Vitamin B6 in the Treatment of Tardive Dyskinesia: A Double-Blind, Placebo-Controlled, Crossover Study
Abstract
OBJECTIVE: The authors’ goal was to conduct a double-blind trial of vitamin B6 in the treatment of tardive dyskinesia in patients with schizophrenia. METHOD: Fifteen inpatients with schizophrenia who met research diagnostic criteria for tardive dyskinesia were randomly assigned to treatment with either vitamin B6 or placebo for 4 weeks in a double-blind crossover paradigm. The Extrapyramidal Symptom Rating Scale was used to assess patients weekly. RESULTS: Mean scores on the parkinsonism and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale were significantly better in the third week of treatment with vitamin B6 than during the placebo period. CONCLUSIONS: Vitamin B6 appears to be effective in reducing symptoms of tardive dyskinesia.
There are several reports regarding the use of vitamin B6 in the treatment of the patients suffering from neuroleptic-induced movement disorders (1–4). With one exception (1), the dose of vitamin B6 was relatively low (100–500 mg/day), and the subjects experienced some benefit. The current study is the first report to our knowledge of a double-blind trial of vitamin B6 in the treatment of tardive dyskinesia in patients with schizophrenia or schizoaffective disorder.
Method
Fifteen inpatients with schizophrenia or schizoaffective disorder (four men and 11 women) who had been receiving a stable psychotropic regimen for at least 1 month and who fulfilled diagnostic criteria for tardive dyskinesia were included in this study. The patients’ age range was 28–71 years. All were free of any concurrent medical or neurological disorders as well as evidence of substance or alcohol abuse. None had received vitamin treatment. Written informed consent and institutional review board ethics committee approval were obtained. All patients received the regular balanced hospital diet.
All patients received traditional or atypical neuroleptics; 11 received oral preparations, and four received injectable long-acting neuroleptics (mean dose=490 mg/day chlorpromazine equivalents). Twelve patients received combination therapy.
The study design was double-blind, with crossover and placebo control. Vitamin B6 or placebo were added to the patients’ ongoing treatment for 4 weeks each and then crossed over after a 1-week washout period to allow for return to normal levels of vitamin B6(5).
Doses of all psychotropic medication were kept unchanged throughout the study. The dose of vitamin B6 was increased by 100 mg/week from 100 mg/day to 400 mg/day in twice-daily divided doses.
We used the parkinsonism, dystonia, and dyskinetic movement subscales of the Extrapyramidal Symptom Rating Scale (6) to assess tardive dyskinesia. Assessments were taken at baseline and repeated every week by the same investigator at the same time of day in order to rule out any influence of diurnal fluctuation of the tardive dyskinesia symptoms (7). The interrater reliability (kappa) in Extrapyramidal Symptom Rating Scale scores from baseline to week 4 was good (intraclass correlation coefficient=0.92). A 20% reduction in Extrapyramidal Symptom Rating Scale scores from baseline to week 4 was taken to represent no response, 21%–40% as minimal improvement, 41%–60% as moderate improvement, and more than 61% as marked improvement.
Plasma levels of vitamin B6 were assessed at baseline and every other week by radioenzymatic assay of plasma pyridoxal-5′-phosphate (normal range=20–120 nmol/liter) (8). The raters were kept blind to the results.
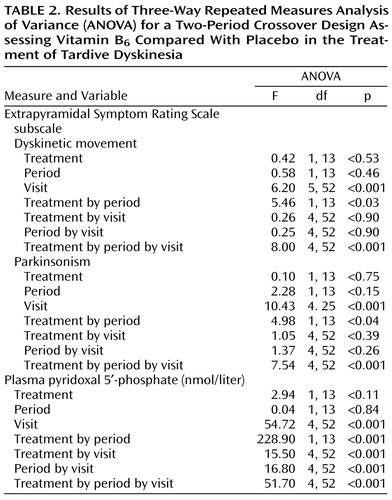
To compare effects in groups, in periods, and across all visits, variables were analyzed by three-way repeated measures analysis of variance (ANOVA) for a two-period crossover design. Post hoc comparisons employed the Scheffé post hoc test.
Results
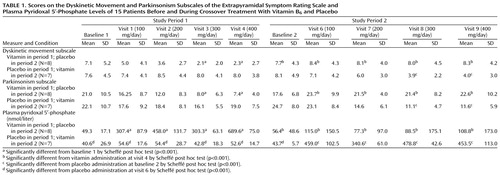
All patients received the maximal dose of 400 mg/day of vitamin B6 without adverse effects. At both baselines, there were no significant differences between groups receiving placebo or the vitamin in dyskinetic movement subscale scores, parkinsonism subscale scores, or pyridoxal-5′-phosphate levels (Table 1 and Table 2).
Three-way repeated measures ANOVA for the two-period crossover design revealed significant differences between vitamin B6 and placebo groups in scores on the dyskinetic movement subscale in period 1 and period 2.
The improvements in dyskinetic movement subscale scores after 3 and 4 weeks of treatment with vitamin B6 in period 1 were 69.2% (SD=14.4%) and 68.6% (SD=23.0%). In period 2 the improvements were 38.2% (SD=46.1%) and 32.8% (SD=57.0%), respectively. (The percentage of improvement was calculated on the basis of individual scores rather than means for the entire group.)
In period 1, treatment with vitamin B6 caused a significant improvement in parkinsonism subscale scores, first observed in the third week of the treatment (dose of vitamin B6 was 300 mg/day) and continuing till the end of this phase of treatment. In period 2, vitamin B6 also caused a significant improvement in parkinsonism scores with vitamin B6 doses of 300 mg/day. The improvements on the parkinsonism subscale after 3 and 4 weeks of treatment with vitamin B6 in period 1 were 51.6% (SD=43.8%) and 57.1% (SD=30.1%) and in period 2 were 52.0% (SD=20.8%) and 51.6% (SD=22.8%), respectively.
After the washout period following vitamin treatment, the dyskinetic movement and parkinsonism subscale scores on the Extrapyramidal Symptom Rating Scale returned to baseline levels.
Baseline plasma pyridoxal-5′-phosphate levels were in the normal range in 11 patients (20–120 nmol/liter). In one patient, a higher pyridoxal-5′-phosphate level (163.5 nmol/liter) was found, and in four patients pyridoxal-5′-phosphate levels were below normal (5.0–16.2 nmol/liter). In both experiment periods, levels of vitamin B6 were significantly higher after the first week of treatment and continued till the end of the phase. However, we could not find a direct relationship between therapeutic response and pyridoxal-5′-phosphate level or changes in tardive dyskinesia.
Discussion
The results of this small study suggest that vitamin B6 is effective in treating tardive dyskinesia at doses from 300 mg/day. Vitamin B6 was effective during the treatment period only.
There have been several uncontrolled controversial reports about treatment of tardive dyskinesia with vitamin B6(1–4, 9). Our results are consistent with previous reports suggesting the beneficial effect of pyridoxine on movement disorders (1–4).
We were not able to establish a correlation between pyridoxal-5′-phosphate levels and changes in two subscales of the Extrapyramidal Symptom Rating Scale. We assume, therefore, that serum pyridoxal-5′-phosphate is not directly associated with tardive dyskinesia and that serum levels of vitamin B6 do not correlate directly with pyridoxine-dependent activity of biogenic amines.
The mechanisms by which pyridoxine attenuates the symptoms of tardive dyskinesia are not completely understood. Pyridoxyl-5-PO4, derived from dietary pyridoxine, serves as a cofactor in the enzymatic decarboxylation of dopa to dopamine (10) and other metabolic transformations, including γ-aminobutyric acid, serotonin, and melatonin (11, 12).
One possible explanation for the effects observed in this study is that they are due to the antioxidant and free radical scavenger activities of vitamin B6(2, 13). Free radicals have been implicated in a variety of neuropsychiatric conditions, many of which are marked by the gradual development of psychopathologic symptoms and movement disorders. There is evidence that radical-induced damage may be important in tardive dyskinesia and, possibly, in schizophrenia as well (14). Because vitamin B6 takes part in almost all the possible mechanisms proposed to underlie tardive dyskinesia, it appears to make sense that it could alleviate symptoms of tardive dyskinesia.
To our knowledge, this is the first double-blind study examining the treatment of tardive dyskinesia with vitamin B6. The potential usefulness of vitamin B6 in treating tardive dyskinesia could be of clinical importance because it has no side effects in small doses (15). Furthermore, to date there is no other effective treatment for this troublesome and sometimes incapacitating condition.
Our study group was rather small, and further studies should involve larger numbers of patients and a long-term study design. In retrospect, we feel that it would have been possible to limit the study to a single period, without the crossover phase, since the results from period 1 were statistically significant. Moreover, it is necessary to examine the effect of concomitant use of vitamin B6 and risperidone or olanzapine in patients with tardive dyskinesia.
 |
 |
Received Feb. 15, 2000; revisions received June 5 and Aug. 31, 2000, and Jan. 3 and Feb. 22, 2001; accepted March 6, 2001. From the Division of Psychiatry, Ministry of Health Be’er Sheva Mental Health Center, Faculty of Health Sciences Ben-Gurion University of the Negev. Address reprint requests to Dr. Lerner, Be’er-Sheva Mental Health Center, P.O. Box 4600, Be’er-Sheva, 84170, Israel; [email protected] (e-mail).
1. DeVeaugh-Geiss J, Manion L: High-dose pyridoxine in tardive dyskinesia. J Clin Psychiatry 1978; 39:573-575Medline, Google Scholar
2. Sandyk R, Pardeshi R: Pyridoxine improves drug-induced parkinsonism and psychosis in a schizophrenic patient. Int J Neurosci 1990; 52:225-232Crossref, Medline, Google Scholar
3. Lerner V, Liberman M: Movement disorders and psychotic symptoms treated with pyridoxine: a case report (letter). J Clin Psychiatry 1998; 59:623-624Crossref, Medline, Google Scholar
4. Lerner V, Kaptsan A, Miodownik C, Kotler M: Vitamin B6 in treatment of tardive dyskinesia: a preliminary case series study. Clin Neuropharmacol 1999; 22:241-243Medline, Google Scholar
5. Dalton K, Dalton M: Characteristics of pyridoxine overdose neuropathy syndrome. Acta Neurol Scand 1987; 76:8-11Crossref, Medline, Google Scholar
6. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
7. Hyde TM, Egan MF, Brown RJ, Weinberger DR, Kleinman JE: Diurnal variation in tardive dyskinesia. Psychiatry Res 1995; 56:53-57Crossref, Medline, Google Scholar
8. Camp VM, Chipponi J, Faraj BA: Radioenzymatic assay for direct measurement of plasma pyridoxal 5′-phosphate. Clin Chem 1983; 29:642-644Medline, Google Scholar
9. Crane GE, Turek IS, Kurland A: Failure of vitamin to reverse the l-dopa effect in patients on a dopa decarboxylase inhibitor. J Neurol Neurosurg Psychiatry 1971; 34:682-686Crossref, Medline, Google Scholar
10. Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG (eds): Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, 1992Google Scholar
11. Dreyfus PM, Geel SE: Vitamins and nutritional deficiencies, in Basic Neurochemistry. Edited by Siegel GJ, Alberts RW, Agranoff BW, Katzman R. Boston, Little, Brown, 1981, pp 661-679Google Scholar
12. Viswanathan M, Siow YL, Paulose CS, Dakshinamurti K: Pineal indoleamine metabolism in pyridoxine-deficient rats. Brain Res 1988; 473:37-42Crossref, Medline, Google Scholar
13. Cabrini L, Bergami R, Fiorentini D, Marchetti M, Landi L, Tolomelli B: Vitamin B6 deficiency affects antioxidant defences in rat liver and heart. Biochem Mol Biol Int 1998; 46:689-697Medline, Google Scholar
14. Lohr JB: Oxygen radicals and neuropsychiatric illness: some speculations. Arch Gen Psychiatry 1991; 48:1097-1106Google Scholar
15. Beckett A: Debate continues on vitamin B6 (letter). Lancet 1998; 352:62Crossref, Medline, Google Scholar



