, Merico A, Pizzolato G: Effects of acute and chronic treatment with fluoxetine on regional glucose cerebral metabolism in rats: implications for clinical therapies. Brain Res 2000; 854:35-41Crossref, Medline, Google Scholar
71. Cudennec A, Duverger D, Serrano A, Scattfon B, MacKenzie ET: Influence of ascending serotonergic pathways on glucose use in the conscious rat brain, II: effects of electrical stimulation of the rostral raphe nuclei. Brain Res 1988; 444:227-246Crossref, Medline, Google Scholar
72. Beck AT, Rush AJ, Shaw BF, Emery G: Cognitive Therapy of Depression. New York, Guilford, 1979Google Scholar
73. Teasdale JD: Negative thinking in depression: cause, effect, or reciprocal relationship? Adv Behav Res Ther 1983; 5:3-25Crossref, Google Scholar
74. Schwartz JM: A role for volition and attention in the generation of new brain circuits. J Consciousness Studies 1999; 6:115-142Google Scholar
75. Norman WH, Miller IW, Keitner GI: Relationship between dysfunctional cognitions and depressive subtypes. Can J Psychiatry 1987; 32:194-198Medline, Google Scholar
76. DeRubeis RJ, Evans MD, Hollon SD, Garvey MJ, Grove WM, Tuason VB: How does cognitive therapy work? J Consult Clin Psychol 1990; 58:862-869Crossref, Medline, Google Scholar
77. Teasdale JD: Emotional processing, three modes of mind and the prevention of relapse in depression. Behav Res Ther 1999; 37(suppl 1):S53-S77Google Scholar
78. Free ML, Oei TP, Appleton CJ: Biological and psychological processes in recovery from depression during cognitive therapy. Behav Ther Exp Psychiatry 1998; 29:213-226Crossref, Medline, Google Scholar
79. Rush AJ, Beck AT, Kovacs M, Weissenburger J, Hollon SD: Comparison of the effects of cognitive therapy and pharmacotherapy on hopelessness and self-concept. Am J Psychiatry 1982; 139:862-866Link, Google Scholar
80. Segal ZV, Gemar M, Williams S: Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. J Abnorm Psychol 1999; 108:3-10Crossref, Medline, Google Scholar
81. Jensen MP, Karoly P: Motivation and expectancy factors in symptoms perception: a laboratory study of the placebo effect. Psychosom Med 1991; 53:144-152Crossref, Medline, Google Scholar
82. Brody H: The doctor as therapeutic agent: a placebo effect research agenda, in The Placebo Effect: An Interdisciplinary Exploration. Edited by Harrington A. Cambridge, Mass, Harvard University Press, 1997, pp 77-92Google Scholar
83. Reynaert C, Janne P, Vause M, Zdanowicz N, Lejeune D: Clinical trials of antidepressants: the hidden face: where locus of control appears to play a key role in depression outcome. Psychopharmacology (Berl) 1995; 119:449-454Crossref, Medline, Google Scholar
84. Kirsch I: Specifying nonspecifics: psychological mechanisms of placebo effects, in The Placebo Effect: An Interdisciplinary Exploration. Edited by Harrington A. Cambridge, Mass, Harvard University Press, 1997, pp 166-186Google Scholar
85. Ehlers CL, Kupfer DJ, Frank E, Monk TH: Biological rhythms and depression: the role of zeitgebers and zeitstorers. Depression 1993; 1:285-293Crossref, Google Scholar
86. Ehlers CL, Frank E, Kupfer DJ: Social zeitgebers and biological rhythms. Arch Gen Psychiatry 1988; 45:948-952Crossref, Medline, Google Scholar
87. Schatzberg AF, Kraemer HC: Use of placebo control groups in evaluating efficacy of treatment of unipolar major depression. Biol Psychiatry 2000; 47:736-744Crossref, Medline, Google Scholar
88. Brown WA, Johnson MF, Chen MG: Clinical features of depressed patients who do and do not improve with placebo. Psychiatry Res 1992; 41:203-214Crossref, Medline, Google Scholar
89. Bialik RJ, Ravindran AV, Bakish D, Lapierre YD: A comparison of placebo responders and nonresponders in subgroups of depressive disorder. J Psychiatry Neurosci 1995; 20:265-270Medline, Google Scholar
90. Casper RC, Tollefson GD, Nilsson ME: No gender differences in placebo responses of patients with major depressive disorder. Biol Psychiatry 2001; 49:158-160Crossref, Medline, Google Scholar
91. Vogt BA, Pandya DN: Cingulate cortex of the rhesus monkey, II: cortical afferents. J Comp Neurol 1987; 262:271-289Crossref, Medline, Google Scholar
92. Crino PB, Morrison JH, Hof PR: Monoamine innervation of cingulate cortex, in Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Edited by Vogt BA, Gabriel M. Boston, Birkhauser, 1993, pp 285-310Google Scholar
93. Maddock RJ: The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 1999; 22:310-316Crossref, Medline, Google Scholar
94. Dew MA, Reynolds CF III, Mulsant B, Frank E, Houck PR, Mazumdar S, Begley A, Kupfer DJ: Initial recovery patterns may predict which maintenance therapies for depression will keep older adults well. J Affect Disord 2001; 65:155-166Crossref, Medline, Google Scholar
95. Reimherr FW, Ward MF, Byerley WF: The introductory placebo washout: a retrospective evaluation. Psychiatry Res 1989; 30:191-199Crossref, Medline, Google Scholar
96. Senn S: Are placebo run ins justified? Br Med J 1997; 314:1191-1193Crossref, Medline, Google Scholar
97. Hrobjartsson A, Gotzsche PC: Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001; 344:1594-1602Crossref, Medline, Google Scholar
98. Mayberg HS: Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997; 9:471-481Crossref, Medline, Google Scholar



