Glutamate and Glutamine Measured With 4.0 T Proton MRS in Never-Treated Patients With Schizophrenia and Healthy Volunteers
Abstract
OBJECTIVE: This in vivo 1H magnetic resonance spectroscopy study examined levels of glutamate, glutamine, and N-acetylaspartate in patients experiencing their first episode of schizophrenia. METHOD: Localized in vivo 1H spectra were acquired at 4.0 T from the left anterior cingulate and thalamus of 21 never-treated patients with schizophrenia and 21 comparable healthy volunteers. RESULTS: The level of glutamine was significantly higher in the left anterior cingulate cortex and thalamus of the patients with schizophrenia than in the healthy subjects. No differences were found between groups in the levels of other metabolites in the anterior cingulate or thalamus. CONCLUSIONS: Higher than normal glutamine levels in the left anterior cingulate and thalamus provide in vivo evidence of greater than normal glutamatergic activity proposed by glutamatergic models of schizophrenia. In contrast to other studies in chronically ill patients, no differences were seen in the levels of N-acetylaspartate in either location, suggesting that the findings in patients with chronic schizophrenia may be related to the effect of medication or the progression of the illness.
Glutamatergic models have highlighted the role of the anterior cingulate and other parts of the limbic system in the pathophysiology of schizophrenia (1–3). However, glutamatergic models of schizophrenia cannot be fully tested without in vivo measures of glutamate metabolism. Levels of glutamate, glutamine, N-acetylaspartate, total creatine, choline-containing compounds, taurine, scyllo-inositol, and myo-inositol can be measured simultaneously by using in vivo short-echo-time 1H magnetic resonance spectroscopy (MRS) (4). In our previous 1.5 T 1H MRS study (5), we found higher levels of glutamine and similar levels of N-acetylaspartate in a 4.5-cc left medial prefrontal region of never-treated patients with schizophrenia compared with healthy volunteers.
The use of high-field MR scanners (≥3.0 T) increases the spectral signal-to-noise ratio, enabling the collection of spectroscopic data from smaller volumes while maintaining quantification precision (4). In this study, we report what is to our knowledge the first short-echo-time 1H MRS findings at 4.0 T from 1.5-cc volumes of the left anterior cingulate and thalamus in never-treated patients with schizophrenia and healthy volunteers. We hypothesized that, compared with healthy volunteers, never-treated patients with schizophrenia would have higher levels of glutamine in the left anterior cingulate, supporting our earlier findings in a lower-field study (5), and higher levels of glutamine in the left thalamus because the left anterior cingulate and the left thalamus are functionally connected by glutamatergic neurons.
Method
Twenty-one never-treated patients experiencing their first episode of schizophrenia and 21 healthy volunteers participated in the study after giving informed written consent according to the guidelines of the Review Board for Health Sciences Research Involving Human Subjects at the University of Western Ontario. The mean age of the 21 patients was 26 years (SD=7); 14 were male and seven were female. The mean age of the healthy subjects was also 26 (SD=7), and 14 were male and seven were female.
All patients and healthy volunteers were assessed with the Structured Clinical Interview for DSM-IV (SCID) (6) by two interviewers who arrived at a consensual diagnosis. Fourteen of the patients were classified as having paranoid, one as having disorganized, and six as having undifferentiated schizophrenia. The patients’ mean total scores on the Scale for the Assessment of Negative Symptoms (SANS) (7) and the Scale for the Assessment of Positive Symptoms (SAPS) (8) were 43 (SD=9) and 35 (SD=11), respectively. A mean of 21 months (SD=24) had elapsed since the first positive symptoms in the patients. Five patients had received a mean of 1.9 mg (SD=1.2) of lorazepam in the 24 hours before the scan, and one patient had taken paroxetine for 3 weeks, which was discontinued 48 hours before the scan.
The mean parental education level of the highest educated parent, rated on a 4-point scale (5), was 2.3 (SD=1.1) for the patients and 2.7 (SD=1.2) for the healthy volunteers. According to a handedness questionnaire (5), all subjects were right-handed with the exception of four patients who were rated left-handed or ambidextrous and three volunteers who were rated left-handed. None of the patients or volunteers had a history of head injury, drug or alcohol abuse in the year before the scan, or serious medical illnesses based on information provided during the SCID. Routine clinical anatomical images detected no gross abnormalities in the patients.
In vivo short-echo 1H-stimulated echo acquisition mode spectra were obtained from all subjects by using a Varian/Siemens Unity Inova 4.0 T MR scanner (Varian, Palo Alto, Calif.; Siemens, Erlangen, Germany) (TR=2000 msec, TE=20 msec, TM=30 msec, dwell time=500 μsec). A water-suppressed (256 averages) and two water-unsuppressed (16 averages) acquisitions were recorded from two 1.5-cc voxels—one containing mostly gray matter from the left anterior cingulate and one from the left medial thalamus. Lineshape-corrected and water-subtracted spectra were fitted in the time domain by using a priori knowledge from 12 metabolite solutions and a constrained Levenberg-Marquardt minimization algorithm (4).
Sixty-four contiguous 2.75-mm-thick transverse slices were acquired by using a three-dimensional sequence of fast low-angle shots (TR=11 msec, TE=7 msec) to localize the volumes of interest and to assess the amount of gray matter, white matter, and CSF within each voxel according to a histogram-based technique (ANALYZE software [9]). The amplitude of each water-unsuppressed spectrum was corrected for the relative gray matter, white matter, and CSF content of the voxel. Metabolite levels were normalized to this amplitude. Metabolites and macromolecules with coefficients of variation less than 75% were compared between groups with analyses of variance. Correlation between metabolite levels and clinical scores (SANS and SAPS) was evaluated with the Pearson product moment correlation coefficient; significance was set at p=0.001 because no a priori hypotheses were made.
Results
One left anterior cingulate data set was rejected because of excessive patient motion and two left thalamus data sets were unavailable because of premature termination of the examination. The parallel sets from corresponding volunteers were removed from the statistical analysis. The mean metabolite levels for both regions studied as well as the significant differences between groups are presented in Figure 1.
Not shown is the significantly higher level of unidentified macromolecules at 3.03 ppm (F=8.68, df=1, 38, p=0.005) and 3.05 ppm (F=9.43, df=1, 38, p=0.004) in the left anterior cingulate of never-treated patients than in healthy volunteers. None of the correlations reached the p=0.001 level.
Discussion
A significantly higher than normal level of glutamine in the left anterior cingulate and thalamus in never-treated patients with schizophrenia is consistent with our earlier 1.5 T study in which we found significantly higher than normal levels of this metabolite within a larger region including tissue from the left medial prefrontal cortex in a different group of never-treated patients with schizophrenia. It is possible that no differences were seen in the levels of glutamate between never-treated patients and healthy volunteers because the glutamate measured by 1H MRS includes both the metabolic and transmitter pools (10). A high level of glutamine suggests greater than normal glutamatergic activity, consistent with glutamatergic models of schizophrenia (1–3), because most of the physiologically active glutamate is derived from glutamine (10). Released glutamate is taken up by astrocytes, where it is converted to glutamine, transported back to the presynaptic neuron, and reconverted to glutamate (10). Thus, higher levels of glutamine could also indicate less glutamatergic activity if there was an abnormality in the conversion of glutamine to glutamate.
There were no differences between patients and volunteers in N-acetylaspartate levels in either the anterior cingulate or the thalamus. This observation contrasts with several reports indicating lower levels of N-acetylaspartate within similar regions in medicated patients with chronic schizophrenia (11–13). Some differences might be accounted for by the methods used to quantify this metabolite and the effect of medication. However, it is also possible that N-acetylaspartate, glutamate, and glutamine levels may fall over time as the result of glutamate-induced neuronal degeneration. In keeping with this suggestion is a report by Omori et al. (14), which demonstrated a trend for lower levels of N-acetylaspartate and glutamate assessed with 7.0 T 1H MRS in postmortem thalamic tissue of patients with schizophrenia than in tissue from control subjects. Follow-up in vivo 1H MRS examinations in our first-episode patients are necessary to substantiate this possibility.
Presented in part at the International Society for Magnetic Resonance in Medicine meeting in Glasgow, Scotland, April 21–27, 2001, and the International Congress on Schizophrenia Research, Whistler, B.C., Canada, April 28–May 5, 2001. Received Jan. 18, 2002; revision received May 15, 2002; accepted May 22, 2002. From the Department of Nuclear Medicine and Magnetic Resonance, St. Joseph’s Health Care, London, Ont., Canada; the Laboratory for Functional Magnetic Resonance Research, John P. Robarts Research Institute, London, Ont., Canada; the Department of Psychiatry and Department of Psychology, University of Western Ontario, London; and King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia. Address reprint requests to Dr. Williamson, Department of Psychiatry, University Campus, London Health Sciences Centre, 339 Windermere Rd., London, ON Canada N6A 5A5; [email protected] (e-mail). Supported in part by grant MT-12078 from the Canadian Institutes of Health Research.
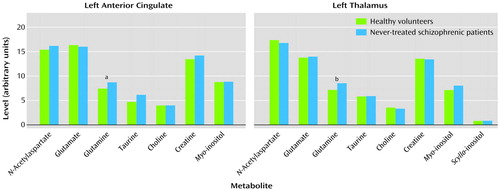
Figure 1. Brain Metabolite Levels Measured for Healthy Volunteers and Never-Treated Patients With Schizophrenia
aN=20 for schizophrenia group. Significant group effect (F=5.21, df=1, 38, p=0.03).
bN=19 for schizophrenia group. Significant group effect (F=5.54, df=1, 36, p=0.02).
1. Javitt DC, Zukin SR: Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 1991; 148:1301-1308Link, Google Scholar
2. Olney JW, Farber NB: Glutamate receptor dysfunction in schizophrenia. Arch Gen Psychiatry 1995; 52:998-1007Crossref, Medline, Google Scholar
3. Carlsson A, Hansson LO, Waters N, Carlsson ML: A glutamatergic deficiency model of schizophrenia. Br J Psychiatry 1999; 174:2-6Crossref, Google Scholar
4. Bartha R, Drost DJ, Menon RS, Williamson PC: Comparison of the quantification precision of human short echo time 1H spectroscopy at 1.5 and 4.0 Tesla. Magn Reson Med 2000; 44:185-192Crossref, Medline, Google Scholar
5. Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, Canaran MacFabe G, Rylett RJ, Neufeld RWJ: Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1997; 54:959-965Crossref, Medline, Google Scholar
6. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1997Google Scholar
7. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
8. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1983Google Scholar
9. Robb RA: Biomedical Imaging, Visualization, and Analysis. New York, John Wiley & Sons, 1999Google Scholar
10. Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG: In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Phil Trans R Soc London B 1999; 354:1165-1167Crossref, Medline, Google Scholar
11. Deicken RF, Zhou L, Schuff N, Weiner MW: Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr Res 1997; 27:65-71Crossref, Medline, Google Scholar
12. Deicken RF, Johnson C, Eliaz Y, Schuff N: Reduced concentrations of thalamic N-acetylaspartate in male patients with schizophrenia. Am J Psychiatry 2000; 157:644-647Link, Google Scholar
13. Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA: Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res 2000; 41:389-395Crossref, Medline, Google Scholar
14. Omori M, Pearce J, Komoroski RA, Griffin WST, Mrak RE, Husain MM, Karson CN: In vitro 1H-magnetic resonance spectroscopy of postmortem brains with schizophrenia. Biol Psychiatry 1997; 42:359-366Crossref, Medline, Google Scholar



