Placebo-Controlled Trial of d-Cycloserine Added to Conventional Neuroleptics, Olanzapine, or Risperidone in Schizophrenia
Abstract
OBJECTIVE: The authors investigated the clinical effects of d-cycloserine when added to treatment with conventional neuroleptics, olanzapine, or risperidone for treatment-resistant schizophrenia. METHOD: Twenty-four patients participated in a double-blind, placebo-controlled, 6-week crossover trial with d-cycloserine, 50 mg/day, added to their fixed dose of antipsychotic medication. Clinical ratings were performed every 2 weeks. RESULTS: d-Cycloserine treatment was well tolerated and resulted in a significant reduction in negative symptoms (mean=15%). The degree of improvement did not differ between patients treated with conventional neuroleptics and those treated with olanzapine or risperidone. CONCLUSIONS: These data support the efficacy of the addition of 50 mg/day of d-cycloserine to treatment with conventional neuroleptics and suggest that therapeutic benefits may also be attained when d-cycloserine is added to olanzapine or risperidone.
Enhancement of glutamatergic neurotransmission by means of administration of compounds having agonistic activity at the glycine recognition site associated with the N-methyl-d-aspartate (NMDA) receptor may be an innovative pharmacological strategy for schizophrenia. Significant reductions in negative symptoms have been registered following adjuvant treatment with the full agonists glycine and d-serine at the glycine site in patients treated with conventional antipsychotics (1, 2) but not in patients receiving clozapine (3, 4).
d-Cycloserine, an antituberculosis drug that is a relatively selective partial agonist at the NMDA receptor, may improve negative symptoms in patients treated with conventional antipsychotics (5–7) but not clozapine (8, 9) when administered at a dose of 50 mg/day. The use of the newer atypical antipsychotics risperidone and olanzapine has increased dramatically recently, and the effectiveness of NMDA agonists used in combination with these agents is unknown. The aim of this study was to evaluate the therapeutic potential of d-cycloserine in patients receiving either conventional antipsychotics or the newer atypical antipsychotics.
Method
The study was approved by the appropriate institutional review boards. Twenty-four medicated inpatients meeting DSM-IV criteria for schizophrenia and who were clinically stable and free of any other axis I diagnoses or significant medical illness were enrolled in the study. Diagnosis was established on the basis of semistructured psychiatric interviews, review of all available medical records, and confirmation by two board-certified psychiatrists. Patients fulfilled criteria for treatment resistance used in previous trials of glycine (1) and d-cycloserine (7) and had been receiving stable doses of medication for at least 3 months before study entry. Medication doses remained fixed throughout the study.
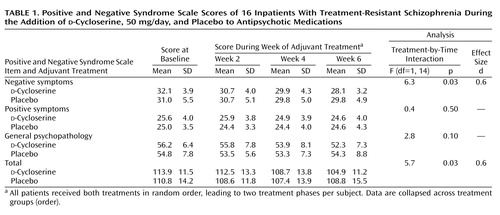
After complete description of the study, written informed consent was obtained from all participating patients. The double-blind, placebo-controlled, crossover study consisted of two random-order 6-week treatment arms (d-cycloserine, 50 mg/day, or placebo), separated by a 2-week adjuvant treatment washout, as described elsewhere (7). Patients were assessed biweekly with the Positive and Negative Syndrome Scale (10), Hamilton Depression Rating Scale, Simpson-Angus Rating Scale (11), and Abnormal Involuntary Movement Scale (AIMS) (12) performed by one trained research psychiatrist. CBC and SMA-20 measures were assessed biweekly throughout the study.
Results
Four patients were withdrawn from the study because they developed common viral infections (one during d-cycloserine administration and three during placebo administration). Four additional patients were withdrawn because their symptoms grew worse (two during d-cycloserine administration and two during placebo administration).
Six of the 16 patients who completed the study were women and 10 were men. Their mean age was 40.0 years (SD=12.1), the mean duration of their illness was 28.2 years (SD=11.2), and the mean duration of their current hospitalization was 6.4 years (SD=2.1). Eight patients were randomly assigned to receive d-cycloserine during the first treatment phase; eight received placebo during this phase. Repeated measures multivariate analyses of variance were performed with within-subject factors of treatment phase (placebo versus d-cycloserine) and time within phase (baseline versus week 6) and a between-subjects factor of treatment order.
These analyses demonstrated a significant, moderate effect size for a treatment-by-time interaction for the Positive and Negative Syndrome Scale negative symptoms score and total score (Table 1), indicating significant therapeutic efficacy of d-cycloserine in this patient group. Treatment with d-cycloserine led to a 15.2% reduction (SD=10.5%) in Positive and Negative Syndrome Scale negative symptoms score (95% confidence interval [CI]=10%–21%), a 10.0% reduction (SD=12.2%) in Positive and Negative Syndrome Scale general psychopathology score (95% confidence interval [CI]=3%–16%), and a 10.6% reduction (SD=6.8%) in Positive and Negative Syndrome Scale total score (95% CI=7%–14%). (Calculation of the percent reduction in Positive and Negative Syndrome Scale scores took into account the fact that items on this scale are scored 1–7 and that a score of 1=no symptoms.)
Reductions in negative symptoms score (t=5.76, df=15, p<0.001), general psychopathology score (t=3.13, df=15, p=0.007), and total score (t=6.23, df=15, p<0.001) of the Positive and Negative Syndrome Scale during d-cycloserine treatment were all significant at 6 weeks compared with baseline. No symptoms improved significantly during placebo treatment. Two of 16 patients showed a greater than 20% improvement in negative symptoms score during d-cycloserine treatment, but no patients showed this level of improvement during the placebo treatment phase.
To verify whether the data from the eight patients who did not complete the study might modify these findings, we performed an additional intent-to-treat analysis, in which the last ratings of all withdrawn patients were carried across to the end of the study. In this larger study group (N=24), the d-cycloserine therapeutic effect was also significantly greater than the effect of placebo for Positive and Negative Syndrome Scale negative symptoms score (F=5.1, df=1, 23, p<0.03) and total score (F=9.7, df=1, 23, p=0.005).
Half of the patients who completed the study were receiving atypical antipsychotics (five were receiving olanzapine and three were receiving risperidone); the other half were receiving conventional neuroleptics as their baseline treatment. When medication type was used as a between-subjects factor in a repeated measures analysis of variance for symptom reduction during each treatment phase, no significant interaction of typical versus atypical medications and reduction in symptoms emerged. Patients receiving atypical antipsychotics showed a significant improvement in negative symptoms score during d-cycloserine treatment (F=18.7, df=1, 7, p=0.003). The magnitude of this improvement (12.2%, SD=5.9%) was not significantly different from the magnitude of the improvement in negative symptoms score during d-cycloserine treatment among patients receiving conventional antipsychotics (17.0%, SD=14.0%) (t=0.59, df=14, p=0.60). The reduction in Positive and Negative Syndrome Scale total scores during d-cycloserine treatment for patients receiving atypical antipsychotics (11.6%, SD=4.7%) was also not significantly different from the corresponding value for patients receiving typical antipsychotics (10.0%, SD=8.0%) (t=0.57, df=14, p=0.60).
No significant change was found for Positive and Negative Syndrome Scale positive symptoms score during d-cycloserine treatment, and no Positive and Negative Syndrome Scale scores decreased significantly during placebo treatment. Hamilton depression scale, Simpson-Angus Rating Scale, and AIMS scores did not change significantly throughout the study. Further, the improvement in negative symptoms score (F=7.33, df=1, 11, p=0.02, d=0.8) during the d-cycloserine treatment phase remained significant following covariation for Hamilton depression scale, Simpson-Angus Rating Scale, and AIMS scores. No significant effect was found for treatment order, and there was no significant interaction of treatment order by time.
d-Cycloserine was well tolerated throughout the study and was not associated with changes in serum chemistry or hematological values.
Discussion
The primary finding of this study is that d-cycloserine treatment led to a significant reduction in negative symptoms across subjects (mean=15.2%) in a group of inpatients with treatment-resistant schizophrenia. This reflects a moderate-sized statistical effect, similar to that observed in other studies of d-cycloserine (6, 7) and smaller than the large changes in effect size observed in studies of NMDA full agonists added to typical antipsychotics (1, 2). As in previous studies with d-cycloserine (6) and other NMDA agonists (1, 2), the observed improvement in negative symptoms was not accompanied by exacerbation of positive symptoms and could not be explained by changes in other symptom clusters or extrapyramidal symptoms. These findings support the hypothesis that NMDA agonists may affect primarily negative symptoms of schizophrenia.
To our knowledge, this is the first study to evaluate the effectiveness of d-cycloserine in combination with newer atypical antipsychotics. Patients receiving risperidone and olanzapine improved significantly during d-cycloserine treatment, as did the group as a whole, indicating that NMDA-based treatments may be effective even among patients receiving newer atypical antipsychotics. This preliminary finding contrasts with the reported exacerbation of negative symptoms during d-cycloserine treatment among patients receiving clozapine (8, 9) and warrants further, larger-scale investigation.
 |
Presented in part at the 5th Annual Meeting of the Israel Society for Biological Psychiatry, Kfar Giladi, March 16–18, 1999. Received Sept. 21, 2000; revisions received Feb. 1 and July 26, 2001; accepted Aug. 17, 2001. From Ezrath Nashim-Herzog Memorial Hospital; the Department of Psychiatry, Hadassah Medical School-Hebrew University, Jerusalem; Nathan S. Kline Institute for Psychiatric Research, Orangeburg, N.Y.; and the Department of Psychiatry, New York University, New York. Address reprint requests to Dr. Heresco-Levy, Ezrath Nashim-Herzog Memorial Hospital, P.O.B. 35300, Jerusalem 91351, Israel; [email protected] (e-mail). Supported by grants from the National Alliance for Research on Schizophrenia and Depression (Dr. Heresco-Levy); the Scottish-Rite Schizophrenia Research Program, Northern Masonic Jusrisdiction of the United States (Dr. Heresco-Levy); and NIMH grant MH-01439 (Dr. Javitt).
1. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M: Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999; 56:29-36Crossref, Medline, Google Scholar
2. Tsai G, Yang P, Chung LC, Lange N, Coyle JT: d-Serine added to antipsychotics for the treatment of enduring negative symptoms of schizophrenia. Biol Psychiatry 1998; 44:1081-1089Crossref, Medline, Google Scholar
3. Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC: Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry 2000; 157:826-828Link, Google Scholar
4. Tsai GE, Yang P, Chung L-C, Tsai I-C, Tsai C-W, Coyle JT: d-Serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry 1999; 156:1822-1826Abstract, Google Scholar
5. Goff DC, Tsai G, Manoach DS, Coyle JT: Dose-finding trial of d-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry 1995; 152:1213-1215Link, Google Scholar
6. Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT: A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:21-27Crossref, Medline, Google Scholar
7. Heresco-Levy U, Javitt DC, Ermilov M, Silipo G, Shimoni J: Double-blind, placebo-controlled, crossover trial of d-cycloserine adjuvant therapy for treatment-resistant schizophrenia. Int J Neuropsychopharmacol 1998; 1:131-135Crossref, Medline, Google Scholar
8. Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT: d-Cycloserine added to clozapine for patients with schizophrenia. Am J Psychiatry 1996; 153:1628-1630Link, Google Scholar
9. Goff DC, Henderson DC, Evins AE, Amico E: A placebo-controlled crossover trial of d-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry 1999; 45:512-514Crossref, Medline, Google Scholar
10. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
11. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11-19Crossref, Medline, Google Scholar
12. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534-537Google Scholar



