Frontal White Matter Microstructure and Treatment Response of Late-Life Depression: A Preliminary Study
Abstract
OBJECTIVE: This study tested the hypothesis that microstructural abnormalities in white matter areas of the brain containing frontostriatal tracts are associated with a low rate of remission of geriatric depression. METHOD: Thirteen older patients with major depression received open, but controlled, treatment with citalopram at a target daily dose of 40 mg for 12 weeks. Diffusion tensor imaging was used to determine fractional anisotropy in preselected white matter regions. RESULTS: Survival analysis with Cox’s proportional hazards model revealed that lower fractional anisotropy of the right and the left frontal white matter regions 15 mm above the anterior commissure–posterior commissure plane was associated with a low remission rate after age was considered. Remission was not significantly associated with fractional anisotropy of lower frontal regions or a temporal region. CONCLUSIONS: Microstructural white matter abnormalities lateral to the anterior cingulate may be associated with a low rate of remission of geriatric depression.
Frontostriatal dysfunction may contribute to depression (1–3) and influence its course. Executive impairment, an expression of frontostriatal dysfunction, was reported to predict a poor (4) and unstable (5) antidepressant response of geriatric major depression. White matter hyperintensities, i.e., regions of greater signal intensity shown by magnetic resonance imaging (MRI), are associated with executive dysfunction and with chronicity of geriatric depression (6).In younger patients, remission of depression is associated with metabolic increases in dorsal cortical regions (1) and decreases in ventral limbic and paralimbic structures (7). Persistence of elevated amygdala metabolism during remission of depression may be a predictor of relapse (1). Hypometabolism of the rostral anterior cingulate is associated with treatment-resistant depression (7). It has been proposed (1, 7) that disruption in dorsal cortical-ventral limbic regulation perpetuates depression.
On the basis of these findings, we hypothesized that microstructural white matter abnormalities lateral to the anterior cingulate, compromising limbic-cortical balance, are associated with a low likelihood of remission of geriatric depression.
Method
Subjects at the Cornell Intervention Research Center who agreed to MRI and signed written consent statements were consecutively recruited for this study. They were aged 60–77 years, met the Research Diagnostic Criteria for unipolar major depression, and had a score of 18 or higher on the 24-item Hamilton Depression Rating Scale. Exclusion criteria were other axis I disorders, dementia, metastatic cancer, brain tumors, myocardial infarction within the 3 months before the study, delirium, stroke, Parkinson’s disease, and multiple sclerosis.
The subjects were assessed with the Schedule for Affective Disorders and Schizophrenia and the 24-item Hamilton depression scale. Overall cognitive impairment was determined by the total score on the Mini-Mental State Examination (MMSE), and executive dysfunction was measured with the initiation/perseveration domain of the Mattis Dementia Rating Scale (8) and the Stroop Color-Word Response Inhibition Test (9). Medical burden was assessed with the Cumulative Illness Rating Scale—Geriatrics (10); the psychiatric domain was not included.
Diffusion tensor imaging was performed at Nathan Kline Institute, Orangeburg, N.Y., by using a 1.5-T Siemens Vision MR and an echo-planar double-spin echo sequence with TR=6000 msec, TE=100 msec, 5-mm-thick slices, skip 0, alignment with the anterior commissure–posterior commissure (AC-PC) plane, 24-cm field of vision, 128×128 matrix reconstructed to a 256×256 b matrix for which b=1000 sec/mm2. The tensor was computed from seven images: the b=0 sec/mm2 image and six images with b=1000 sec/mm2 with gradients applied in six noncollinear directions. Six maps of the apparent diffusion coefficient were computed. Then six independent elements of the diffusion tensor were determined, the eigenvalues and eigenvectors were computed, and a scalar measure, fractional anisotropy, was assessed (11). Fractional anisotropy yields values between 0 (i.e., isotropic or unrestricted diffusion, as in CSF) and 1 (i.e., anisotropic or constrained diffusion due to barriers, as in organized white matter fibers).
Circular regions of interest of fixed sizes were positioned bilaterally in the frontal white matter of five consecutive images, from 15 mm above to 5 mm below the AC-PC plane. The size of the regions of interest at 15 mm, 10 mm, and 5 mm above the AC-PC line was 84.4 mm2, and the size on and 5 mm below the AC-PC line was 43.5 mm2. We hypothesized that low fractional anisotropy at regions of interest 15 and 10 mm above the AC-PC plane is associated with a low remission rate. The lower frontal regions and a temporal white matter region of interest adjacent to the hippocampus were used to evaluate the specificity of the hypothesized relationships.
Survival analysis with Cox proportional hazards was used to examine the relationship of fractional anisotropy to remission. Student’s t test and chi-square were used to examine demographic and clinical differences at baseline, and Spearman’s rank-order correlation was used to study the relationship of fractional anisotropy to executive function. Two-tailed alpha levels of significance were used.
Results
Of the 13 subjects (60–77 years old), eight were treated with 40 mg/day of citalopram (target), one with 30 mg, and four with 20 mg. Five subjects remained depressed, and eight achieved remission, defined as no longer meeting the DSM-IV criteria for a depressive disorder and having a score less than 10 on the Hamilton depression scale for at least 2 weeks.
Survival analysis revealed that higher fractional anisotropy of the right frontal white matter 15 mm above the AC-PC plane was associated with the occurrence of remission (χ2=4.02, df=1, p<0.05) (Figure 1), even when the effect of age on remission was taken into consideration (χ2=4.36, df=1, p<0.04). A similar relationship was noted for fractional anisotropy of the left frontal white matter 15 mm above the AC-PC plane, but it fell short of statistical significance (χ2=3.67, df=1, p=0.06) (Figure 1). The relationship of frontal fractional anisotropy 10 mm above the AC-PC line approached significance (right: χ2=2.97, df=1, p<0.08; left: χ2=1.77, df=1, p<0.18) after the effect of age was taken into consideration (right: χ2=3.20, df=1, p<0.07; left: χ2=4.42, df=1, p<0.04). Remission was not significantly associated with fractional anisotropy of frontal regions below the 10-mm plane (5 mm, 0 mm, –5 mm) or the temporal region.
There were no significant differences between the patients with remitted (N=8) and nonremitted (N=5) depression in the dose of citalopram, baseline severity of depression (Hamilton depression scale total score), overall cognitive impairment (MMSE total score), or education. The subjects in remission had more normal scores on executive function tests than those without remission (initiation/perseveration: t=3.04, df=11, p<0.05; Stroop: t=2.42, df=11, p<0.05). The scores on the Stroop test were associated with fractional anisotropy 15 mm above the AC-PC plane in frontal regions on the right side (Spearman’s rank correlation: rs=0.65, df=10, p<0.03) but not the left (rs=0.31, df=10, p<0.36) and were also significant at 10 mm above the AC-PC plane on both the right (rs=0.78, df=10, p<0.005) and left (rs=0.66, df=10, p<0.03). Correlations with the Mattis Dementia Rating Scale domain of initiation/perseveration were not significant for fractional anisotropy 15 mm above the AC-PC plane on the right (rs=0.54, df=11, p<0.06) or left (rs=0.44, df=11, p<0.15) or at 10 mm above the AC-PC plane on the right (rs=0.42, df=11, p<0.18) or left (rs=0.38, df=11, p<0.22).
Discussion
The principal finding of this study is that lower fractional anisotropy at the frontal white matter located 15 mm, and perhaps 10 mm, above the AC-PC line is associated with a lower rate of remission of geriatric major depression after treatment with citalopram. No such relationship was identified in lower frontal regions or in a temporal region. To our knowledge, this is the first study to localize microstructural white matter abnormalities associated with lower likelihood of remission of depression.
White matter 15 mm and 10 mm above the AC-PC plane is located laterally to the anterior cingulate at the level of the middle frontal gyrus and contains fibers of the anterior cingulate and the dorsolateral pathways (12). Compromised white matter in these regions may interfere with the reciprocal regulation of dorsal neocortical-ventral limbic structures and lead to a “disconnection syndrome” with poor antidepressant response. This view is further supported by the association of lower fractional anisotropy in these regions with executive dysfunction, an abnormality noted to predict poor response of depression to treatment (3, 4).
The theoretical value of these findings is that they provide a step in understanding the neural systems needed for antidepressant response. If these observations are confirmed, subsequent diffusion tensor imaging studies can use fiber mapping to identify with greater precision specific frontostriatal pathways interfering with treatment response.
The clinical value of this study is that it can guide the selection of cognitive tests for studies of prediction of treatment response. Tests of specific frontostriatal functions, validated through neuroimaging, are available. If shown to predict treatment response, such tests can be used in treatment planning.
The principal limitation of this study is its small study group. Despite this limitation, the localization of microstructural abnormalities can guide studies of specific pathways associated with treatment response and generate pharmacological investigations of novel agents targeting depressed elderly patients with abnormalities in frontostriatal systems.
Received Aug. 6, 2001; revision received April 9, 2002; accepted May 9, 2002. From the Department of Psychiatry, Weill Medical College, Cornell University; and the Nathan Kline Institute, Orangeburg, N.Y. Address reprint requests to Dr. Alexopoulos, Department of Psychiatry, Weill Medical College of Cornell University, 21 Bloomingdale Rd., White Plains, NY 10605; [email protected] (e-mail). Supported by NIMH grants MH-42819, MH-51842, MH-49762, and MH-19132 to Dr. Alexopoulos; by NIMH grant MH-53313 to Dr. Lim; and by support to Dr. Alexopoulos from Forest Pharmaceuticals, the Sanchez Foundation, and the Dr. I Foundation.
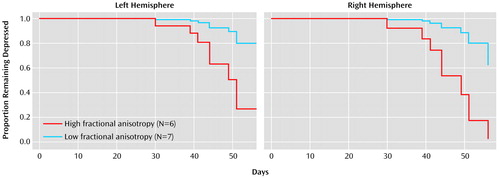
Figure 1. Time to Remission for 13 Depressed Elderly Subjects in Relation to Fractional Anisotropy in a White Matter Axial Region Located 15 mm Above the Anterior Commissure–Posterior Commissure Planea
aHigh and low values for fractional anisotropy were divided at the median value. Eight subjects achieved remission, and five remained depressed.
1. Drevets WC: Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813-819Crossref, Medline, Google Scholar
2. MacFall JR, Payne ME, Provenzale JE, Krishnan KRR: Medial orbital frontal lesions in late-onset depression. Biol Psychiatry 2000; 49:803-806Crossref, Google Scholar
3. Kumar A: Neuroanatomy of late-life mood disorders. Economics of Neuroscience 2001; 3:44-48Google Scholar
4. Kalayam B, Alexopoulos G: Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry 1999; 56:713-718Crossref, Medline, Google Scholar
5. Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Hull J, Kakuma T: Executive dysfunction increases the risk for relapse and recurrence of geriatric depression. Arch Gen Psychiatry 2000; 57:285-290Crossref, Medline, Google Scholar
6. Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B: Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry 1995; 37:151-160Crossref, Medline, Google Scholar
7. Mayberg HS: Depression and frontal-subcortical circuits: focus on prefrontal-limbic interactions, in Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. Edited by Lichter DC, Cummings JL. New York, Guilford, 2001, pp 177-206Google Scholar
8. Mattis S: Dementia Rating Scale. Odessa, Fla, Psychological Assessment Resources, 1989Google Scholar
9. Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC: Neuropsychological test norms above age 55. Clin Neuropsychol 1996; 10:262-278Crossref, Google Scholar
10. Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF III: Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237-248Crossref, Medline, Google Scholar
11. Basser PJ: Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995; 8:333-344Crossref, Medline, Google Scholar
12. Middleton FA, Strick PL: A revised neuroanatomy of frontal-subcortical circuits, in Frontal Subcortical Circuits in Psychiatric and Neurological Disorders. New York, Guilford, 2001, pp 44-58Google Scholar



