Smaller Frontal Gray Matter Volume in Postmortem Schizophrenic Brains
Abstract
OBJECTIVE: The prefrontal cortex exhibits prominent functional, biochemical, and anatomic abnormalities in schizophrenic patients. However, smaller than normal volume of the frontal lobe has not been found in previous postmortem studies of schizophrenic subjects, and magnetic resonance imaging (MRI) scans of schizophrenic subjects have not consistently revealed frontal volumetric deficits. The variability in MRI findings may be related partly to difficulty in defining the posterior border of the frontal lobe. In this study, precise measurements of frontal lobe volume from postmortem brains were derived by defining the posterior border according to the brain atlas of Talairach and Tournoux and by applying stereologic methods to estimate gray and white matter volumes. METHOD: Whole, or nearly whole, formalin-fixed left hemispheres from 14 schizophrenic and 19 normal comparison subjects were analyzed. Total cortical gray and white matter volumes, as well as frontal cortical gray and white matter volumes, were measured by using the Cavalieri method. RESULTS: Only frontal gray matter volume was significantly smaller in the schizophrenic subjects than in the comparison subjects (12% difference). The differences between groups in total gray and white matter volumes and frontal white matter volume (6%–8% smaller in the schizophrenic subjects than in the comparison subjects) did not reach statistical significance. CONCLUSIONS: The smaller frontal gray matter volume observed in schizophrenic brains suggests that pathology of the frontal lobe may be more severe than that of the three posterior lobes and may account for the prominence of prefrontal dysfunction associated with schizophrenia.
Schizophrenic patients exhibit marked prefrontal cortical dysfunction, including working memory impairment, thought disorder, and eye tracking abnormalities, as well as more subtle perceptual and sensorimotor deficits that are indicative of widespread cortical dysfunction (1–3).
Early postmortem studies suggested that brains from schizophrenic patients were smaller in weight and had a smaller cortical volume relative to normal brains and to brains from patients with affective disorder (4, 5). Likewise, initial structural analyses of schizophrenic patients with magnetic resonance imaging (MRI) uncovered global deficits in cortical gray matter (6, 7). More recently, some MRI analyses have found selective or more pronounced deficits of frontal gray matter in schizophrenic brains (8–12). However, smaller frontal lobe gray matter volume has not been reported consistently in MRI studies of schizophrenic patients (13, 14). This inconsistency may be partly attributed to difficulty in defining the posterior border of the frontal lobe. Arbitrary landmarks, such as the genu of the corpus callosum or level of the optic chiasm, have often been used to demarcate the posterior extent of the frontal or prefrontal cortex, although the relationship of these structures to the frontal cortex is highly variable (15). In addition, MRI analyses frequently rely on automated segmentation programs to define the border between gray and white matter, creating a boundary that is dependent on scanner settings.
To our knowledge, only three postmortem studies have examined cortical volume in schizophrenic patients (16–18). Although these studies did not find significantly smaller frontal or prefrontal gray matter volumes in the schizophrenic patients, compared to normal subjects, the negative findings could be related to the small number of subjects (16, 17), the inclusion of aged brains (17, 18), or inclusion of both right and left hemisphere tissue samples (16, 17).
In the study reported here, postmortem volumetric analyses were performed on a series of whole, or nearly whole, left formalin-fixed hemispheres from schizophrenic patients and normal comparison subjects. Measurements of total gray matter volume, total white matter volume, frontal gray matter volume, and frontal white matter volume were performed on 2-cm-thick slabs cut in the coronal plane. The posterior boundary of the frontal lobe was defined as a plane bisecting the line connecting the anterior and posterior commissures, an accurate and consistent approximation of the central sulcus in the human brain, according to the brain atlas of Talairach and Tournoux (15).
Method
A total of 47 brains were examined in this study. Thirty-seven brains were obtained from the Harvard Brain Tissue Resource Center, Belmont, Mass.; 10 brains were obtained from the Section on Neuropathology, Clinical Brain Disorders Branch, Intramural Research Program, National Institute of Mental Health (NIMH), Bethesda, Md. All brains were procured in accordance with procedures established by the institutional review boards of the respective institutes and were screened for neuropathology both macro- and microscopically. Fourteen brains were eliminated from the study for the following reasons: insufficient medical records for a definitive diagnosis (N=7), final diagnosis other than schizophrenia (N=5), secondary diagnosis of multiple sclerosis (N=1), and family history of schizophrenia in a comparison subject (N=1). Demographic data for the remaining 19 normal comparison subjects and 14 schizophrenic patients are shown in Table 1.
The diagnosis of schizophrenia was ascertained via postmortem, retrospective review of the subjects’ medical records and application of either the Feighner criteria, for the brains from the Harvard Brain Tissue Resource Center (F. Benes, personal communication, 2001), or DSM-IV, for the brains provided by NIMH (J. Kleinman, personal communication, 2001). All subjects with schizophrenia in this study had a chronic illness of at least 6 months’ duration without subsequent return to premorbid levels of psychosomatic adjustment, did not show signs of depression or mania consistent with affective disorder, and exhibited either delusions and/or hallucinations, significant communication deficits related to lack of logical thinking, or both. Under the Feighner criteria, “probable schizophrenia” is distinguished from “schizophrenia” by meeting only two of five of the following demographic criteria, whereas three or more must be met for a diagnosis of schizophrenia: 1) single, 2) poor premorbid social adjustment or work history, 3) family history of schizophrenia, 4) absence of alcoholism or drug abuse within 1 year of onset of psychosis, and 5) onset of illness before age 40. One schizophrenic patient (subject 21) had a secondary diagnosis of alcohol abuse. The brains of two patients were neuroleptic-free (subjects 23 and 27), and the brain of one other patient (subject 20) was essentially neuroleptic-naive, as this subject had received medication for only 1 day.
Whole, or nearly whole, formalin-fixed left hemispheres were shipped to the Department of Neurobiology at Yale University School of Medicine. Lateral and medial views of the hemispheres were photographed, and the brains were then dissected into 2-cm-thick coronal slabs by using a custom-made brain slicing apparatus. The first coronal cut was made on a plane perpendicular to the line connecting the anterior and posterior commissures and was positioned at the midpoint of this line (Figure 1). Subsequent cuts were made anterior and posterior to this initial cut at 2-cm intervals. This dissection yielded 8–10 coronal brain slabs. As the posteriormost slab did not have a posterior cut surface, 7–9 slab faces per brain were analyzed. The posterior faces of these slabs were photographed and printed at approximately 2× magnification; gray and white matter compartments were easily distinguished and outlined with a fine black marker (Figure 2). All photographs were coded so that the investigators who performed the measurements were blind to the subject’s diagnosis. A point-counting grid was placed over each photograph, and the number of points on the grid (spacing=1 cm) falling on gray and white matter was recorded. The Cavalieri method (19) was used to determine the total gray matter volume and the total white matter volume. Frontal gray matter volume and frontal white matter volume were estimated by using the same procedure but only for slabs that included the mid-commissural cut (Figure 2, panels D and L) and tissue anterior to the mid-commissural cut. Five brains (one from a comparison subject and four from schizophrenic patients) were missing small areas of the medial temporal cortices; therefore, only measurements of frontal gray matter volume and frontal white matter volume were performed for those brains. The coefficient of error, calculated by dividing the standard error of the mean by the mean (19), for all measurements in this study ranged from 0.032 to 0.043.
Measurement reliability was assessed by using a subset of four brains (two normal, two schizophrenic) for total gray matter volume, total white matter volume, frontal gray matter volume, and frontal white matter volume. The Pearson correlation coefficients for the four volumes were 0.99, 1.00, 0.97, and 0.78, respectively. Two-tailed significance values for the correlations (N=4) were <0.02, 0.003, <0.03, and 0.22, respectively. Similar results were obtained with Kendall’s tau-B and Spearman’s rho.
A Student’s t test was performed after Pearson correlation to determine whether the normal comparison group and the schizophrenic group differed in age, postmortem interval, brain storage time in formalin, or brain weight. In addition, regression analyses were performed to assess possible correlations between measured parameters and age, postmortem interval, storage time in formalin, or brain weight. Significant correlations were found between brain weight and total gray matter volume (r=0.69, t=–3.93, df=27, p=0.001), total white matter volume (r=0.57, t=2.80, df=27, p=0.01), frontal gray matter volume (r=0.58, t=3.70, df=32, p=0.001), and frontal white matter volume (r=0.61, t=3.84, df=32, p=0.001), and between storage time in formalin and total gray matter volume (r=–0.61, t=–3.93, df=27, p=0.001), total white matter volume (r=–0.52, df=27, p=0.01), frontal gray matter volume (r=–0.45, t=–3.08, df=32, p=0.005), and frontal white matter volume (r=–0.39, t=–2.65, df=32, p<0.02). Accordingly, an analysis of covariance (ANCOVA) was performed on these volumes, with diagnosis as the factor and brain weight and storage time in formalin as covariates. Possible gender differences in brain weight, total gray matter volume, total white matter volume, frontal gray matter volume, and frontal white matter volume were analyzed with ANCOVA, with storage time in formalin as the covariate. Since the study group included some brain samples with incomplete hemispheres, volumes for each region were analyzed by independent ANCOVAs. As an additional control, volumes for each region for the study group subset with complete hemispheres were analyzed by repeated measures ANCOVA. Data were initially analyzed with gender as a factor. However, observed power estimates were low. Moreover, nearly all of the gender-based variance was accounted for by using brain weight as a covariate. As a consequence, except for the gross overview noted below, gender differences were not considered in this study. For all statistical analyses, p<0.01 was considered significant, although 0.1<p<0.01 are also reported.
Results
Demographic Variables
The comparison and schizophrenic brains did not differ with respect to age at time of subject’s death, storage time in formalin, or weight (Table 1). The postmortem interval was longer for the schizophrenic brains than for the normal comparison brains in the full series of brains, but not in the subset of whole brains (Table 1).
Gray and White Matter Volumes
Frontal gray matter volume was 12% smaller in the schizophrenic brains than in the normal brains (Table 2). No significant differences were found in total gray matter volume, total white matter volume, or frontal white matter volume (Table 2). Analysis of frontal gray matter volume in the smaller group of whole brains (N=18 for normal subjects; N=10 for schizophrenic subjects) indicated that frontal gray matter volume was smaller in the schizophrenic group, but the difference did not reach significance (diagnosis-by-region interaction: F=2.64, df=3, 72, p=0.056) (Figure 3). Cortical gray matter volume for the posterior lobes, i.e., parietal, temporal, and occipital lobes combined, did not differ between the normal and schizophrenic brains (Figure 3).
Gender Differences
The brains of male subjects weighed 12% more (F=14.22, df=1, 28, p=0.001) and had marginally larger total gray matter (17%) (F=6.31, df=1, 23, p=0.02) and total white matter volumes (15%) (F=3.90, df=1, 23, p=0.06) than the brains of female subjects. Frontal gray matter volume was 15% greater and frontal white matter volume was 13% greater in male than in female subjects, although the differences reached only marginal significance (frontal gray matter volume: F=4.49, df=1, 28, p=0.04; frontal white matter volume: F=3.70, df=1, 28, p=0.07). Gray matter volume of the posterior lobes was 30% greater (F=12.37, df=1, 23, p=0.002) in male subjects than in female subjects, whereas posterior white matter volume was 19% greater in male subjects than in female subjects, only a marginally significant difference (F=4.13, df=1, 23, p=0.054).
Discussion
In the full group of brains in this study, only the frontal gray matter volume was significantly smaller (12% smaller) in the schizophrenic subjects than in the comparison subjects. Total gray matter volume of the schizophrenic brains was 8% smaller than that of the comparison brains, but this difference was not significant. However, with a larger number of subjects, a significant difference in whole gray matter volume may have been detected. These observations are consistent with the findings of several MRI studies indicating a preferential or selective deficit in gray matter volume in the frontal lobes (8–12) and with those of recent MRI analyses focusing specifically on subdivisions of the prefrontal cortex (20–22). However, smaller frontal gray matter volume is far from a universal finding in schizophrenia research. Indeed, only about half the MRI studies have reported smaller frontal gray matter volume in schizophrenia (13, 14). McCarley et al. (14) suggested that the 8% smaller prefrontal cortical width we previously described (23) is near the limit that can be detected with the resolution of current MRI technology, resulting in both positive and negative reports in the literature. As schizophrenia is a heterogeneous disease, cohort differences certainly might also contribute to the inconsistency between studies. Finally, imprecision in the definition of the frontal lobe may account for some of the variability in findings. Arbitrary anatomical landmarks, such as the corpus callosum or optic chiasm, have often been used to determine the posterior border of the frontal lobe. In this study, we elected to use the midpoint of the anterior commissure–posterior commissure line to define the posterior margin of the frontal lobe, a delineation that has been shown to represent an accurate approximation of the central sulcus (15). In addition, the border between gray and white matter, a boundary that is dependent on scanner settings with MRI, is readily discernible on slabs of formalin-fixed brain tissue. Thus, accurate discrimination of the frontal lobe gray matter compartment in a cohort that did not include elderly subjects (>76 years old) may have enabled us to detect relatively small volumetric differences in schizophrenic and comparison brains.
Comparison With Previous Postmortem Studies
Previous postmortem studies of the whole frontal lobe (16), prefrontal gray matter (17), or frontal gray matter (18) have not reported significantly smaller volumes in schizophrenic brains, although small numbers of subjects (16, 17), inclusion of aged subjects (17, 18), and inclusion of both brain hemispheres (16, 17) may account for the negative findings. Brain volume has been shown to undergo significant age-related reduction, with the frontal lobes exhibiting more severe volumetric deficits with age than the other cortical lobes (24–26). Moreover, aging is not uniform across individuals. Aged rats have been shown to exhibit functional and anatomic heterogeneity. Some aged rats do not differ from young rats with respect to hippocampal morphology or function, while others show deficits in learning capacity and concomitant changes in hippocampal volume (27). In humans, dendritic patterns in aged subjects show similar variability (28). Therefore, simple age-matching does not compensate for the changes occurring in elderly subjects. A recent study found that the age-related changes in frontal lobe volume were indistinguishable from the pathology found in schizophrenic subjects (29). As a result, inclusion of aged comparison subjects is likely to mask volumetric findings in the schizophrenic cohort or, at the very least, to increase the variability in both comparison and schizophrenic groups, making it less likely that differences will be detected.
Despite differences between our findings and earlier findings with respect to schizophrenic patients, our volumetric measurements for the comparison brains are very similar to those found in previous postmortem studies that used sulcal landmarks to define the boundaries of the frontal lobe (16–18). For instance, Pakkenberg (16) reported a frontal lobe volume of 211.6 cm3, representing gray and white matter volumes combined, in normal comparison subjects. A later study of normal human brains (30) reported a frontal lobe volume of 213 cm3. The combined frontal gray and white matter volumes for the comparison subjects in the present study totaled 210.5 cm3. Although a somewhat smaller frontal lobe volume (174.4 cm3) was reported by Highley et al. (18), the cingulate gyrus was not included in their definition of the frontal lobe. Thus, the agreement between the frontal lobe volume measured in our study and that of previous reports using sulcal landmarks is quite good. The volume of comparison subjects’ prefrontal gray matter volume reported by Thune et al. (17) (62.5 cm3) is significantly smaller than the volume we found for whole frontal lobe gray matter (114.7 cm3), but that should be expected.
Technical Considerations
We chose to use the definition of Talairach and Tournoux (15) for the posterior border of the frontal lobe for two reasons. First, we wanted to dissect the brains as uniformly as possible, and this was best achieved by making the first cut at the midpoint of the anterior commissure–posterior commissure line. In addition to rendering brain slabs that were cut at a standard angle, our protocol also generated a random first cut. If we had begun cutting from the frontal pole, the first cut would not have been random. Second, once the brains were cut, identification of the central sulcus would have been difficult, because the central sulcus, like the plane of dissection, is oriented in the coronal plane and basically traverses the posterior face of the block just anterior to the first cut. It should be noted that the Sylvian fissure was used to define the boundary between the frontal and temporal cortices; therefore, while it might be possible that some parietal cortex was included in delineation of the frontal lobe, our definition of frontal lobe absolutely did not include any anterior temporal cortices.
Possible Confounding Factors
Storage time in formalin was the only factor that was significantly correlated with gray and white matter volumes. These correlations probably reflect differences in storage time between the Harvard Brain Tissue Resource Center and NIMH cohorts, as storage time in formalin was longer in the NIMH brains, averaging a little more than 4 years. Nevertheless, storage time in formalin was included as a covariate in the ANCOVAs for these measures, and the resulting analysis showed that the selective deficit in frontal gray matter volume remained significant.
The slightly longer postmortem intervals in the schizophrenic group relative to the comparison group also probably cannot account for the differences in frontal gray matter volume observed in this study, because the deficit in the entire schizophrenic group was also found in the smaller subgroup of whole brains, even though the schizophrenic and comparison groups had no difference in postmortem interval. Moreover, postmortem interval did not show a significant correlation with any of the measured parameters in the regression analysis.
Most of the schizophrenic patients in this study had received typical antipsychotic drugs. Changes in the volume of subcortical structures have previously been shown to be related to medication (31–33). Therefore, the possibility that long-term exposure to antipsychotic drugs may account for the smaller volume of cortical gray matter must be considered. However, the findings for the three schizophrenic patients in this study who were essentially neuroleptic-free revealed the same selective frontal gray matter volume deficit (12%) that was found for the schizophrenic group as a whole. Likewise, smaller gray matter volumes in specific regions of the prefrontal cortex have been found in neuroleptic-naive patients (21), indicating that the deficit in gray matter volume is a pathologic correlate of the disease and not a medication effect. Moreover, cortical gray matter was not reduced in thickness in nonhuman primates treated with antipsychotic drugs for a period of 6 months (34).
Exclusion of the schizophrenic patient with a secondary diagnosis of alcohol abuse did not alter the results of the study: frontal gray matter volume was still significantly smaller in the schizophrenic group without this patient (13% smaller than in the comparison group).
Prominence of Frontal Lobe Pathology
The study results indicate that the difference in gray matter volume between schizophrenic and normal brains was greater in the frontal lobe than in more posterior lobes. While these findings lend further support to several MRI studies that have indicated a preferential or selective deficit in gray matter volume in the frontal lobes (8–12), it is noteworthy that circumscribed regions of the mesial temporal cortex (35–39) and superior temporal gyrus (40–43) in schizophrenic patients have been shown to exhibit smaller volumes that are comparable in extent to the frontal lobe deficit reported here. Thus, a finer-grained analysis of volume in the posterior cortices may have revealed small regional deficits in gray matter volume of the temporal or parietal lobes that would rival the reductions observed in the frontal lobe as a whole. Nonetheless, it is an important observation that the magnitude of the volumetric deficits in gray matter volume mirror the functional deficits to a large extent. For example, cognitive deficits on “prefrontal” tasks that involve abstract thinking, working memory, and contextually dependent behaviors are more pronounced in schizophrenia than are deficits in visual processing (3), thereby correlating with the greater reduction of frontal than occipital lobe gray matter. Indeed, if we had been able to delineate the prefrontal cortex in our analysis, the deficit in schizophrenic patients might have been more pronounced.
Data from the present study support a growing literature suggesting that the frontal lobe, and the prefrontal cortex in particular, is a focal point for pathology in schizophrenia. Although few in number, postmortem studies that have examined both prefrontal and more posterior cortices in schizophrenic brains have generally found specific prefrontal deficits (44, 45) or more marked pathology in the prefrontal cortex (23). However, postmortem assay of excitatory amino acid concentrations and the levels of related enzymes have found abnormalities in hippocampal and prefrontal, but not parietal or cingulate, cortices (46). Paired temporal and frontal pathology has also been observed with proton magnetic resonance spectroscopy of N-acetylaspartate concentrations in the schizophrenic brain, revealing bilateral reductions of N-acetylaspartate in the hippocampus and dorsolateral prefrontal cortex (47). Thus, the cortical pathology in schizophrenia is nonuniform and seems to selectively involve prefrontal and medial temporal cortices.
Finally, the loss of prefrontal gray matter is consistent with the now substantial evidence for selective changes in prefrontal cell morphology or function rather than a loss of cell number per se (3, 48). Thus, although we cannot exclude the possibility that small pockets of neuronal degeneration are present in the cerebral cortex, the volumetric deficit in the frontal lobe most likely represents a smaller than normal number of the structures associated with neuronal connectivity, i.e., axonal processes, synaptic terminals, and dendritic arbors and spines.
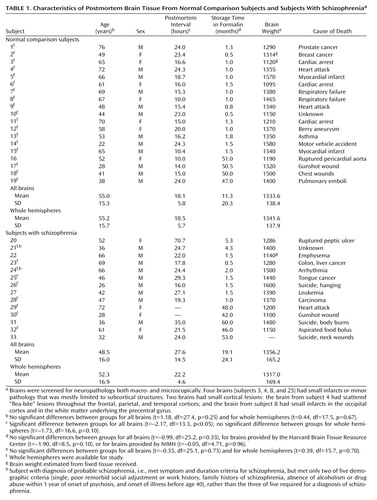 |
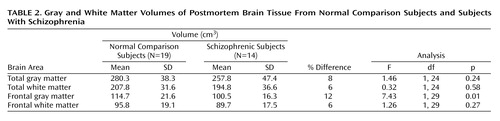 |
Presented at the seventh International Congress on Schizophrenia Research, Santa Fe, N.M., April 17–21, 1999. Received Oct. 19, 2001; revision received June 20, 2002; accepted July 9, 2002. From the Department of Neurobiology, Yale University School of Medicine; and the Clinical Brain Disorders Branch, NIMH, Bethesda, Md. Address reprint request to Dr. Selemon, Department of Neurobiology, Yale University School of Medicine, P.O. Box 208001, New Haven, CT 06520-8001; [email protected] (e-mail). Supported by NIHM grants MH-44866 and MH-59329 and a grant from the Theodore and Vada Stanley Foundation. The authors thank Dr. Francine M. Benes, Harvard Brain Tissue Resource Center (HBTRC) (supported by U.S. Public Health Service grant MH/NS 31862), for provision and diagnosis of the HBTRC brains; Dr. Ian Paul for statistical advice; Dr. Douglas Gelowitz for help in preparing the figures; and JoAnn Coburn for technical assistance.
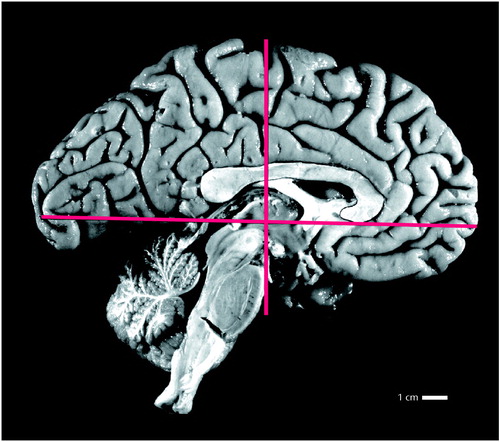
Figure 1. Sagittal Midhemispheric View of a Left Brain Hemisphere Showing Approximation of the Posterior Border of the Frontal Lobea
aThe vertical line, perpendicular to the midpoint of the anterior commissure–posterior commissure line, forms an accurate approximation of the posterior border of the frontal lobe, according to the brain atlas of Talairach and Tournoux (15).
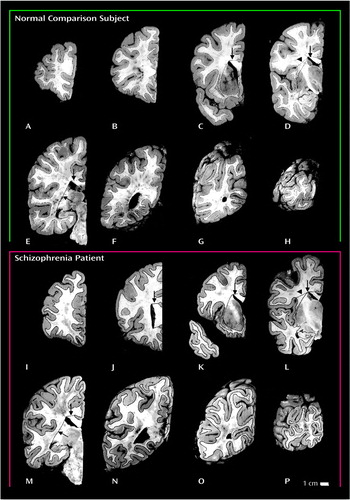
Figure 2. Coronal Slabs Showing Gray and White Matter Components in Postmortem Brains From a Normal Comparison Subject and a Subject With Schizophreniaa
aSlabs were 2 cm thick. For both brains, the posteriormost slabs, which did not have cut faces, and the schizophrenic subject’s anteriormost slab, which was very small, are not shown. Frontal and temporal areas of the brain were delineated on the mid-commissural slabs (panels D and L) by a line drawn from the lateral corner of the anterior horn of the lateral ventricle to the depths of the dorsal limb of the Sylvian fissure (arrowheads). Lines drawn from the lateral corner of the anterior horn of lateral ventricle to the depth of the callosal sulcus (panels C–E and J–M) and to the lateral corner of the temporal horn of the lateral ventricle (panels E and M) separated the cortical white matter from the corpus callosum and subcortical structures, respectively (arrows).
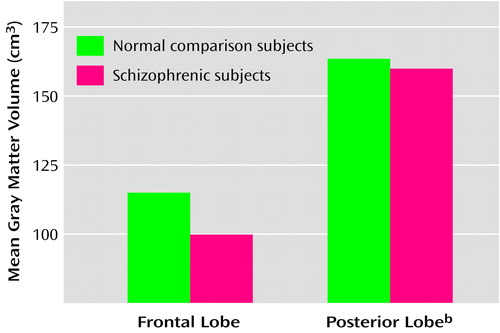
Figure 3. Mean Gray Matter Volume of the Frontal and Posterior Lobes in Postmortem Brains of Normal Comparison Subjects (N=18) and Subjects With Schizophrenia (N=10)a
aData for brains in which the whole left hemisphere was available for study.
bThe posterior lobe consists of the parietal, temporal, and occipital lobes.
1. Goldman-Rakic PS: Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory, in Psychopathology and the Brain. Edited by Carroll BJ, Barrett JE. New York, Raven Press, 1991, pp 1-23Google Scholar
2. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437-458Crossref, Medline, Google Scholar
3. Selemon LD: Regionally diverse cortical pathology in schizophrenia: clues to the etiology of the disease. Schizophr Bull 2001; 27:349-377Crossref, Medline, Google Scholar
4. Brown R, Colter N, Corsellis N, Crow TJ, Frith CD, Jagoe R, Johnstone EC, Marsh L: Postmortem evidence of structural brain changes in schizophrenia: difference in brain weight, temporal horn area, and parahippocampal gyrus compared with affective disorder. Arch Gen Psychiatry 1986; 43:36-42Crossref, Medline, Google Scholar
5. Pakkenberg B: Post-mortem study of chronic schizophrenic brains. Br J Psychiatry 1987; 151:744-752Crossref, Medline, Google Scholar
6. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195-205Crossref, Medline, Google Scholar
7. Harvey I, Ron MA, Du Boulay G, Wicks D, Lewis SW, Murray RM: Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med 1993; 23:591-604Crossref, Medline, Google Scholar
8. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V II, O’Leary DS, Ehrhardt JC, Yuh WTC: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763-1769Crossref, Medline, Google Scholar
9. Nopoulos P, Torres I, Flaum M, Andreasen NC, Ehrhardt JC, Yuh WTC: Brain morphology in first-episode schizophrenia. Am J Psychiatry 1995; 152:1721-1723Link, Google Scholar
10. Sullivan EV, Lim KO, Mathalon D, Marsh L, Beal M, Harris D, Hoff AL, Faustman WO, Pfefferbaum A: A profile of cortical gray matter volume deficits characteristic of schizophrenia. Cereb Cortex 1998; 8:117-124Crossref, Medline, Google Scholar
11. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537-547Crossref, Medline, Google Scholar
12. Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK: Mapping of grey matter changes in schizophrenia. Schizophr Res 1999; 35:1-14Crossref, Medline, Google Scholar
13. Yurgelin-Todd DA, Kinney DK, Sherwood AR, Renshaw PF: Magnetic resonance in schizophrenia. Semin Clin Neuropsychiatry 1996; 1:4-19Medline, Google Scholar
14. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099-1119Crossref, Medline, Google Scholar
15. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. Stuttgart, Germany, Georg Thieme, 1988Google Scholar
16. Pakkenberg B: Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical dissectors. Biol Psychiatry 1993; 34:768-772Crossref, Medline, Google Scholar
17. Thune JJ, Uylings HBM, Pakkenberg B: Total number of neurons in the prefrontal cortex in schizophrenics and controls. J Psychiatr Res 2001; 35:15-21Crossref, Medline, Google Scholar
18. Highley JR, Walker MA, Esiri MM, McDonald B, Harrison PJ, Crow TJ: Schizophrenia and the frontal lobes: post-mortem stereological study of tissue volume. Br J Psychiatry 2001; 178:337-343Crossref, Medline, Google Scholar
19. Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ: Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand 1988; 96:379-394Crossref, Medline, Google Scholar
20. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049-1055Link, Google Scholar
21. Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC: Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 2000; 57:761-768Crossref, Medline, Google Scholar
22. Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelin-Todd D, Kikinis R, Jolesz FA, McCarley RW: Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex 2001; 11:374-381Crossref, Medline, Google Scholar
23. Selemon LD, Rajkowska G, Goldman-Rakic PS: Abnormally high neuronal density in the schizophrenia cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995; 52:805-818Crossref, Medline, Google Scholar
24. Haug H, Eggers R: Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging 1991; 12:336-338Crossref, Medline, Google Scholar
25. Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD: Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997; 7:268-282Crossref, Medline, Google Scholar
26. Jernigan TL, Archibald SL, Fennema-Nostestine C, Gamst AC, Stout JC, Hesselink JR: Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 2001; 22:581-594Crossref, Medline, Google Scholar
27. Rapp PR, Stack EC, Gallagher M: Morphometric studies of the aged hippocampus, I: volumetric analysis in behaviorally characterized rats. J Comp Neurol 403:459-470Google Scholar
28. Jacobs B, Scheibel AB: A quantitative dendritic analysis of Wernicke’s area in humans, I: lifespan changes. J Comp Neurol 1993; 327:83-96Crossref, Medline, Google Scholar
29. Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Satin Louis LA, Cancro R: Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res Neuroimaging 2001; 107:61-73Crossref, Medline, Google Scholar
30. Pakkenberg B, Gundersen HJ: Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 1997; 384:312-320Crossref, Medline, Google Scholar
31. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430-1436Link, Google Scholar
32. Keshavan MS, Bagwell WW, Haas GI, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
33. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711-1717Link, Google Scholar
34. Selemon LD, Lidow MS, Goldman-Rakic PS: Increased volume and glial density in primate prefrontal cortex associated with chronic antipsychotic drug exposure. Biol Psychiatry 1999; 46:161-172Crossref, Medline, Google Scholar
35. Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia: a systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 1998, 172:110-120Google Scholar
36. Nelson D, Nelson MA, Saykin AJ, Flashman LA, Riordan HJ: Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Arch Gen Psychiatry 1998, 55:433-440Google Scholar
37. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET: Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16-25Link, Google Scholar
38. Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236-246Crossref, Medline, Google Scholar
39. Whitworth AB, Honeder M, Kremser C, Kemmler G, Felber S, Hausman A, Wanko C, Wechdorn H, Aichner F, Stuppaeck CH, Fleischhacker WW: Hippocampal volume reduction in male schizophrenic patients. Schizophr Res 1998; 31:73-81Crossref, Medline, Google Scholar
40. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457-1462Link, Google Scholar
41. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med 1992; 327:604-612Crossref, Medline, Google Scholar
42. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelin-Todd D, Kikinis R, Jolesz A, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia. Arch Gen Psychiatry 2000; 57:692-699Crossref, Medline, Google Scholar
43. Sanfilipo M, Lafargue T, Rusinek, H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A: Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 2000; 57:471-480Crossref, Medline, Google Scholar
44. Glantz LA, Lewis DA: Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry 1997; 54:943-952Crossref, Medline, Google Scholar
45. Glantz LA, Lewis DA: Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 2000; 57:65-73Crossref, Medline, Google Scholar
46. Tsai G, Passani LA, Slusher BS, Carter T, Baer L, Kleinman JE, Coyle JT: Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry 1995; 52:829-836Crossref, Medline, Google Scholar
47. Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CTW, Frank JA, Tedeschi G, Weinberger DR: Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1996; 153:1554-1563Link, Google Scholar
48. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17-25Crossref, Medline, Google Scholar



