Cholinergic Activity in Autism: Abnormalities in the Cerebral Cortex and Basal Forebrain
Abstract
OBJECTIVE: Measures of cholinergic transmitter activity were investigated in patients with autism because of reported neuropathological abnormalities in cholinergic nuclei in the basal forebrain. METHOD: Levels of cholinergic enzyme and receptor activity were measured in the frontal and parietal cerebral cortex of deceased autistic adults, similarly aged normal adults without mental retardation, and nonautistic mentally retarded adults. The immunoreactivity levels of brain-derived neurotrophic factor and nerve growth factor were measured in the basal forebrain. RESULTS: There were no differences between the autistic and comparison groups in choline acetyltransferase or acetylcholinesterase activity in the cerebral cortex and basal forebrain or in muscarinic M2 receptor or α-bungarotoxin binding within the cortex. Cortical M1 receptor binding was up to 30% lower than normal in the autistic subjects, and the difference reached significance in the parietal cortex. In both the parietal and frontal cortices, differences in nicotinic receptors assessed by [3H]epibatidine binding were significant and extensive (65%–73% lower in the autistic group than in the normal subjects); there were no differences in nicotine binding in the basal forebrain. Immunochemical analysis indicated lower levels of both the α4 and β2 nicotinic receptor subunits in the parietal cortex. The M1 receptor abnormality was not evident in the nonautistic group with mental retardation, although the lower [3H]epibatidine binding was apparent. In the basal forebrain, the level of brain-derived neurotrophic factor in the autistic group was three times as high as the level of the normal group. CONCLUSIONS: These neurochemical abnormalities implicate the cholinergic system in developmental disorders such as autism and suggest the potential for intervention based on cholinergic receptor modulation.
There is as yet no etiology-based treatment or cure for autism. Neurotransmitter signaling systems are relevant to symptom etiology and treatment and play a critical role in brain development. Investigations of autism have included only a few transmitters, including serotonin, dopamine, noradrenaline, and several neuropeptides (1). These studies have mostly involved measurements in blood or cerebrospinal fluid and response to pharmaceutical agents.
To our knowledge, the cholinergic system has not previously been investigated neurochemically in autism, and yet there have been reports of pathological abnormalities in basal forebrain (septal) cholinergic neurons, such as larger than normal size and numbers in children and small size and numbers in adults (2). Cholinergic afferents innervate the cerebral cortex during the most dynamic periods of neuronal differentiation and synapse formation, suggesting they play a regulatory role in these events (3). In the rodent cortex, cholinergic innervation is the latest of the modulatory afferent innervations to mature (4). In the human cerebral cortex and hippocampus, measures of cholinergic activity, including levels of choline acetyltransferase (the enzyme synthesizing the transmitter) and high-affinity nicotinic receptor binding, demonstrate substantial changes postnatally (5, 6), during the early postnatal period, when symptoms of autism first become manifest (7). Disruption of cholinergic innervation during early postnatal development (e.g., neonatal basal forebrain cholinergic lesions in rats) results in delayed cortical neuronal development and permanent changes in cortical cytoarchitecture and cognitive function (3). Abnormalities in cortical cytomorphology, including abnormal thalamic afferent distribution in layer IV (8–10), are said to resemble pathologies associated with developmental disorders resulting in mental retardation (11).
In view of the neuropathological evidence for involvement of the basal forebrain cholinergic system in autism, and the importance of this system in cortical development, cholinergic biomarkers were investigated in the basal forebrain and cerebral cortex (which receives a dense innervation from basal forebrain cholinergic neurons) in autistic individuals. Using available brain bank material, we analyzed tissue samples for the following biomarkers: 1) choline acetyltransferase biochemical activity, which is specific for cholinergic neurons; its decline is used as a standard measure of cholinergic neuronal loss or dysfunction (12); 2) acetylcholinesterase activity as a relatively specific cholinergic marker; 3) cholinergic receptors located pre- and postsynaptically, including muscarinic M1 and M2 and high- and low-affinity nicotinic subtypes; and 4) both nerve growth factor and brain-derived neurotrophic factor, which control cholinergic neuronal function.
Method
Subjects
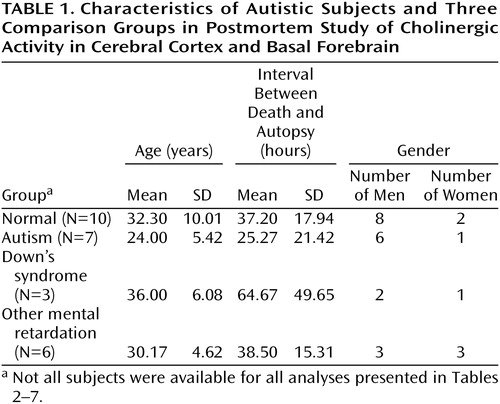
Autopsy tissue was examined from seven adults with autism meeting the DSM-IV criteria (which include delay or abnormal functioning in at least one of the following: social interaction, language, or symbolic or imaginative play). Clinical details were elicited from the parents by using the revised Autism Diagnostic Interview (13). Ratings for the different interview areas were as follows: communication, nonverbal, 13–21; social, 22–25; behaviors, 5–10; and development, 2–5. For all subjects for whom information was available, there was clinical evidence of mental retardation (IQ, <20 to <50), and for 50% there was evidence of epilepsy. The causes of death for the autistic subjects were cardiac arrest, pneumonia, asphyxia, chronic renal failure, and fire. Comparisons were made with 10 normal individuals without mental retardation, with six individuals affected by other congenital cerebral disorders involving intellectual impairment (associated with birth trauma, viral encephalopathy, congenital cerebellar degeneration, or congenital epilepsy), and with three adults with Down’s syndrome. There was no history of mental retardation in the normal group, and the causes of death were respiratory failure (traffic accident), asphyxia (laryngeal injury), drug overdose, myocardial infarction or left ventricular failure, and pneumonia. Age, postmortem delay, and gender are provided in Table 1. There were no significant differences between the groups in mean age or postmortem delay.
Frozen tissue was obtained from the Newcastle Brain Bank (United Kingdom), Harvard Brain Tissue Resource Center (United States), and University of Miami Brain and Tissue Bank for Developmental Disorders (United States); the samples for five of the seven autistic subjects were from the Harvard center. For each of the Harvard subjects, written consent approved by the McLean Hospital institutional review board was obtained from the legal next of kin, authorizing donation of tissue to the bank for use in research. For the Newcastle series, permission for postmortem study and for brain donation were obtained by prior consent from the next of kin, in accordance with the regulations of the North Tyneside Health Authorities Joint Ethics Committee.
For the Newcastle series, 1-cm coronal sections of the left hemisphere were snap frozen in liquid chlorodifluoromethane and stored at –70°C; the right hemisphere was fixed in paraformaldehyde. Tissue from the Harvard Brain Tissue Resource Center was subdissected at –20°C from whole hemispheres (three right and two left) originally frozen intact. The cortical tissue included the frontal cortex (Brodmann’s area 9) and parietal cortex (Brodmann’s area 39). The basal forebrain included the area just caudal to the anterior commissure and ventral to the globus pallidus, within the region of the substantia innominata.
Receptor Autoradiography
Frozen tissue blocks were subdissected at –20°C and mounted onto aluminum chucks. Then 10-μm cryostat sections were cut at –12°C and thaw mounted onto slides coated with Vectabond (Vector Laboratories, Burlingame, Calif.). The sections were air dried at room temperature for 2 hours before storage at –70°C. Adjacent sections were used to compare total with nonspecific binding; those determinations were made in duplicate and single, respectively.
Muscarinic M1 receptor binding was measured by using 2 nM [3H]pirenzepine with incubation for 1 hour; binding was assessed in the presence and absence of 1 μM atropine (14). For M2 receptor binding, 7 nM [3H]AFDX-384 (2,3-dipropylamine) was used with displacement by 1 μM atropine and incubation for 1 hour (15). For epibatidine binding to the nicotinic receptor (α3/β2 or α4/β2 subunit), sections were incubated with 1 nM (±)[3H]epibatidine for 3 hours at room temperature, and nonspecific binding was assessed in the presence of 1 μM cytisine (6). [125I]α-Bungarotoxin binding (α7 subunit) was measured by using 1.2 nM radioligand, and nonspecific binding was measured in the presence of 2.5 mM nicotine during both the pre-incubation period (30 minutes) and incubation (2 hours) (6). The sections were washed and dried after incubation and exposed to high-performance autoradiography film with high and low tritium standards for 3 weeks for pirenzepine, 2 months for AFDX-384, 3 months for epibatidine, and 1 week for α-bungarotoxin. After development, the images were quantified by using a Lynx densitometry system (Applied Imaging International, Newcastle Upon Tyne, U.K.).
Membrane Receptor Binding
For nicotine binding to the basal forebrain, membranes were prepared (16) and incubated in triplicate with 2 nM [3H]nicotine with or without unlabeled 1 mM nicotine. The membranes were collected on Whatman GF/C glass fiber filters (presoaked in 0.3% polyethyleneimine) by using a PHD cell harvester (model 2000, Cambridge Technology, Watertown, Mass.). The filters were washed three times in buffer, and the beta radiation was counted by using a scintillation counter.
For the determination of the concentrations producing 50% inhibition (IC50) of epibatidine and pirenzepine binding, washed membrane pellets were prepared from subjects for whom sufficient tissue from the parietal cortex was available (three normal subjects and two autistic subjects). Protein was measured by the method of Lowry et al. (17). For epibatidine binding, resuspended membranes were pre-incubated with 1 × 10–12 to 10–8 M unlabeled epibatidine, before the addition of 1 nM [3H]epibatidine, and incubated for 3 hours at room temperature. For the M1 receptor, 1 × 10–11 to 10–4 M unlabeled pirenzepine and 2 nM [3H]pirenzepine were used with 1 hour of incubation. The membrane suspensions were filtered through Whatman GF/C filters (presoaked in 0.5% polyethyleneimine), and beta radiation was determined by using a liquid scintillation counter. Binding was analyzed by using nonlinear regression and assuming a one-site competition model (GraphPad Prism, GraphPad Software, San Diego).
Nicotinic Receptor Subunits
In select samples of the parietal cortex, nicotinic receptor subunits α3, α4, α7, and β2 were quantified by using Western blotting (18). The polyclonal antibodies used were as follows: antihuman nicotinic acetylcholine receptor α3 antibody (antibody 607), against the carboxy terminus of the nicotinic acetylcholine receptor (19); sc-1772 raised against the carboxy terminus of the nicotinic acetylcholine receptor α4 subunit (Santa Cruz Biotechnology, Santa Cruz, Calif.); sc-1447, against the carboxy terminus of the α7 subunit (Santa Cruz Biotechnology); and antihuman nicotinic acetylcholine receptor β2 subunit raised in rabbit against a human epitope, corresponding to an amino acid sequence in the cytoplasmic loop region at position 350–452 between the M3 and M4 transmembrane domains (20). Membrane pellets, prepared as already described, were subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis and electroblotting onto polyvinylidene fluoride membranes as described previously (18). The samples were evaluated by Western blotting between three and five times.
Choline Acetyltransferase
Representative samples of gray matter (including all cortical layers) were removed, and 10% homogenates of these and basal forebrain samples were prepared in 0.32 M sucrose containing 0.5% Triton X-100 at 4°C. Duplicate or triplicate samples were incubated at 37°C for 20 minutes with [14C]acetyl coenzyme A by using, for the cortical samples, an adaptation (21) of the method of Fonnum (22) and, for the basal forebrain samples, the original Fonnum method (22).
Acetylcholinesterase
For histochemical analyses of the cortical samples, 10-μm cryostat sections were fixed in formal calcium and incubated with acetylthiocholine iodide with staining intensification as previously described (23). Acetylcholinesterase biochemical activity in basal forebrain homogenates was measured in duplicate according to the method of Ellman et al. (24) in the presence of 1 × 10–5 M iso-octamethyldiphosphoramide, a specific inhibitor of butyrylcholinesterase activity.
Nerve Growth Factor and Brain-Derived Neurotrophic Factor
Two-site enzyme immunoassays were performed to measure endogenous levels of brain-derived neurotrophic factor and nerve growth factor. Forebrain samples were sonicated in 50 mM Tris HCl, pH 7.0, 150 mM NaCl, 1% bovine serum albumin, 1% Triton X-100, 4 μg/ml aprotinin and 0.5 mM phenylmethylsulfonyl fluoride, 2 mM EDTA, 0.1 mM benzethonium chloride, and 0.05% sodium azide, and they were then centrifuged (15,000 g) for 10 minutes. Nerve growth factor and brain-derived neurotrophic factor standards (1 to 500 pg/ml) were used to generate standard curves. The immunoplates were coated for 2 hours with purified monoclonal antibodies to nerve growth factor (0.67 μg/ml) and brain-derived neurotrophic factor (100 ng/well), and tissue extracts (in duplicate with 10 mM CaCl2) were added. The trophic factors were quantified according to modifications of previously published methods (25, 26).
Radiochemicals
[3H]Pirenzepine (85.6 Ci/mmol), [3H]AFDX-384 (106.5 Ci/mmol), (±)[3H]epibatidine (33.8 Ci/mmol), (3-[125I]iodotyrosyl)-α-bungarotoxin (150 Ci/mmol), [3H]nicotine (81.5 Ci/mmol), and [14C]acetyl coenzyme A (58.9 mCi/mmol) were purchased from New England Nuclear Life Science Products, Inc. (Boston).
Statistical Analysis
Since the different brain areas are functionally distinct, they were analyzed separately. Values for cortical nicotinic and muscarinic binding were analyzed by two-way analysis of variance (ANOVA) (general linear model, Minitab release 12 [Minitab, State College, Pa.]) with group and cortical layer as factors. If a statistically significant effect was demonstrated for group and/or cortical layer but there was no significant interaction between the factors, the data were analyzed as suggested by Kinnear and Gray (27), by one-way ANOVA followed by post hoc analysis using Fisher’s pairwise comparison, with significance set at p<0.05 (Minitab release 12). Values for the forebrain were analyzed by t tests.
Results
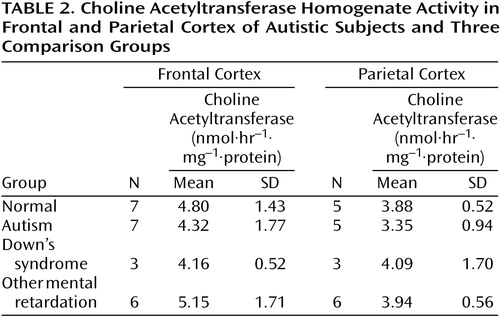
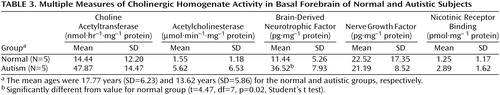
There were no significant abnormalities in the autistic or nonautistic groups of the presynaptic marker, choline acetyltransferase, in the frontal cortex, parietal cortex, or basal forebrain (Table 2 and Table 3), although there was a nonsignificant tendency for the activity to be higher in the basal forebrain in the autistic group. Since the values for cortical choline acetyltransferase activity did not differ significantly among groups, a detailed analysis of the acetylcholinesterase histochemical reactivity in the cortex was not undertaken. It was established by semiquantitative macroscopic assessment that the histochemical activity levels were similar in the autistic and normal groups. In the basal forebrain there was also no significant difference in the acetylcholinesterase activity measured biochemically, although, as with choline acetyltransferase, there was a nonsignificant tendency toward greater activity in the autistic group (Table 3). Of the two neurotrophin measurements investigated in the basal forebrain, the levels of nerve growth factor did not differ significantly, but the levels of brain-derived neurotrophic factor were significantly higher in the autistic group, three times as high as in the normal comparison group (Table 3).
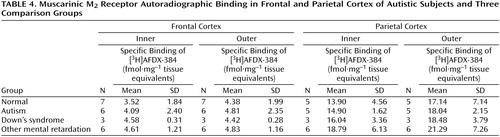
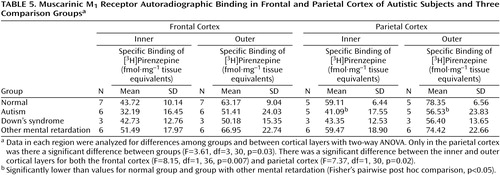
Binding to the M2 receptor in the two cortical areas did not differ substantially between the autistic and other groups (Table 4). In contrast, cortical M1 receptor binding was lower in the autistic group (Table 5, Figure 1, Figure 2>). This difference was apparent in both cortical areas and in both the outer and inner cortical layers, and for the parietal cortex the autistic group was significantly different from both the normal group and the group with other mental retardation. There was, with the exception of one case, no overlap in M1> receptor binding in the inner parietal cortex between the autistic and normal group or group with other mental retardation. The IC50 values for pirenzepine displacement were 1.86×10–9 and 2.03×10–9 M in the two autistic subjects, whereas the mean for the three normal subjects was 1.68×10–9 M (SD=0.28×10–9).
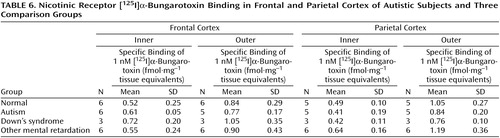
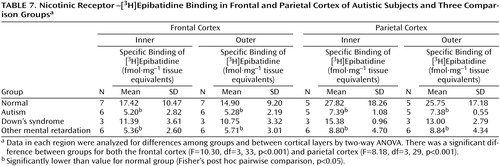
With respect to nicotinic receptor binding, there was no abnormality of α-bungarotoxin binding in either cortical area in the autistic group (Table 6). By contrast, in both cortical areas the autistic group had significantly and substantially lower epibatidine binding (to 27%–35% of normal) than was found for the normal comparison group (Table 7, Figure 3, Figure 4); the differences were apparent in both the outer and inner cortical layers. Epibatidine binding was also significantly lower in the nonautistic mental retardation group, but not in the Down’s syndrome group, than in the normal group. It is unlikely that the lower receptor level is due to a difference in tobacco use between the groups. Although smoking histories were not available for the series, if 50% of the normal group smoked (a rate higher than for the general population) and none of the autistic subjects smoked, the expected difference in epibatidine binding—based on a smoking-induced doubling of binding in the normal human cortex (28)—would not exceed 35%. Moreover, in the basal forebrain, [3H]nicotine binding was normal (Table 3). IC50 values for epibatidine binding in the parietal cortex were 7.9×10–10 M and 2.3×10–10 M for the two normal subjects and 9.1×10–10 M and 10.7×10–10 M for the two autistic subjects. According to Western blotting of samples from the parietal cortex, there was lower α4 and β2, but not α3 or α7, immunoreactivity in autism. The α4 immunoreactivity was in two cases 2.2% and 5.1% of the mean value for the normal group, and β2 immunoreactivity was 18.1% and 43.1%.
There was no difference in cortical epibatidine or pirenzepine binding between normal subjects whose samples had been slow or snap frozen. The mean values for the inner frontal cortex for five snap-frozen and two slow-frozen samples were, respectively, 16.1 and 21.0 fmol·mg–1 for epibatidine binding and 39.9 and 42.7 fmol·mg–1 for pirenzepine binding.
Discussion
This study indicates the potential importance of specific cholinergic receptors and neurotrophins in developmental neurobiology. The findings indicate that although cholinergic enzyme markers are normal in autism, many nicotinic (high-affinity) and moderate muscarinic M1 receptor measures in the cerebral cortex are lower than normal, and the level of brain-derived neurotrophic factor in the basal forebrain is higher than normal.
Normal Presynaptic Cholinergic Activity
In relation to the original hypothesis of basal forebrain cholinergic dysfunction, the finding of normal choline acetyltransferase activity both in the frontal and parietal cortex and in the basal forebrain suggests that the presynaptic cholinergic innervation of the cortex is structurally intact in autistic subjects. This indicates either that cholinergic neurons are not depleted or that compensatory axonal sprouting has occurred. In another developmental disorder, Rett’s syndrome, in which levels of choline acetyltransferase and the vesicular acetylcholine transporter are lower than normal in various areas, including the cortex (29, 30), disruption of cholinergic innervation is likely due to developmental or degenerative neuronal abnormalities occurring before or shortly after birth in the absence of compensation.
Low Binding of Muscarinic M1 Receptors
The low M1 receptor binding apparent in the parietal cortex of autistic subjects but not subjects with other types of mental retardation indicates a specific abnormality in cholinoceptive function, since the M1 receptor is located postsynaptically. The binding abnormality reflected a low number of receptors. This could be related to epilepsy, which occurs in up to 40% of autistic children (31), since a low number has been reported in hippocampal sclerosis associated with temporal lobe epilepsy (32). The finding that a similar series of subjects had normal pirenzepine binding in the hippocampus (33), an area particularly susceptible in epilepsy, suggests another basis for the low number of receptors, such as dendritic dysfunction.
Low Binding of High-Affinity Nicotinic Receptors
The lower degree of high-affinity nicotinic receptor binding in the cortex of the autistic subjects was extensive and likely, on the basis of the kinetic and immunochemical evidence, to reflect low receptor numbers. The lower epibatidine but not α-bungarotoxin binding is consistent with the preliminary immunoblot findings of lower immunoreactivity of the nicotinic α4 and β2 subunits. Although values for the α4 subunit and high-affinity nicotinic agonist binding site are elevated as a result of exposure to nicotine (18, 34), the lower levels of receptor binding in the autistic individuals in this study could not be attributed to differences in tobacco use between the different groups.
Relationships between low nicotinic receptor binding and synaptophysin identified in another cerebral disorder, Alzheimer’s disease (35), suggest that the low receptor binding in autism may be associated with abnormal cortical neuronal morphology, possibly involving overextensive synaptic pruning. Knockout (β2) mice lacking nicotinic receptor binding develop a degree of age-related cortical atrophy and neuronal loss in conjunction with cognitive impairment (36). Lower binding at the high-affinity nicotinic receptor agonist site not only in autism but also in the nonautistic mental retardation group could indicate that this condition is not specific to autism and that receptor loss may be a consequence rather than cause of cortical dysfunction. However, developmental brain abnormalities occur in both groups and an overlap in the processes involved would be expected.
Nicotinic agents are analgesic (37), and on the basis of a gene knockout model (38) the α4 subunit has been implicated in pain perception. Low levels of this receptor subtype in autism may thus be associated with the low degree of pain reactivity that is present in the disease (39). Since abnormal galvanic skin responses (peak amplitudes three times normal, number of arousal events per minute two times normal, and absence of a baseline) have been observed in autism (unpublished 2000 study of P. Iversen and H. Ramshandran), and since such responses may depend on the integrity of sympathetic cholinergic neural pathways (40), it may be worth investigating the extent to which the central nervous system cholinergic abnormalities reported here extend to the peripheral nervous system. The finding that, in contrast to epibatidine binding, α-bungarotoxin binding is normal in autism is interesting since the gene coding for the α7 subunit is located close to the q11-15 region of chromosome 15 (41), which is considered to be one of the likely sites of genetic abnormalities in autism (42).
High Level of Brain-Derived Neurotrophic Factor
The finding that the level of brain-derived neurotrophic factor was significantly higher than normal in the basal forebrain can be interpreted in various ways. Since brain-derived neurotrophic factor is up-regulated by cholinergic activity in developing rat hippocampus (43), it is unlikely that the abnormality is due to developmental cholinergic dysfunction. One explanation for the high level, since neurotrophins influence the development and function of basal forebrain cholinergic neurons (3, 44), is that it reflects a regional compensatory mechanism. This may underlie the finding that levels of cholinergic biomarkers, such as choline acetyltransferase in the cortex and basal forebrain, were normal or even high. An alternative explanation is that overexpression of brain-derived neurotrophic factor is an intrinsic component of the autism disease process. The recent finding (45) of high blood levels of brain-derived neurotrophic factor, among three brain peptides or proteins (brain-derived neurotrophic factor, vasoactive intestinal peptide, and calcitonin gene-related peptide), in 62 of 64 newborn autistic and other mentally retarded individuals suggests an intrinsic rather than compensatory mechanism.
Comparison With Schizophrenia
The overall pattern of cholinergic abnormalities in autism—low levels of muscarinic and nicotinic receptors in conjunction with normal levels of presynaptic cholinergic markers in the cortex—is more similar to that seen in schizophrenia than to patterns in other disorders involving a cholinergic abnormality, such as Rett’s syndrome, Alzheimer’s or Parkinson’s disease, head injury, or vascular dementia. There is in common with schizophrenia a sparing of the presynaptic marker, choline acetyltransferase, but low levels of muscarinic and nicotinic receptors, although in schizophrenia the α7 subtype may also be involved (46, 47). In schizophrenia there is also a high level of brain-derived neurotrophic factor but not nerve growth factor in cortical areas (48). There is an extensive overlap in clinical symptoms between autism and schizophrenia, both behaviorally and cognitively, e.g., in conceptual abnormalities (49). The same neural systems are likely to be involved in both, although differing in developmental staging and etiology.
Conclusions
If the low level of cortical nicotinic receptors is consistently observed and clinically relevant, therapeutic strategies could include receptor agonists, such as nicotine, which has already been applied in Tourette’s disorder with amelioration of symptoms (50). Such treatment could also be disease modifying. Nicotine significantly increases branching of both axons and dendrites in cultured hippocampal neurons (51). In adolescent rat brain, nicotine exposure results in persistent up-regulation of nicotinic receptors in a variety of brain areas, including the cortex (52), and in adult mouse brain chronic nicotine exposure promotes retention of nicotinic receptors that decline in aging (53).
On the basis of the present findings, the role of the cholinergic system in autism should be investigated further, with respect to other brain regions, additional cholinergic receptor subtypes, and clinical correlates and in younger individuals.
 |
 |
 |
 |
 |
 |
 |
Received June 30, 2000; revision received Dec. 5, 2000; accepted Feb. 1, 2001. From Centre Development in Clinical Brain Ageing, Newcastle General Hospital; the Lilly Research Centre, Windleham, Surrey, U.K.; Cure Autism Now, Los Angeles, Calif.; the Children’s Neurology Service, Harvard Medical School and Massachusetts General Hospital, Boston; and the Division of Neural Systems, Memory and Aging, University of Arizona, Tucson. Address reprint requests to Dr. Perry, Centre Development in Clinical Brain Ageing, MRC Building, Newcastle General Hospital, Newcastle Upon Tyne NE4 6BE, U.K.; [email protected] (e-mail). Supported by a project grant from Cure Autism Now. The authors thank the Autism Research Foundation, Harvard Brain Tissue Resource Center, University of Maryland Brain and Tissue Bank for Developmental Disorders, and University of Miami Brain and Tissue Bank for Developmental Disorders; Dr. Susan Folstein, whose research staff carried out the autism diagnostic interviews; and Dr. Cecilia Gotti, who provided the α3 nicotinic receptor subunit antibody.
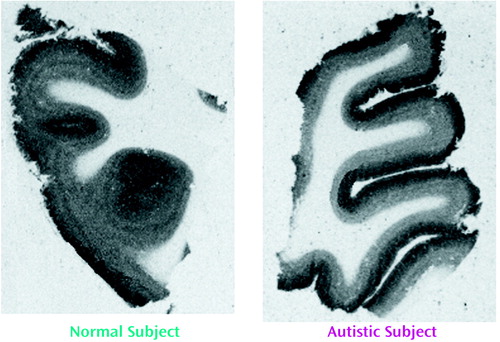
Figure 1. [3H]Pirenzepine Binding to Parietal Cortex of Representative Normal Subject and Autistic Subject
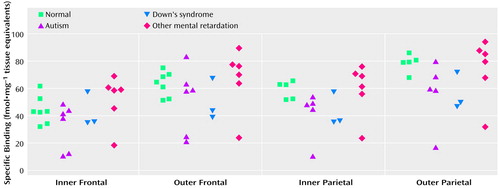
Figure 2. Individual Levels of [3H]Pirenzepine Binding in Inner and Outer Layers of Frontal and Parietal Cortex From Autistic Subjects and Three Comparison Groups
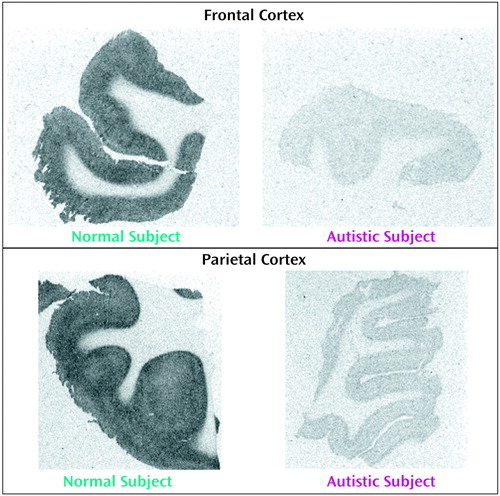
Figure 3. [3H]Epibatidine Binding to Frontal and Parietal Cortex of Representative Normal Subject and Autistic Subject
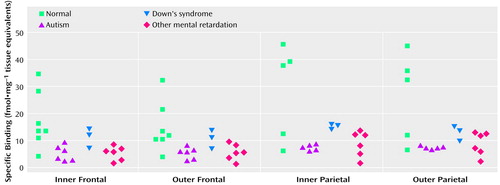
Figure 4. Individual Levels of [3H]Epibatidine Binding in Inner and Outer Layers of Frontal and Parietal Cortex From Autistic Subjects and Three Comparison Groups
1. Tsai LY: Psychopharmacology in autism. Psychosom Med 1999; 61:651-665Crossref, Medline, Google Scholar
2. Bauman ML, Kemper TL: Neuro-anatomic observations of the brain in autism, in The Neurobiology of Autism. Edited by Bauman ML, Kemper TL. Baltimore, Johns Hopkins University Press, 1994, pp 119-145Google Scholar
3. Hohmann CF, Berger-Sweeney J: Cholinergic regulation of cortical development and plasticity: new twists to an old story. Perspect Dev Neurobiol 1998; 5:401-425Medline, Google Scholar
4. Uhlings CG, Van Eden CG, Parnaveles JG, Kaalskeek A: The prenatal and postnatal development of the rat cerebral cortex, in The Cerebral Cortex of the Rat. Edited by Kolb B, Tees RC. Cambridge, Mass, MIT Press, 1992, pp 35-75Google Scholar
5. Court JA, Perry EK, Johnson M, Piggott MA, Kerwin JM, Perry RH, Ince PG: Regional patterns of cholinergic and glutamate activity in the developing and aging human brain. Dev Brain Res 1993; 74:73-82Crossref, Medline, Google Scholar
6. Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJM, Piggott MA, Oakley AE, Ince PG, Perry EK, Perry RH: Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Dev Brain Res 1997; 101:93-105Crossref, Medline, Google Scholar
7. Lainhart JE, Piven J: Diagnosis, treatment and neurobiology of autism in children. Curr Opin Pediatrics 1995; 7:392-400Crossref, Medline, Google Scholar
8. Hohmann CF, Kwiterovich C, Oster-Granite ML, Coyle JT: Newborn basal forebrain lesions disrupt cortical cytodifferentiation as visualized by rapid Golgi staining. Cereb Cortex 1991; 1:143-157Crossref, Medline, Google Scholar
9. Hohmann CF, Wilson L, Coyle JT: Efferent and afferent connections of mouse sensory-motor cortex following transient cholinergic deafferentation at birth. Cereb Cortex 1991; 1:158-172Crossref, Medline, Google Scholar
10. Sengstock GJ, Johnson KB, Jantzen PT, Meyer EM, Dunn AJ, Arendash GW: Nucleus basalis lesions in neonatal rats induce a selective hypofunction and cognitive deficits during adulthood. Brain Res 1992; 90:163-174Google Scholar
11. Huttenlocher PR: Synaptic and dendritic development and mental defect, in Brain Mechanisms in Mental Retardation: Based Upon a Symposium. Edited by Buchwald NA, Brazier MA. San Diego, Academic Press, 1975, pp 123-139Google Scholar
12. Wenk GL, Stoehr JD, Quintana G, Mobley SL, Wiley RG: Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. J Neurosci 1994; 14:5986-5995Google Scholar
13. Lord C, Rutter M, Le Couteur A: Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24:659-685Crossref, Medline, Google Scholar
14. Perry EK, Court J, Goodchild R, Griffiths M, Johnson M, Lloyd S, Piggott M, Spurden D, Ballard C, Jaros E, McKeith I, Perry R: Clinical neurochemistry: new opportunities for research in brain ageing based on brain bank material. J Neural Transm 1998; 105:915-933Crossref, Medline, Google Scholar
15. Crook JM, Dean B, Pavey G, Copolov D: The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci 1999; 64:1761-1771Google Scholar
16. Nordberg A, Alafuzoff I, Winblad B: Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res 1992; 31:103-111Crossref, Medline, Google Scholar
17. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with Folin phenol reagent. J Biol Chem 1951; 193:265-275Medline, Google Scholar
18. Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, Mamalaki A, Tsouloufis T, Tzartos S, Ballard C, Perry RH, Perry EK: Alpha4 but not alpha3 and alpha7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer’s disease. J Neurochem 1999; 73:1635-1640Google Scholar
19. Gotti C, Briscini L, Verderio C, Oortgiesen M, Balestra B, Clementi F: Native nicotinic acetylcholine receptors in human IMR32 neuroblastoma cells: functional, immunological and pharmacological properties. Eur J Neurosci 1995; 7:2083-2092Google Scholar
20. Beattie RE, Volsen SG, Smith D, McCormack AL, Gillard SE, Burnett JP, Ellis SB, Gillespie A, Harpold MM, Smith W: Preparation and purification of antibodies specific to human neuronal voltage-dependent calcium channel subunits. Brain Res Brain Res Protoc 1997; 1:307-319Crossref, Medline, Google Scholar
21. Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE: Neurotransmitter abnormalities in senile dementia. J Neurol Sci 1977; 34:247-265Crossref, Medline, Google Scholar
22. Fonnum F: Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J 1975; 115:465-472Google Scholar
23. Perry RH, Blessed G, Perry EK, Tomlinson BE: Histochemical observations on cholinesterase activities in the brains of elderly normal and demented patients. Age Aging 1980; 9:9-16Crossref, Medline, Google Scholar
24. Ellman GL, Courtney KD, Andres V Jr, Featherstone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7:88-95Crossref, Medline, Google Scholar
25. Korsching S, Thoenen H: Two-site immunoassay for nerve growth factor. Methods Enzymol 1987; 147:167-185Crossref, Medline, Google Scholar
26. Nawa H, Carnahan J, Gall C: BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci 1995; 7:1527-1535Google Scholar
27. Kinnear PR, Gray CD: SPSS for Windows Made Simple. Hove, UK, Lawrence Erlbaum Associates, 1994Google Scholar
28. Court J, Lloyd S, Thomas N, Piggott M, Marshall EF, Morris CM, Lamb H, Perry RH, Johnson M, Perry E: Effects of tobacco on nicotinic receptors in human brain. Neuroscience 1998; 87:63-70Crossref, Medline, Google Scholar
29. Wenk GL, Naidu S, Casanova MF, Kitt CA, Moser H: Altered neurochemical markers in Rett’s syndrome. Neurology 1991; 41:1753-1756Google Scholar
30. Wenk GL, Mobley SL: Choline acetyltransferase activity and vesamicol binding in Rett syndrome and in rats with nucleus basalis lesions. Neuroscience 1996; 73:79-84Crossref, Medline, Google Scholar
31. Minshew NJ, Sweeney JA, Bauman M: Neurological aspects of autism, in Handbook of Autism and Pervasive Developmental Disorders. Edited by Cohen D, Volkmar F. New York, John Wiley & Sons, 1997, pp 344-369Google Scholar
32. Pennell PB, Burdette DE, Ross DA, Henry TR, Albin RL, Sackellares JC, Frey KA: Muscarinic receptor loss and preservation of presynaptic cholinergic terminals in hippocampal sclerosis. Epilepsia 1999; 40:38-46Crossref, Medline, Google Scholar
33. Blatt GJ, Fitzgerald CM, Killiany RJ, Kemper TL, Bauman ML: Neurotransmitter receptor density in the hippocampal formation in human autistic and normal brains. Abstracts of the Society for Neuroscience 1999; 25:489Google Scholar
34. Paterson D, Nordberg A: Neuronal nicotinic receptors in human brain. Prog Neurobiol 2000; 81:75-111Crossref, Google Scholar
35. Sabbagh MN, Reid RT, Corey-Bloom J, Rao TS, Hansen LA, Alford M, Masliah E, Adem A, Lloyd GK, Thal LJ: Correlation of nicotinic binding with neurochemical markers in Alzheimer’s disease. J Neural Transm 1998; 105:709-717Crossref, Medline, Google Scholar
36. Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux J-P: Increased neurodegeneration during ageing in mice lacking high affinity nicotine receptors. EMBO J 1999; 18:1235-1244Google Scholar
37. Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, Bitner RS, Diaz A, Dickenson AH, Porsolt RD, Williams M, Arneric SP: Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science 1998; 279:77-81Crossref, Medline, Google Scholar
38. Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, Huchet M, Damaj MI, Changeux JP: Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 1999; 398:805-810Crossref, Medline, Google Scholar
39. Tordjman S, Antoine C, Cohen DJ, Gauvain-Piquard A, Carlier M, Roubertoux P, Ferrari P: Study of the relationships between self-injurious behavior and pain reactivity in infantile autism. Encephale 1999; 25:122-134Medline, Google Scholar
40. Magnifico F, Misra VP, Murray NM, Mathias CJ: The sympathetic skin response in peripheral autonomic failure—evaluation in pure failure, pure cholinergic dysautonomia and dopamine-beta-hydroxylase deficiency. Clin Auton Res 1998; 8:133-138Crossref, Medline, Google Scholar
41. Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S: Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHRNA7). Genomics 1994; 19:379-381Crossref, Medline, Google Scholar
42. Lamb JA, Moore J, Bailey A, Monaco AP: Autism: recent molecular genetic advances. Hum Mol Genet 2000; 4:861-865Crossref, Google Scholar
43. da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D: Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci 1993; 13:3818-3826Google Scholar
44. Hashimoto Y, Abiru Y, Nishio C, Hatanaka H: Synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor on cultured basal forebrain cholinergic neurons from postnatal 2-week old rats. Brain Res Dev Brain Res 1999; 115:25-32Crossref, Medline, Google Scholar
45. Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Hansen RL, Phillips TM: Neuropeptides and neurotrophins in neonatal blood of children with autism, mental retardation, or cerebral palsy (abstract). Neurology 2000; 54(suppl 3):A247Google Scholar
46. Perry R, Perry E: The cholinergic system in Alzheimer’s disease, in Biochemistry of Dementia. Edited by Roberts PJ. London, John Wiley & Sons, 1980, pp 135-183Google Scholar
47. Freedman R, Hall M, Adler LE, Leonard S: Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 1995; 38:22-33Crossref, Medline, Google Scholar
48. Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, Koizumi S, Wakabayashi K, Takahashi H, Someya T, Nawa H: Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 2000; 5:293-300Crossref, Medline, Google Scholar
49. Pilowsky T, Yirmiya N, Arbelle S, Mozes T: Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophr Res 2000; 42:145-155Crossref, Medline, Google Scholar
50. Sandberg PR, Silver AA, Shytle RD, Philipp MK, Cahill DW, Fogelson HM, McConville BJ: Nicotine for the treatment of Tourette’s syndrome. Pharmacol Ther 1997; 74:21-25Crossref, Medline, Google Scholar
51. Audersirk T, Cabell L: Nanomolar concentrations of nicotine and cotinine alter the development of cultured hippocampal neurons via non-acetylcholine receptor-mediated mechanisms. Neurotoxicology 1999; 20:639-646Medline, Google Scholar
52. Trauth JA, Seidler FJ, McCook EC, Slotkin TA: Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res 1999; 851:9-19Crossref, Medline, Google Scholar
53. Rogers SW, Gahring LC, Collins AC, Marks M: Age related changes in neuronal nicotinic acetylcholine receptors subunit α4 expression are modified by long term nicotine administration. J Neurosci 1998; 18:4825-4832Google Scholar



