Antipsychotic Medication Adherence: Is There a Difference Between Typical and Atypical Agents?
Abstract
OBJECTIVE: Pharmacy refill records were used to compare medication adherence in outpatient veterans receiving typical versus atypical antipsychotic medications. METHOD: Consecutive patients meeting selection criteria and receiving prescriptions for haloperidol (N=57), perphenazine (N=60), risperidone (N=80), olanzapine (N=63), and quetiapine (N=28) over a 3-month period were identified from a computerized database. The hospital policy at the time of this study required failure in trials of at least two typical antipsychotics before initiation of an atypical agent. Patients’ adherence with the antipsychotic regimen was calculated by analyzing refill records for up to 12 months. The cumulative mean gap ratio (the number of days when medication was unavailable in relation to the total number of days) and the compliant fill rate (the number of prescription fills indicating adherence in relation to the total number of prescription fills) at 6 and 12 months were calculated. RESULTS: Adherence rates at 6 and 12 months were moderately higher in patients who received atypical antipsychotics than in those who received typical agents. Cumulative mean gap ratios were 23.2% for typical and 14.1% for atypical antipsychotics at 12 months; thus, patients who received typical agents were without medication for an average of 7 days per month, compared with 4 days per month for those who received atypical agents. At 12 months, compliant fill rates were 50.1% for typical and 54.9% for atypical antipsychotics. CONCLUSIONS: Interventions to improve adherence are warranted even for patients who receive atypical antipsychotic medications.
Effective treatment of psychosis involves the use of antipsychotic medication. Yet, reported rates of nonadherence (noncompliance) to antipsychotics range from 20%–89%, with an average rate of approximately 50% (1, 2). In patients with schizophrenia, nonadherence to antipsychotic maintenance treatment leads to psychotic relapse, rehospitalization, and more frequent clinic and emergency room visits (3, 4). The consequences of nonadherence thus contribute significantly to schizophrenia’s estimated annual cost of $33–$65 billion (5, 6).
The improved side effect profile of atypical antipsychotics (i.e., lower incidences of extrapyramidal symptoms [7–10] and tardive dyskinesia [11–12], compared with the incidences for typical antipsychotics) has led investigators to speculate that patients receiving these medications will show greater adherence (13–15). We are aware, however, of only a few published reports comparing typical and atypical antipsychotics in terms of medication adherence. Rosenheck and colleagues (14) evaluated medication continuation and regimen adherence in 423 patients taking haloperidol or clozapine as part of a double-blind, randomized trial. Although the patients who received clozapine continued their medication significantly longer, the treatment groups did not differ in the proportion of pills returned each week (14). Olfson and colleagues (16) examined the effect of antipsychotic type on adherence 3 months after 213 inpatients with schizophrenia or schizoaffective disorder were discharged while receiving typical (84.5% of patients) or atypical (14.5% of patients) antipsychotics. Patients were considered nonadherent if they reported stopping their antipsychotic for 1 week or more. A nonsignificant trend toward increased adherence was reported among patients with prescriptions for atypical antipsychotics (16). Cabeza and colleagues (17) retrospectively studied the relationship of adherence to antipsychotic type (typical agent, clozapine, or risperidone) in 60 inpatients with schizophrenia. Adherence over the past year was rated as adequate or irregular. No significant association was found between adherence and type of antipsychotic (17).
The purpose of the study reported here was to examine adherence to medication regimens of typical versus atypical antipsychotics among Department of Veterans Affairs (VA) outpatients with psychotic disorders. We selected pharmacy refill records to measure adherence because direct measurement of medication consumption in outpatients was not feasible. All of the methods commonly used to evaluate antipsychotic medication adherence in outpatients have limitations (18). Although pharmacy records provide an indirect measure of adherence, their validity for assessing adherence has been previously reported for nonpsychiatric and psychiatric outpatients receiving long-term pharmacotherapy (19).
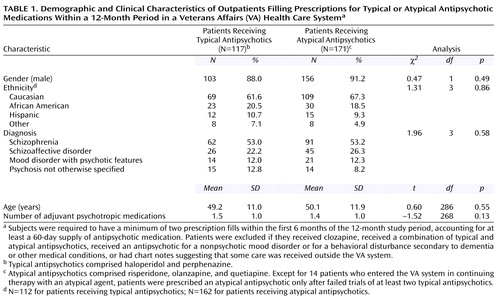
We sought to test the hypothesis that patients with prescriptions for atypical antipsychotics (i.e., risperidone, olanzapine, or quetiapine) would be more adherent than those with prescriptions for conventional neuroleptics (i.e., haloperidol or perphenazine). We also examined the association of certain patient and treatment-related factors with nonadherence. On the basis of the available literature (20–31), we hypothesized that higher daily doses of antipsychotics and a larger number of adjuvant psychotropic medications would be associated with higher rates of nonadherence, but that age, gender, ethnicity, and diagnosis would not be associated with greater nonadherence.
Method
Patient Selection
This protocol was approved by the University of California, San Diego, Human Subjects Committee. By using the VA San Diego Healthcare System pharmacy computer database, we identified more than 1,800 new-fill or refill prescriptions for haloperidol, perphenazine, risperidone, olanzapine, and quetiapine within a 3-month time period. Haloperidol and perphenazine were chosen for this study because they were two of the most commonly prescribed typical antipsychotics in the VA San Diego Healthcare System. Patients with a prescription for clozapine were not included in this study because the VA San Diego Healthcare System required patients who were taking clozapine to have weekly or biweekly clinic visits to receive their medication, which would be likely to result in a higher adherence rate for these patients. At the time of the study, the hospital required that trials of at least two typical antipsychotics must have failed before a trial with an atypical agent could be initiated. Only patients entering the VA San Diego Healthcare System from an outside facility with a documented need for continued therapy with an atypical agent could continue the medication without fulfilling this criterion. In our study group, the large majority of patients who received an atypical agent had previous trials of two or more typical agents that had failed. Only 8.2% of the patients (N=14 of 171) had transferred to the VA San Diego Healthcare System while receiving an atypical antipsychotic.
Within the 3-month time period, we labeled the most recent prescription fill as the “index” fill. This initial group was trimmed to 629 prescriptions for 629 unique patients by eliminating duplicate prescriptions for individual patients and by excluding patients who received any combination of a typical plus an atypical antipsychotic medication. To minimize the heterogeneity of the patient pool, we further restricted the study group by applying the following inclusion criteria: 1) a chart diagnosis of DSM-IV schizophrenia, schizoaffective disorder, mood disorder with psychotic features, or psychosis not otherwise specified and 2) a minimum of two prescription fills within the 6 months before the index fill, accounting for at least a 60-day supply of antipsychotic medication. The diagnostic categories were chosen to represent patients who received continuing antipsychotic therapy for psychotic disorders. Patients were excluded if they received antipsychotics for nonpsychotic mood disorders or behavioral disturbances secondary to dementia or other general medical conditions. In addition, patients were excluded if their charts suggested that they were receiving even part of their medical care outside of the VA system. These selection criteria resulted in a study group of 288 patients, including 57 who were receiving haloperidol, 60 receiving perphenazine, 80 given risperidone, 63 given olanzapine, and 28 given quetiapine.
Data Collection
Demographic and relevant clinical information, including data on age, gender, ethnicity, psychiatric diagnosis, and psychotropic medications received, was collected. Data on prescriptions for antidepressants, mood stabilizers, and sedative-hypnotics were recorded for analysis of the use of adjuvant psychotropic medication. Data on prescriptions for medications to treat extrapyramidal symptoms were also recorded.
Adherence to prescribed regimens was determined by examining computerized medication fill records for a 12-month period. Refill assessments were carried out for up to 12 months beginning with the “baseline study fill” (i.e., the medication fill that occurred up to 6 months before the index fill). Adherence was computed with two previously described methods: 1) the cumulative mean gap ratio and 2) the compliant fill rate (19, 32).
The cumulative mean gap ratio for a specified time period was calculated by dividing the number of days that medication was unavailable for consumption (due to a delayed refill) by the total number of days during the time period (19, 32). The compliant fill rate for a specified time period represents the proportion of total fills that are adherent, i.e., filled at the appropriate time interval. Adherence was assessed by comparing the number of days when the antipsychotic was available for consumption (i.e., day supply) to the number of calendar days between fills. Fills obtained within a period equivalent to 20%–120% of the period covered by the previous prescription were considered adherent (19, 32). A compliant fill rate was determined for each patient on the basis of the assessable fills during the 12 months of observation. However, if a change in therapy, e.g., a dose change or a change to a new medication, required a new prescription to be filled before the end of the period covered by a previous prescription, the premature fill was deemed adherent. Thus, while the compliant fill rate was based on a series of dichotomous assessments of adherence, the cumulative mean gap ratio provided a continuous assessment that detected the magnitude of gaps in therapy and constituted a more clinically meaningful measure. The following are the formulae used in our refill record calculations:
Cumulative Mean Gap Ratio=([Total Number of Days in the Study Period – Total Number of Days that Medication was Available]/Total Number of Days in the Study Period) × 100
Compliant Fill Rate=(Number of Adherent Fills/Total Number of Fills) × 100
Data Analysis
Independent samples t tests were used to compare the mean age, number of adjuvant psychotropic medications, compliant fill rate, and cumulative mean gap ratio for patients receiving typical antipsychotics (haloperidol and perphenazine) versus those receiving atypical agents (risperidone, olanzapine, and quetiapine). If significant differences between the typical and atypical antipsychotic groups were found, one-way analysis of variance with Scheffé’s post hoc tests was used to compare data for specific typical and atypical medications. Chi-square analysis was used to compare data on diagnoses, ethnicity, and gender between groups. Yates’s corrections were employed for all two-by-two chi-square tests. To address our secondary study questions about the association of patient-related factors with nonadherence, Pearson product-moment correlation was used to examine the relationship between cumulative mean gap ratio data and age, daily dose of medication, and number of adjuvant psychotropic medications. Student’s t tests were performed to evaluate the association of cumulative mean gap ratio data with ethnicity and diagnosis. Adherence rates were calculated at 6 and 12 months, but only 12-month adherence rates were used to answer our secondary study questions. All the statistical tests were two-tailed, and significance was defined as alpha=0.05.
Results
Patient and Treatment Characteristics
Comparisons among patients who received specific antipsychotic medications and between patients who received typical versus atypical medications revealed no significant group differences in gender, age, ethnicity, psychiatric diagnosis, and number of adjuvant psychotropic medications (Table 1). In the study group, 22.2% of the patients who received atypical antipsychotics (N=38) and 11.1% of patients who received typical agents (N=13) had at least one psychiatric hospitalization during the 1-year study period (χ2=5.07, df=1, p=0.03). The mean total number of days of antipsychotic use analyzed per patient was 304.9 (SD=80.6). No differences existed among patients receiving specific medications in the mean total number of days of antipsychotic therapy analyzed (F=1.51, df=4, 283, p=0.20). The median total daily doses of antipsychotic medications, calculated by using each patient’s highest prescribed dose for the 12-month period, were 8 mg of haloperidol (range=1–40), 12 mg of perphenazine (range=2–48), 4 mg of risperidone (range=0.5–12), 12.5 mg of olanzapine (range=5–30), and 400 mg of quetiapine (range=50–600).
Adherence Rates
On the basis of the cumulative mean gap ratio method, the patients with prescriptions for atypical antipsychotics had significantly smaller gaps in therapy compared to those with prescriptions for typical antipsychotics at 6 months (cumulative mean gap ratio=12.2%, SD=19.4%, compared with 22.9%, SD=25.9%) (t=–3.81, df=202, p=0.001, unequal variances) and at 12 months (cumulative mean gap ratio=14.1%, SD=18.4%, compared with 23.2%, SD=25.0%) (t=–3.34, df=200, p=0.001, unequal variances). Thus, patients receiving typical agents were without medication for approximately 7 days per month, while those receiving atypical antipsychotics were without medication for approximately 4 days per month. Significant differences in the cumulative mean gap ratio existed at 6 months among some of the five medications included in the analysis (F=4.63, df=4, 283, p=0.001). Olanzapine had a significantly lower gap ratio (mean=10.3%, SD=19.8%) compared to haloperidol (mean=25.5%, SD=29.0%) (p=0.008, Scheffé). No other significant differences in cumulative mean gap ratio existed between individual antipsychotics at 6 or 12 months. Risperidone had a lower gap ratio (mean=13.9%, SD=21.0%) than haloperidol at 6 months (mean=25.5%, SD=29.0%), but the difference was not significant (p=0.06, Scheffé).
On the basis of the compliant fill rate method, the patients with prescriptions for atypical antipsychotics had a significantly higher adherence rate at 6 months (mean=57.4%, SD=33.4%) than the patients with prescriptions for typical agents (mean=49.9%, SD=33.3%) (t=1.97, df=286, p=0.05). At 12 months, patients receiving atypical agents had a higher adherence rate (mean=54.9%, SD=26.0%) than those receiving typical agents (mean=50.1%, SD=30.6%), but the difference was not significant (t=1.59, df=286, p=0.11). No significant differences among individual antipsychotics in compliant fill rates were found at 6 or 12 months (Figure 1).
We also examined the proportions of patients in each group who had been receiving maintenance therapy (i.e., had received the same antipsychotic for at least 180 days before the study period). The typical antipsychotic group had more patients receiving maintenance therapy (80.4%, N=90 of 112) than the atypical antipsychotic group (69.0%, N=100 of 145); the difference approached but did not reach statistical significance (χ2=3.68, df=1, p=0.055). When the data for patients who did not receive maintenance therapy were excluded, the 12-month adherence rates for typical and atypical agents (cumulative mean gap ratio: 21.0%, SD=23.9%, and 12.8%, SD=16.5%, respectively [t=–2.84, df=158, p=0.005, unequal variances]; compliant fill rate: 51.9%, SD=30.0%, and 55.8%, SD=25.2%, respectively [t=1.01, df=182, p=0.31, unequal variances]) were similar to the rates for the entire study group.
Factors Associated with Adherence
Age, total daily dose of antipsychotic, and number of adjuvant psychotropic medications were not significantly correlated with the cumulative mean gap ratio (r<±0.20, df=26–286, p=0.12–0.95 for all correlations). No differences in adherence were found between Caucasians and non-Caucasians (cumulative mean gap ratio=16.1%, SD=20.1%, versus 21.1%, SD=24.7%) (t=–1.71, df=161, p=0.09, unequal variances), between patients with psychosis with a mood component and those with schizophrenia or psychosis not otherwise specified (cumulative mean gap ratio=18.6%, SD=23.9%, versus 17.4%, SD=20.5%) (t=–0.44, df=286, p=0.66), or between patients who received anticholinergic medication for treatment of extrapyramidal symptoms at any time during the 12-month period of refill assessment and those who did not receive anticholinergics (cumulative mean gap ratio=19.5%, SD=22.2%, versus 16.9%, SD=21.5%) (t=0.94, df=286, p=0.35).
Discussion
We confirmed our hypothesis that patients with prescriptions for atypical antipsychotics would have a higher rate of adherence to their medication regimen than those receiving conventional agents. However, at 12 months, statistically significant differences in adherence between those groups were seen in only the cumulative mean gap ratio, and the significant differences in adherence for individual medications that were seen at 6 months (greater adherence for olanzapine than for haloperidol) were not present at 12 months. These results demonstrate the importance of longer adherence assessments. Although adherence rates were numerically highest for quetiapine, no definitive conclusions can be drawn because the number of subjects who received quetiapine was small. The refill rates observed in this study highlight the pervasive, problematic degree of antipsychotic nonadherence in patients with psychotic disorders, including those who have prescriptions for atypical agents.
As we postulated, we found no significant associations between nonadherence and age, gender, ethnicity, or diagnosis. We did not, however, confirm our hypothesis that patients taking higher total daily doses of antipsychotic or more adjuvant psychotropic medications would have lower rates of adherence.
Our findings are both similar to and different from those of Rosenheck et al. (14), Olfson et al. (16), and Cabeza et al. (17). The results for comparisons between adherence with typical antipsychotics and adherence with atypical antipsychotics in the previous studies ranged from no significant difference (17), to differences that were nonsignificant but approached significance (16), to a significant difference in one of the two measures of adherence (14). These discrepancies may be accounted for by differences in methods, including differences in adherence measures, definitions of adherence, specific medications included in the study, frequency of assessments, and treatment settings. The strengths of the present study include the length of assessment period (12 months) and the use of objective definitions of adherence based on refill records (the cumulative mean gap ratio and the compliant fill rate). Obtaining pharmacy refill information was an unobtrusive method of data collection, allowing a naturalistic estimate of adherence (18). In addition, the very low cost to patients of antipsychotic medications in the VA system likely minimized any effect of financial burden on refill rates, and the exclusion of patients who were likely to have received medical care outside the VA San Diego Healthcare System increased the likelihood that the pharmacy records were complete. Finally, it is noteworthy that the adherence rate among the patients with prescriptions for typical neuroleptics (compliant fill rate=50%) was very similar to the rates reported in previous reviews (1, 2).
The higher rates of adherence calculated for patients with prescriptions for atypical antipsychotics become more clinically meaningful when one considers the antipsychotic prescriptions policy in effect at the VA San Diego Healthcare System at the time this study was completed. Previous studies have reported that patients with more severe psychotic symptoms have higher rates of antipsychotic nonadherence (22, 30, 33). In our study group, significantly more patients who received atypical agents had one or more psychiatric hospitalizations, compared to the patients who received typical antipsychotics. The VA policy might have contributed to an underestimation of the effect of atypical antipsychotics on adherence because more patients with treatment-refractory illness and more treatment-intolerant patients may have had prescriptions for atypical agents. However, this explanation is speculative, and a plausible argument could be made to support the idea that the group receiving atypical antipsychotics included adherent patients because of factors that were unaccounted for, such as living situation and medication supervision status. Because we did not have data on psychopathology or side effects, we were unable to evaluate whether and to what degree these factors influenced rates of adherence. Additional studies will be needed to evaluate differences among the individual atypical antipsychotics in risk factors for nonadherence. Future studies should examine the clinical significance of the modest differences observed in adherence among typical and atypical antipsychotics.
We should point out several limitations of this study. For example, refill records do not directly measure medication intake. At the present time, however, no gold standard exists for the measurement of medication adherence (18). Moreover, all other methods of adherence assessment have specific limitations. Patient self-reports are by their very nature subjective. The accuracy of both pill counts and serum drug levels, measures that may seem less subject to bias, can also be influenced by patients’ behavior, either intentionally or unintentionally. Newly developed performance-based tests to evaluate medication management skills, such as the Medication Management Ability Assessment, are promising, but do not measure adherence directly (34). Rates of adherence based on pharmacy refill records have been reported to correlate with other adherence behaviors (e.g., appointment keeping), serum drug levels, and drug effects such as blood pressure control (35–38). In addition, although refill records provided an indirect measure of adherence, they enabled us to calculate gaps in therapy when patients were late in refilling their supply of medication, which demonstrated widespread underuse of medications. Finally, we attempted to improve the comprehensiveness of pharmacy refill records by excluding patients who received medical care outside of the VA system.
Another limitation was the nonrandomized nature of this study. Nonetheless, to reduce selection bias, we included all patients who met the selection criteria for this naturalistic study. Because the study used a retrospective design, we were unable to analyze the relationship of adherence to antipsychotic efficacy or side effects. In addition, although we did not find significant relationships between nonadherence and age, gender, ethnicity, or number of adjuvant psychotropic medications, we were unable to measure other potentially relevant patient-related factors, such as insight, medication supervision status, or substance abuse. Finally, our results may not generalize to non-VA patients.
In summary, on the basis of pharmacy refill records, VA outpatients with prescriptions for atypical antipsychotics had greater medication adherence, compared to patients with prescriptions for typical agents. Nonadherence was considerable, however, even among the patients receiving atypical antipsychotics. Future research in this area should focus on developing effective interventions to improve medication adherence in patients receiving antipsychotic medications.
 |
Presented in part at the 14th annual meeting of the American Association for Geriatric Psychiatry, San Francisco, Feb. 24–26, 2001. Received Feb. 16, 2001; revision received June 18, 2001; accepted July 31, 2001. From the Department of Psychiatry, University of California, San Diego; and the Pharmacy and Psychiatry Services, Veterans Affairs (VA) San Diego Healthcare System. Address reprint requests to Dr. Dolder, Geriatric Psychiatry Intervention Research Center (116A-1), VA San Diego Healthcare System, 3350 La Jolla Village Dr., San Diego, CA 92161; [email protected] (e-mail). Supported in part by the Department of Veterans Affairs and NIMH grants MH-19934, MH-49671, MH-43693, and MH-59101. Dr. Lacro and Dr. Jeste have been consultants to and have received grant support for other projects from AstraZeneca, Bristol-Meyers-Squibb, Eli Lilly, Janssen, and Pfizer; however, no pharmaceutical industry support was received for this study. The authors thank Shahrokh Gholshan, Ph.D., for assistance with the statistical analysis.
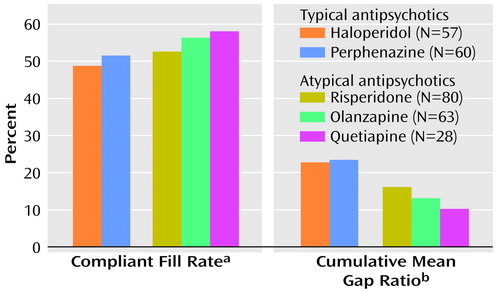
Figure 1. Medication Adherence Rates at 12-Month Follow-Up for Outpatients Filling Prescriptions for Typical and Atypical Antipsychotic Medications in a Veterans Affairs (VA) Health Care System
aPercentage of total medication fills that occurred at time-appropriate intervals. No significant difference between patients prescribed typical and atypical antipsychotics (F=0.84, df=4, 283, p=0.50).
bPercentage of total study days during which medication was unavailable because of a delayed refill. Significant difference between patients prescribed typical and atypical antipsychotics (F=3.61, df=4, 283, p=0.007). No significant differences between individual antipsychotics (p=0.12–1.00, Scheffé).
1. Fenton WS, Blyler CR, Heinssen RK: Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull 1997; 23:637-651Crossref, Medline, Google Scholar
2. Young JL, Zonana HV, Shepler L: Medication noncompliance in schizophrenia: codification and update. Bull Am Acad Psychiatry Law 1986; 14:105-122Medline, Google Scholar
3. Weiden PJ, Olfson M: Cost of relapse in schizophrenia. Schizophr Bull 1995; 21:419-429Crossref, Medline, Google Scholar
4. Terkelsen KG, Menikoff A: Measuring the costs of schizophrenia: implications for the post-institutional era in the US. Pharmacoeconomics 1995; 8:199-222Crossref, Medline, Google Scholar
5. Wyatt RJ, Henter I, Leary MC, Taylor E: An economic evaluation of schizophrenia: 1991. Soc Psychiatry Psychiatr Epidemiol 1995; 30:196-205Medline, Google Scholar
6. Rice DP: The economic impact of schizophrenia. J Clin Psychiatry 1999; 60:4-6Google Scholar
7. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789-796Crossref, Medline, Google Scholar
8. Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994; 151:825-835Link, Google Scholar
9. Tollefson GD, Beasley CM Jr, Tamura RN, Tran PV, Potvin JH: Blind, controlled, long-term study of the comparative incidence of treatment-emergent tardive dyskinesia with olanzapine or haloperidol. Am J Psychiatry 1997; 154:1248-1254Link, Google Scholar
10. Small JG, Hirsch SR, Arvanitis LA, Miller BG, Link CG: Quetiapine in patients with schizophrenia: a high- and low-dose double-blind comparison with placebo. Arch Gen Psychiatry 1997; 54:549-557Crossref, Medline, Google Scholar
11. Jeste DV, Lacro JP, Bailey A, Rockwell E, Harris J, Caligiuri MP: Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999; 47:716-719Crossref, Medline, Google Scholar
12. Jeste DV, Rockwell E, Harris MJ, Lohr JB, Lacro J: Conventional vs newer antipsychotics in elderly patients. Am J Geriatr Psychiatry 1999; 7:70-76Crossref, Medline, Google Scholar
13. Marder SR: Facilitating compliance with antipsychotic medication. J Clin Psychiatry 1998; 59:21-25Medline, Google Scholar
14. Rosenheck R, Chang S, Choe Y, Cramer J, Xu W, Thomas J, Henderson W, Charney D: Medication continuation and compliance: a comparison of patients treated with clozapine and haloperidol. J Clin Psychiatry 2000; 61:382-386Crossref, Medline, Google Scholar
15. Arana GW: An overview of side effects caused by typical antipsychotics. J Clin Psychiatry 2000; 61:5-11Google Scholar
16. Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden PJ: Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv 2000; 51:216-222Link, Google Scholar
17. Cabeza IG, Amador MS, Lopez CA, Chavez MG: Subjective response to antipsychotics in schizophrenic patients: clinical implications and related factors. Schizophr Res 2000; 41:349-355Crossref, Medline, Google Scholar
18. Vitolins MZ, Rand CS, Rapp SR, Ribisl PM, Sevick MA: Measuring adherence to behavioral and medical interventions. Control Clin Trials 2000; 21:188S-194SCrossref, Medline, Google Scholar
19. Steiner JF, Prochazka AV: The assessment of refill compliance using pharmacy records: methods validity, and applications. J Clin Epidemiol 1997; 50:105-116Crossref, Medline, Google Scholar
20. Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT Jr: Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry 1998; 155:751-760Link, Google Scholar
21. Buchanan A: A two-year prospective study of treatment compliance in patients with schizophrenia. Psychol Med 1992; 22:787-797Crossref, Medline, Google Scholar
22. Dixon L, Weiden P, Torres M, Lehman A: Assertive community treatment and medication compliance in the homeless mentally ill. Am J Psychiatry 1997; 154:1302-1304Link, Google Scholar
23. Heyscue BE, Levin GM, Merrick JP: Compliance with depot antipsychotic medication by patients attending outpatient clinics. Psychiatr Serv 1998; 49:1232-1234Link, Google Scholar
24. Nageotte C, Sullivan G, Duan N, Camp PL: Medication compliance among the seriously mentally ill in a public mental health system. Soc Psychiatry Psychiatr Epidemiol 1997; 32:49-56Crossref, Medline, Google Scholar
25. Owen RR, Fischer EP, Booth BM, Cuffel BJ: Medication noncompliance and substance abuse among patients with schizophrenia. Psychiatr Serv 1996; 47:853-858Link, Google Scholar
26. Pan PC, Tantam D: Clinical characteristics, health beliefs and compliance with maintenance treatment: a comparison between regular and irregular attenders at a depot clinic. Acta Psychiatr Scand 1989; 79:564-570Crossref, Medline, Google Scholar
27. Agarwal MR, Sharma VK, Kumar K, Lowe D: Non-compliance with treatment in patients suffering from schizophrenia: a study to evaluate possible contributing factors. Int J Soc Psychiatry 1998; 44:92-106Crossref, Medline, Google Scholar
28. Razali MS, Yahya H: Compliance with treatment in schizophrenia: a drug intervention program in a developing country. Acta Psychiatr Scand 1995; 91:331-335Crossref, Medline, Google Scholar
29. Garavan J, Browne S, Gervin M, Lane A, Larkin C, O’Callaghan E: Compliance with neuroleptic medication in outpatients with schizophrenia; relationship to subjective response to neuroleptics; attitudes to medication and insight. Compr Psychiatry 1998; 39:215-219Crossref, Medline, Google Scholar
30. Duncan JC, Rogers R: Medication compliance in patients with chronic schizophrenia: implications for the community management of mentally disordered offenders. J Forensic Sci 1998; 43:1133-1137Medline, Google Scholar
31. Smith TE, Hull JW, Goodman M, Hedayat-Harris A, Willson DF, Israel LM, Munich RL: The relative influences of symptoms, insight, and neurocognition on social adjustment in schizophrenia and schizoaffective disorder. J Nerv Ment Dis 1999; 187:102-108Crossref, Medline, Google Scholar
32. Hamilton RA, Briceland LL: Use of prescription-refill records to assess patient compliance. Am J Hosp Pharm 1992; 49:1691-1696Medline, Google Scholar
33. Corriss DJ, Smith TE, Hull JW, Lim RW, Pratt SI, Romanelli S: Interactive risk factors for treatment adherence in a chronic psychotic disorders population. Psychiatry Res 1999; 89:269-274Crossref, Medline, Google Scholar
34. Patterson TL, Lacro J, McKibbin CL, Davidson K, Jeste DV: Direct observation of medications management (MMAA): results from a new performance-based test in older outpatients with schizophrenia. J Clin Psychopharmacol (in press)Google Scholar
35. Deyo RA, Inui TS, Sullivan B: Noncompliance with arthritis drugs: magnitude, correlates, and clinical implications. J Rheumatol 1981; 8:931-936Medline, Google Scholar
36. Peterson GM, McLean S, Millingen KS: Determinants of patient compliance with anticonvulsant therapy. Epilepsia 1982; 23:607-613Crossref, Medline, Google Scholar
37. Steiner JF, Koepsell TD, Fihn SD, Inui TS: A general method of compliance assessment using centralized pharmacy records. Med Care 1988; 26:814-823Crossref, Medline, Google Scholar
38. Steiner JF, Fihn SD, Blair B, Inui TS: Appropriate reductions in compliance among well-controlled hypertensive patients. J Clin Epidemiol 1991; 44:1361-1371Crossref, Medline, Google Scholar



