Double-Blind, Placebo-Controlled Comparison of Intramuscular Olanzapine and Intramuscular Haloperidol in the Treatment of Acute Agitation in Schizophrenia
Abstract
OBJECTIVE: The authors evaluated the comparative efficacy and safety of intramuscular olanzapine, intramuscular haloperidol, and intramuscular placebo for the treatment of acute agitation in schizophrenia. METHOD: Hospitalized patients with schizophrenia received one to three injections of intramuscular olanzapine, 10 mg, intramuscular haloperidol, 7.5 mg, or intramuscular placebo over a 24-hour period. Agitation was measured with the excited component of the Positive and Negative Syndrome Scale and two additional scales. RESULTS: According to scores on the excited component of the Positive and Negative Syndrome Scale, both intramuscular olanzapine and intramuscular haloperidol reduced agitation significantly more than intramuscular placebo 2 and 24 hours following the first injection. Intramuscular olanzapine reduced agitation significantly more than intramuscular haloperidol 15, 30, and 45 minutes following the first injection. No patients treated with intramuscular olanzapine experienced acute dystonia, compared with 7% of those who were treated with intramuscular haloperidol. No significant QTc interval changes were observed in any patients. CONCLUSIONS: Intramuscular olanzapine represents a rapid, effective, and safe treatment for acute agitation in schizophrenia.
Intramuscular typical neuroleptics are frequently required to treat acute agitation in schizophrenia, particularly when speed of action and compliance are issues (1). These agents, however, may be associated with undesirable side effects, including acute dystonia (2) and QTc prolongation (3). There is currently no intramuscular atypical antipsychotic available in the United States. Oral olanzapine has a favorable profile with regard to acute dystonia (4) and QTc prolongation (5). We hypothesized that intramuscular olanzapine, 10 mg, would not be inferior to intramuscular haloperidol, 7.5 mg, and would be superior to intramuscular placebo for reducing agitation in schizophrenia.
Method
The study subjects were male and female patients who were 18 years old or older, who had a DSM-IV diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder, and who had an excited component score greater than or equal to 14 on the Positive and Negative Syndrome Scale with a score of 4 or more on at least one item (1–7-point scale) (6). The patients (N=311) were assessed in hospitals in Australia, Austria, Belgium, Canada, the Czech Republic, France, Greece, Hungary, Israel, the Republic of South Africa, Spain, the United Kingdom, and the United States. They were randomly assigned to receive one to three injections over a 24-hour period. One group received intramuscular injections of olanzapine, 10 mg; one group received haloperidol, 7.5 mg; and one group received placebo. Both drugs and placebo were administered in identical, color-blinded, translucent standard syringes, and raters and study personnel were blind to treatment assignment. Optional second and third injections were given 2 or more and 4 or more hours following the first injection, respectively.
All patients were considered by the investigators to be clinically agitated and appropriate candidates for intramuscular treatment. Pregnant or lactating women and patients with serious medical illnesses in which pharmacotherapy posed a substantial clinical risk or confounded diagnosis were excluded. All patients provided written informed consent after the procedure(s) had been fully explained.
Olanzapine dosing was determined on the basis of a preliminary open-label trial (7). Haloperidol dosing was chosen following a literature review and discussions with ethical boards, regulatory authorities, and participating psychiatrists. Other medications affecting the central nervous system and prophylactic anticholinergics were prohibited. Benzodiazepines were allowed 1 or more hours following a second and/or third injection.
To evaluate agitation, the excited component of the Positive and Negative Syndrome Scale (tension, uncooperativeness, hostility, poor impulse control, and excitement) (6) was completed at 15, 30, 45, 60, 90, and 120 minutes following the first injection and less frequently thereafter, with the 2-hour rating the primary outcome measure determined a priori. The Agitated Behavior Scale (8) and the Agitation Calmness Evaluation Scale, a single-item, 9-point scale (e.g., 1=marked agitation, 4=normal behavior, 9=unarousable) (Eli Lilly and Company), were also used. For 24 hours, safety was evaluated by solicited and spontaneously reported adverse events and ECGs.
We planned to randomly assign 300 or more patients to olanzapine, haloperidol, or placebo in a ratio of 2:2:1, respectively. The lower limit of noninferiority was defined a priori as 40% of the observed mean change from baseline to 2 hours following the first haloperidol injection. A lower boundary of the one-sided 97.5% confidence interval of zero or less but greater than the lower limit indicated no between-treatment difference and noninferiority. Analysis of variance assessed the efficacy of olanzapine compared with placebo. The last-observation-carried-forward response rate was defined a priori as a reduction of 40% or more in scores on the excited component of the Positive and Negative Syndrome Scale 2 hours following the first injection. Fisher’s exact test was used to evaluate categorical data.
Results
Patients received olanzapine (N=131), haloperidol (N=126), or placebo (N=54); 285 patients (91.6%) completed the study. There were no significant between-group baseline demographic or illness differences. The mean age of the patients was 38.2 years (SD=11.6, range=18–72); their mean age at onset of illness was 24.4 (SD=8.5, range=7–58). There were no between-group differences in baseline scores on the excited component of the Positive and Negative Syndrome Scale, the Agitated Behavior Scale, and the Agitation Calmness Evaluation Scale (mean=18.4, SD=3.4, mean=27.6, SD=6.1, and mean=2.6, SD=0.8, respectively, for the olanzapine-treated patients; mean=18.2, SD=3.2, mean=26.9, SD=5.3, and mean=2.5, SD=0.7, respectively, for the haloperidol-treated patients; mean=18.4, SD=3.5, mean=28.5, SD=7.2, and mean=2.4, SD=0.7, respectively, for the patients given placebo).
There were significant differences between patients given olanzapine or haloperidol compared with those given placebo 2 hours after injection in scores on the excited component of the Positive and Negative Syndrome Scale, Agitated Behavior Scale, and Agitation Calmness Evaluation Scale.
Mean changes in excited component scores on the Positive and Negative Syndrome Scale from baseline to 2 hours (adjusted for country differences) were –7.7 (SD=6.1) for patients given olanzapine, –7.6 (SD=5.0) for patients given haloperidol, and –3.6 (SD=5.2) for patients given placebo. The difference between olanzapine and haloperidol was 0.1 units favoring olanzapine (one-sided lower 97.5% confidence limit=–1.2); noninferiority (–1.2 versus –7.6 × 0.4=–3.0) was concluded. Significant differences between olanzapine and haloperidol were observed at 15, 30, and 45 minutes after the first injection (Figure 1). Significant differences between olanzapine and placebo were observed at all postbaseline time points (Figure 1). Significant differences between haloperidol and placebo were observed from 30 minutes onward.
Mean changes in scores from baseline to 2 hours after the first injection on the Agitated Behavior Scale and Agitation Calmness Evaluation Scale (adjusted for country differences) were –8.3 (SD=0.6) and 1.6 (SD=0.1), respectively, for olanzapine-treated patients; –8.2 (SD=0.6) and 1.5 (SD=0.1) for haloperidol-treated patients; and –4.8 (SD=0.9) and 0.6 (SD=0.2) for patients given placebo.
At 24 hours, significant differences were observed between patients given olanzapine, haloperidol, and placebo in the mean change from baseline in the excited component score of the Positive and Negative Syndrome Scale (mean=–6.5, SD=5.3, mean=–6.7, SD=4.6, and mean=–3.1, SD=5.1, respectively) (F=10.7, df=2, 298, p<0.001), the Agitated Behavior Scale score (mean=–6.4, SD=5.9, mean=–6.6, SD=5.3, and mean=–3.7, SD=6.7, for olanzapine, haloperidol, and placebo, respectively) (F=5.5, df=2, 298, p=0.004), and the Agitation Calmness Evaluation Scale score (mean=0.8, SD=1.0, mean=1.1, SD=1.0, and mean=0.6, SD=1.2, respectively) (F=5.5, df=2, 298, p=0.004).
Pairwise comparisons (adjusted for country differences) of haloperidol and olanzapine, olanzapine and placebo, and haloperidol and placebo, respectively, were made in scores on the excited component of the Positive and Negative Syndrome Scale (t=–0.3, df=298, p=0.76; t=–4.2, df=298, p<0.001; t=–4.4, df=298, p<0.001), the Agitated Behavior Scale (t=–0.1, df=298, p=0.91; t=–3.0, df=298, p=0.003; t=–3.1, df=298, p=0.002), and the Agitation Calmness Evaluation Scale (t=2.3, df=298, p=0.02; t=1.3, df=298, p=0.20; t=3.1, df=298, p=0.002).
Significantly more olanzapine-treated patients (73.3%, N=96) (χ2=24.4, df=1, p<0.001) and haloperidol-treated patients (69.0%, N=87) (χ2=20.1, df=1, p<0.001) than placebo-treated patients (33.3%, N=18) responded. There was no significant difference in response rate between patients treated with olanzapine and those treated with haloperidol (χ2=0.4, df=1, p=0.52). Significantly more patients given placebo (38.9%, N=21) than those given olanzapine (16.0%, N=21) (p=0.002, Fisher’s exact test) or haloperidol (19.8%, N=25) (p=0.009, Fisher’s exact test) received benzodiazepines during the 24-hour period.
No olanzapine-treated patient experienced acute dystonia, compared with nine (7.1%) of the haloperidol-treated patients (Fisher’s exact p=0.001). One patient treated with olanzapine (0.8%) experienced an extrapyramidal syndrome, compared with seven (5.6%) patients treated with haloperidol (p=0.03, Fisher’s exact test). Significantly more haloperidol-treated patients (20.6%, N=26) than olanzapine-treated patients (4.6%, N=6) (p<0.001, Fisher’s exact test) or placebo patients (3.7%, N=2) (p=0.003, Fisher’s exact test) received anticholinergics.
At 24 hours, there were no significant QTc interval changes from baseline (mean=–3.0 msec, SD=21.3, for olanzapine; mean=–1.2 msec, SD=24.4, for haloperidol; mean=–3.7 msec, SD=26.1, for placebo) (F=0.3, df=2, 292, p=0.73) or significant between-group differences (t=0.70, df=292, p=0.49, for olanzapine versus haloperidol; t=0.11, df=292, p=0.91, for olanzapine versus placebo).
Discussion
Olanzapine was not inferior to haloperidol in reducing agitation 2 hours following intramuscular injection, and it had a significantly more rapid onset of action. Acute dystonia did not occur among intramuscular-olanzapine-treated patients. Changes in QTc intervals with active treatments were not significantly different from those with placebo. Thus, patients who require rapid reduction in agitation or who refuse oral therapy may benefit from treatment with intramuscular olanzapine, which involves a very low risk of both acute dystonia and QTc prolongation.
Received June 29, 2000; revisions received Oct. 31, 2000, and Jan. 26, 2001; accepted Feb. 12, 2001. From the Lilly Research Centre, Eli Lilly and Company, Surrey, U.K.; the Institute of Psychiatry, University of London, London; Lilly Research Laboratories, Eli Lilly and Company, Indianapolis; Hospital Benito Menni, Sant Boi de Llobregat, Barcelona, Spain; Hospital Clinic i Provincial, Barcelona; and River Edge Hospital, Forest Park, Ill. Address reprint requests to Dr. Breier, Lilly Research Laboratories, Eli Lilly and Company, Lilly Corporate Center, DC 1748, Indianapolis, IN 46285; [email protected] (e-mail).Sponsored by Eli Lilly and Company.
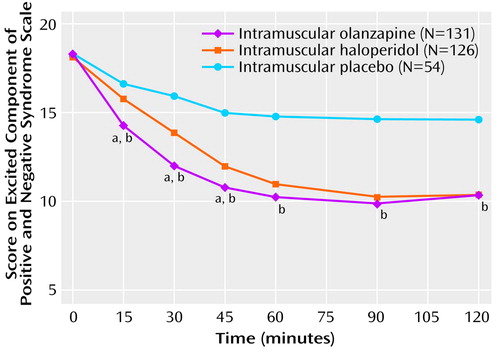
Figure 1. Scores of Inpatients With Schizophrenia Spectrum Disorders on the Excited Component of the Positive and Negative Syndrome Scale From Baseline to 2 Hours After the First Intramuscular Injection of Olanzapine, Haloperidol, or Placebo
aSignificant differences between olanzapine and haloperidol were observed at 15 minutes (t=–3.67, df=298, p<0.001), 30 minutes (t=–3.91, df=298, p<0.001), and 45 minutes (t=–2.50, df=298, p=0.01).
bSignificant differences between olanzapine and placebo were observed at 15 minutes (t=–4.13, df=298, p<0.001), 30 minutes (t=–5.75, df=298, p<0.001), 45 minutes (t=–5.87, df=298, p<0.001), 60 minutes (t=–5.98, df=298, p<0.001), 90 minutes (t=–6.07, df=298, p<0.001), and 120 minutes (t=–4.86, df=298, p<0.05).
1. Milton GV, Jann MW: Emergency treatment of psychotic symptoms. Clin Pharmacokinet 1995; 28:494-504Crossref, Medline, Google Scholar
2. Addonizio G, Alexopoulos GS: Drug-induced dystonia in young and elderly patients. Am J Psychiatry 1988; 145:869-871Link, Google Scholar
3. Buckley NA, Sanders P: Cardiovascular adverse effects of antipsychotic drugs. Drug Saf 2000; 23:215-228Crossref, Medline, Google Scholar
4. Tollefson GD, Beasley CM Jr, Tran P, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME: Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997; 154:457-465Link, Google Scholar
5. Czekalla J, Beasley CM Jr, Dellva MA, Berg PH, Grundy SL: Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. J Clin Psychiatry 2001; 62:191-198Crossref, Medline, Google Scholar
6. Kay SR, Sevy S: Pyramidical model of schizophrenia. Schizophr Bull 1990; 16:537-545Crossref, Medline, Google Scholar
7. Wright P, Kiesler G, Mitchell M, Jewell H, Birkett M, Selemani S, VanWyk C, Brook S: Safety and efficacy of intramuscular (IM) olanzapine in patients with acute nonorganic psychosis (abstract). Schizophr Res 1999; 36:318Google Scholar
8. Corrigan JD, Mysiw WJ: Agitation following traumatic head injury: equivocal evidence for a discrete stage of cognitive recovery. Arch Phys Med Rehabil 1988; 69:487-492Medline, Google Scholar



