The Use of Psychotropic Medications During Breast-Feeding
Abstract
OBJECTIVE: The authors reviewed the risks and benefits regarding the use of psychiatric medications during breast-feeding as they relate to the health and well-being of mothers and their infants. Strategies are discussed to limit infant exposure to a medication while effectively treating the nursing mother. METHOD: A MEDLINE search of the literature since 1955 was conducted to determine the use of psychotropic medications in breast-feeding women. Search items included each of the categories of psychopharmacologic agents as well as each of the agents in association with nursing, breast-feeding, postpartum, lactation, and breast milk. RESULTS: No controlled studies on the safety of psychotropic medications in nursing mothers were found. Case reports and small case series for each of the different psychotropic medications serve as the basis for suggested treatment guidelines for the management of psychiatric illnesses in breast-feeding women. Thus, each case needs to be considered on an individual basis, with a thoughtful analysis of the risks and benefits of nursing and exposure of the infant to medication. The baseline clinical status of the infant should also be reviewed. CONCLUSIONS: Women are vulnerable postpartum to psychiatric disorders and frequently face the need to decide whether to take psychotropic medications while breast-feeding. Should psychiatric medication be indicated, the parents should be provided with the available information regarding the effects of these medications on the neonate. In this way, an informed decision can be made. When psychotropic medication is used during breast-feeding, it is strongly recommended that the infant’s pediatrician be involved in monitoring the infant.
Breast milk offers many advantages to developing infants. The American Academy of Pediatrics endorses breast milk as the best and only source of nutrition necessary for the infant during the first 6 months of life (1). Breast-fed infants have lower rates of gastrointestinal disease, anemia, respiratory ailments, and otitis media (2, 3). In addition, breast-feeding provides a unique opportunity for bonding between infant and mother (4).
The vulnerability for psychiatric illness during the 3 months after delivery raises the possibility that psychotropic medications will be administered (5, 6). Issues to address during analysis of the risks and benefits of psychotropic use during breast-feeding include documented benefits of breast-feeding, the potential adverse impact of untreated maternal mental illness on infant attachment and cognitive and behavioral development, and the effects of untreated mental illness on the mother (7–13). This article reviews the literature on the various classes of psychotropic medications in order to provide the basis for educated treatment planning.
On average, nursing mothers produce 600 to 1,000 ml of milk daily. Factors affecting medication concentration in breast milk are pH, protein content, and lipid content. These vary throughout the postpartum period and at different times during a single feeding, resulting in marked concentration variations in milk aliquots. Milk pH ranges from 6.35 to 7.65 (14). Mature milk is produced approximately 2 weeks after birth. The higher lipid content of hind milk (the milk ejected during the second half of a feeding) makes it likely that the second half will have a higher concentration of maternal medication than the first half (fore milk). Other major factors affecting medication concentration in breast milk include lactose, serum albumin, lysozyme, approximately 30 enzymes, prolactin, and minerals such as calcium and phosphates (15).
The extent to which an infant is exposed to medication is affected by the rate of absorption into maternal circulation, diffusion from maternal circulation to breast milk, and absorption of the agent by the infant. Taking medication immediately after breast-feeding minimizes the amount present in milk and maximizes clearance before the next feeding (16). In vitro studies have demonstrated that full-term neonatal cytochrome P-450 activity is approximately one-half that found in adults (17). Each liver enzyme system matures at a different rate in the developing infant. Thus, different substrates are metabolized at different points in maturation. For example, while glucuronyl transferase activity is mature enough to metabolize bilirubin 3 days after term, it cannot safely degrade chloramphenicol until at least day 10. Hepatic enzyme immaturity is even more pronounced in premature infants. Premature and full-term infants have a diminished capacity to metabolize medications for at least the first 2 weeks of life (17).
While the ratio of kidney weight to body mass in infants is twice that in adults, the newborn kidney is functionally immature. With a glomerular filtration rate only 30%–40% of adult values and tubular secretion 20%–30% of adult function, renal excretion is considerably diminished. Consequently, compounds eliminated through the kidney tend to accumulate in the infant, causing toxic exposure over time (18). For full-term infants, the glomerular filtration rate seen in adults is achieved between the second and fifth months of life (19).
Since the newborn blood-brain barrier is also immature, lipid-soluble agents can be 10–30 times more concentrated in the CSF than in serum (20) and may be higher in infants for a given plasma concentration compared to adults (21). Because body fat storage sites are limited in the neonate, central nervous system concentrations of lipid-soluble substances are greater in newborns than in older infants (22).
Despite the pharmacokinetic complexity and variability of the individual compartmentalized processes just described, most reports do not account for maternal, breast milk, or infant data. Frequently, infant exposure is estimated by measuring breast milk concentrations and assumed average daily milk consumption.
Method
This review comprises an analysis of 95 studies and reports covering 32 psychotropic agents used by nursing mothers. Of the studies, 66 measured infant serum concentrations. In some cases, infant serum concentrations reflected exposure both in utero and through breast milk. The wide range of variability in sensitivity and reliability of a given assay precluded meaningful statistical grouping of individual case reports. Most reports lacked data for concentrations relative to time of dose administration. The interval between medication dose administration and infant feeding times is rarely reported. Finally, where infant clinical status is documented, behavioral evaluation is rarely based on standardized clinical assessment.
To avoid overinterpretation of data with so many limitations, and since the clinical significance of infant serum concentrations of medications is unclear, detailed discussion is reserved only for data that suggest a possible connection between drug and deleterious effects in offspring. Guidelines for the use of psychotropic agents in breast-feeding mothers will be suggested.
Results
Antidepressants
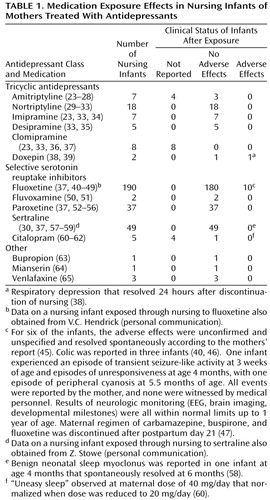
Data from studies of breast-feeding and antidepressants are summarized in Table 1.
Tricyclic antidepressants
Infant serum levels of parent and active metabolites reported in studies of tricyclic antidepressants ranged from nondetectable to less than 28 ng/ml. Despite widely ranging milk-to-plasma ratios and variable infant serum levels of amitriptyline and nortriptyline (either as parent drug or as a metabolite of amitriptyline), there were no reports of adverse effects in the breast-fed infants. Similarly, no adverse effects were reported for the seven infants exposed to imipramine, the five infants exposed to desipramine, or the eight infants exposed to clomipramine.
For doxepin, two single case reports revealed milk-to-plasma ratios of parent and metabolite compounds that were close to or greater than one and measurable serum metabolite concentrations. Respiratory depression occurred in one case (38) but resolved 24 hours after discontinuation of nursing. Since the infant was not exposed to doxepin rechallenge through nursing, it is not possible to state definitively that respiratory compromise was due to doxepin. In the second case (39), no adverse effects were noted in the infant.
Selective serotonin reuptake inhibitors (SSRIs)
Fluoxetine use by breast-feeding mothers has been evaluated in 11 published reports of 190 breast-fed infants (Table 1). In 101 cases, infant sera concentrations were not tested. Of the remaining cases, levels of parent compound and metabolite varied from nondetectable to a single report of 340 ng/ml (40). There were no clear associations between levels in infant sera, maternal dose, and infant age. No adverse effects were noted in 180 of the 190 cases. One study of four infants reported normal neurobehavioral development to 1 year of age (44). Of some concern is a single case of a 6-week-old infant with serum concentrations of fluoxetine that were comparable to maternal concentrations (40); adverse effects reported included excessive crying, decreased sleep, vomiting, and diarrhea that dissipated upon discontinuation of nursing. Adverse effects reported in other studies were transient and by maternal report (45, 46) or were confounded by multiple medications (47). Although a retrospective study indicated poorer weight gain for fluoxetine-exposed infants than age-matched control subjects, weights in the exposed infants were not statistically below the national mean (48). These data highlight the importance of professional monitoring of the clinical status of nursing infants rather than relying on laboratory measurements of serum levels and maternal observations.
Fifteen published reports documented the use of the remaining SSRIs (fluvoxamine, paroxetine, sertraline, and citalopram) in breast-feeding mothers. For sertraline, infant serum levels were nondetectable or less than 5 ng/ml for parent compound; the metabolite concentrations that were measured in four reports were less than 10 ng/ml. In the six paroxetine reports, serum levels were measured in 27 of the 37 breast-fed infants: concentrations ranged from nondetectable for 24 of the infants to less than 20 ng/ml in the remaining three. Citalopram and desmethylcitalopram were measured in infant serum in two of the citalopram case reports (60, 61): concentrations ranged from 2.3 ng/ml to 12.7 ng/ml, and metabolite concentrations were nondetectable. Serum levels were not reported for fluvoxamine. No clear adverse effects were noted in the 49 infants exposed to sertraline, the 37 exposed to paroxetine, or the two exposed to fluvoxamine.
Other antidepressants
Although five infants exposed through nursing to bupropion, mianserin, or venlafaxine did not experience apparent adverse effects, more data are needed before conclusions can be made regarding their safety in breast-feeding. Since infant serum concentrations and clinical status were not reported for the novel antidepressant trazodone (66) or the reversible monoamine oxidase inhibitor moclobemide (67), the safety of these agents in exposed nurslings is unknown.
Anxiolytics
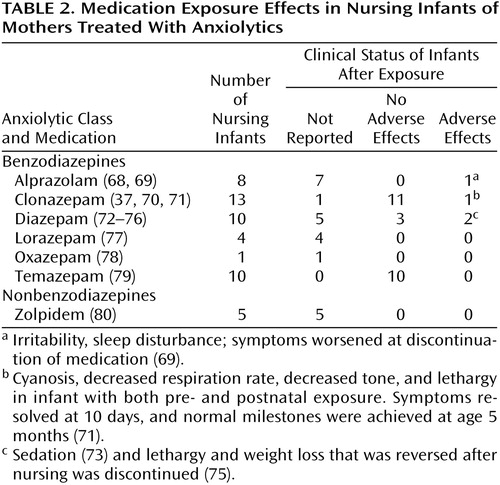
Studies with data on anxiolytic exposure in nursing infants are summarized in Table 2.
For 12 of 13 infants whose mothers were on regimens of clonazepam, exposure was in utero as well as through breast-feeding. In one case of an infant exposed both in utero and through nursing to clonazepam (infant serum level <5 ng/ml), persistent cyanosis was noted at delivery and for the first 10 postpartum days (71). By day 10 breathing normalized, and neurodevelopment appeared to be normal at 5 months (71). For the remaining 11 infants for which clinical status was described, no adverse effects were noted (37).
Only three of 10 infants exposed to diazepam through nursing had data for serum concentrations of parent and metabolite compounds, which varied from nondetectable to 243 ng/ml. Serum levels of both parent and metabolite compounds were lowest in the oldest nursling, an infant of 1 year of age (72), which suggests that older infants have improved metabolic capacity. Clinical status was reported in five infants, with no adverse effects in three (76) and normal development recorded in a fourth despite some apparent sedation (73). In another case report, lethargy and weight loss was reversed following discontinuation of nursing (75). For the 11 infants exposed to oxazepam or temazepam (and its metabolite, oxazepam), no adverse effects were noted.
The remaining reports of medication effects in infants exposed to anxiolytics through breast milk involved treatment with alprazolam, lorazepam, or zolpidem. Infant serum concentrations were not reported, and in the one case where clinical status was reported (69), alprazolam withdrawal appeared upon medication tapering.
Antipsychotics
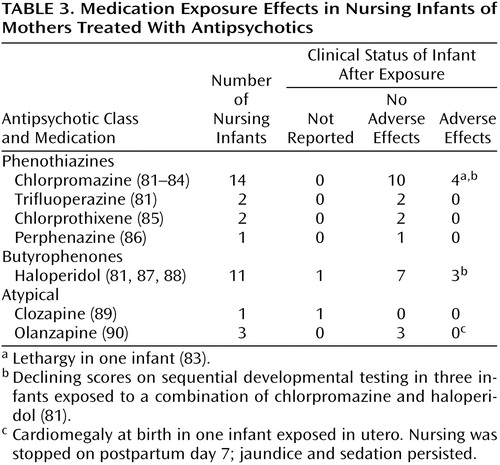
Ten reports have addressed infant exposure to antipsychotic agents through nursing (Table 3). Of 34 clinical status reports of infants exposed to antipsychotics through breast milk, 25 showed no adverse effects. Lethargy was noted in one chlorpromazine-exposed infant (83). Delayed developmental testing at 12–18 months of age was reported for three infants exposed to a combination of haloperidol and chlorpromazine; only one of the three infants had detectable serum levels of neuroleptic (81). In a single case report of clozapine exposure during breast-feeding (89), milk-to-plasma ratios for clozapine revealed that it was concentrated in milk, but since there were no data regarding infant serum levels, the relevance of this is unclear. The early data for olanzapine come from one report of three infants exposed both in utero and through nursing (90); no adverse effects attributable to olanzapine ingestion through breast-feeding were noted.
Mood Stabilizers
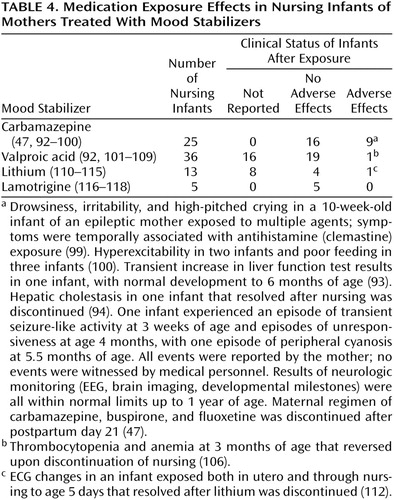
A recent study indicated that postpartum bipolar women who discontinued lithium before pregnancy were almost three times as likely to have a recurrence in the first postpartum month as bipolar control subjects (91). Twenty-eight reports of the use of mood stabilizers in nursing mothers include 79 infants exposed to lithium, carbamazepine, valproate, or lamotrigine through breast-feeding (Table 4). Of 25 cases of carbamazepine exposure in nursing infants, there were two cases of transient hepatic dysfunction (93, 94). The etiology of reported instances of seizure-like activity (47) and drowsiness, irritability, and abnormal crying (99) was impossible to ascribe because of the exposure to a combination of agents through breast milk.
Of 36 valproate-exposed infants, no adverse effects were noted for 19, and clinical status was not reported for 16. Thrombocytopenia and anemia in an exposed 3-month-old infant reversed upon cessation of nursing (106).
Of the 13 infants exposed to lithium, serum concentrations were found to be very high. One infant with a congenital heart murmur, cyanosis, and hypotonia had been exposed to lithium both in utero and through breast milk (112). There were no adverse effects in four other infants, and clinical status was not reported for eight. No adverse effects were reported in five cases of lamotrigine exposure in nursing infants. We found no published data on gabapentin exposure through nursing.
Treatment Guidelines
General Issues
The decision to breast-feed while taking psychotropic medications is complicated. Considerations include known benefits of breast-feeding to infant and mother, wishes of the mother, risk of infant exposure to the medication, and the possibility that a severely depressed, anxious, or psychotic mother may forego treatment rather than give up breast-feeding.
Parents faced with this decision should be educated about possible side effects in their infants. As the association between infant drug levels and clinical status is unclear, clinical monitoring seems to be the best approach to minimize the risk to infants. Before initiating maternal medication, a pediatric evaluation should assess infant baseline behavior, sleep, feeding, and alertness. Metabolism and elimination are more efficient in older infants, who generally sleep for longer intervals, permitting dosing of the mother just after nursing and before the baby’s longest sleep interval. The pediatrician should be educated about potential side effects of medication exposure and interactions with other medications typically prescribed to infants (e.g., antibiotics, nonsteroidal anti-inflammatory agents, acetaminophen). Caution should be exercised when prescribing hepatically metabolized medications (such as acetaminophen) to breast-fed infants whose mothers are treated with psychotropics, since infants with immature liver function may have difficulty eliminating several medications through the same hepatic enzyme system. Mothers taking agents such as paroxetine, fluoxetine, or fluvoxamine should abstain from caffeine, since its metabolism is inhibited by these antidepressants.
The choice of medication depends on diagnosis, past history of the mother, side effect profile, dose flexibility, and pharmacokinetic characteristics that minimize accumulation of the agent in milk and infant serum. Dosage should be as low as possible while still achieving psychiatric remission. Doses so low as to constitute ineffective treatment represent needless exposure by infant to medication.
If a medication is administered on an as-needed basis, short-acting agents are probably preferable. It is best to use medications in which the parent compound does not metabolize into several generations of active compounds. Formula supplementation reduces infant exposure while retaining some breast-feeding benefits.
It is important to note that low or even negligible infant serum medication levels are not necessarily reassuring, since access to the brain and target cell sensitivity may be greater in young infants. Infant clinical status is the most important parameter to follow. Parents should be alerted to the side effect profile of medications, and monthly pediatric clinical monitoring is important to ensure general health and normal pediatric development.
Postpartum Depression
Depression in the postpartum period is disabling. If cognitive behavior therapy or interpersonal therapy is not helpful, or if the condition warrants aggressive early treatment, consideration should be given to ECT. Once diagnosed, postpartum psychiatric disorders are frequently managed with psychotropic medications. As noted by Wisner et al. (119), the use of antidepressants by nursing mothers is often acceptable so long as the mother-infant pair is carefully monitored. Data on the use of tricyclics by nursing mothers suggest that while parent compound levels in infant serum are often below the limits of detection of most commercial laboratories, metabolite levels in infant serum are sometimes detectable. The single case of respiratory distress in an infant exposed to the sedating antidepressant doxepin (38) suggests that it is best to avoid this agent in nursing mothers.
The emerging data for SSRIs is important, since this class comprises the most commonly prescribed antidepressants. The data for fluoxetine are variable and somewhat difficult to interpret. The few adverse effects were generally transient and not verified by medical personnel or objective tests. The lack of adverse effects in 180 fluoxetine-exposed infants justifies its continued use if it has been prescribed antenatally or if there is a history of preferential efficacy with this agent. A previous report suggests that there is little central serotonin reuptake inhibition in infants breast-fed by mothers taking sertraline (120). Although the gradient of antidepressants such as paroxetine tends to increase from fore milk to hind milk (52), the extent to which this is reflected in infant serum depends on the extent of nursing at any single feeding. Furthermore, the lack of adverse effects in 86 breast-fed infants exposed to sertraline or paroxetine and the uniformly low or nondetectable infant serum levels in these infants suggest that these are good choices for nursing mothers with postpartum depression. In the case of sertraline, serum levels peak in infants between 7 and 11 hours after maternal dosing; refraining from nursing during this time period may significantly reduce infant exposure (57). Similar peak levels have not been reported for paroxetine or fluoxetine. As the data for fluvoxamine and citalopram are sparse, these agents are not primary treatment options for breast-feeding mothers.
Postpartum Bipolar Disorder
Treatment of bipolar disorder invariably requires mood stabilizers. Maternal use of lithium and carbamazepine has been associated with serious difficulties in nurslings. There has been a single recorded case of serious blood abnormalities following exposure to sodium valproate through breast milk (106), and this agent has been associated with hepatotoxicity when directly administered to infants (121, 122). Therefore, when administering valproate to breast-feeding mothers, pediatric clinical status, liver enzymes, and platelets should be carefully monitored.
Lithium increases the risk of thyroid dysfunction, cyanosis, poor muscle tone, and ECG changes in infants (19, 114, 123). Because renal clearance is decreased in infants up to at least 5 months of age, use of lithium during breast-feeding is not advisable. Nevertheless, for the bipolar patient who invariably decompensates when lithium is discontinued, use by a nursing mother may be reasonable so long as infant clinical status is carefully monitored and serum concentrations of lithium in the infant are followed.
While the American Academy of Pediatrics suggests that carbamazepine exposure appears safe for breast-feeding infants, two cases of hepatic dysfunction (93, 94) and one case of transient seizure-like activity (47) suggest that it is advisable to monitor liver enzymes, bilirubin, and WBC counts frequently and to assay for carbamazepine levels in exposed infants. There are limited (although reassuring) data on lamotrigine exposure through breast milk and no data for the use of gabapentin by nursing mothers. Since these agents are not first-line medications for bipolar disorder, their use in breast-feeding should be reserved only when no other alternatives remain for effective treatment.
Postpartum Anxiety Disorders
Postpartum anxiety may be ameliorated with nonpharmacologic interventions (e.g., cognitive behavioral therapy, progressive relaxation techniques, environmental stress reduction). When possible, securing a nanny is helpful to reduce sleep deprivation. Occasional low doses of short-acting benzodiazepines such as temazepam or oxazepam are probably safe (124). Reports of adverse side effects for diazepam and withdrawal with alprazolam suggest that these should not be a first option if a benzodiazepine is administered to a breast-feeding mother. Tricyclics (with the exception of doxepin) or SSRIs are also good options for the treatment of panic disorder in breast-feeding mothers.
Postpartum Psychosis
Postpartum psychotic symptoms should be treated with antipsychotics. Psychotic women may be so dysfunctional that they are unable to breast-feed their infants. In cases of infant exposure to antipsychotics through breast-feeding, infant clinical status should be regularly monitored for antipsychotic side effects such as somnolence, muscle rigidity, or tremors. The reports of developmental decline in infants nursed by mothers receiving antipsychotic combinations suggest that a monotherapeutic regimen should be maintained (81). Clozapine-induced fatal agranulocytosis in adults and the lack of data on infant clinical status make it imprudent for mothers treated with this agent to breast-feed their infants. Of note, conception is possible while breast-feeding, and the method of birth control should be documented during the risk-benefit discussion.
Conclusions
Women with postpartum psychiatric disorders often face the dilemma of whether or not to use psychotropic medication while continuing to breast-feed their infants. In such cases, it is important to safeguard the mental health of the mother while at the same time optimizing the emotional and physical well-being of the infant. All psychotropic agents enter breast milk. While these medications pass into infant circulation to varying degrees, a clear relationship between concentration of these medications (and their active metabolites) on infant physiology, behavior, and development is unknown. Therefore, rather than basing decisions regarding the use of medications during breast-feeding on serum levels, it is prudent to carefully monitor the clinical status of infants who are breast-fed by mothers taking psychiatric medications. In the event that the parents or pediatrician become concerned that the infant’s behavior, activity level, or achievement of developmental milestones may be related to medication exposure, serious consideration should be given to weaning. Studies are needed that relate measured levels of medications and metabolites in the sera of breast-fed infants to clinical status and that carefully note infant age and maternal and infant weights at the time of blood sampling. Accurate assessments may then be made of comparative levels of drug exposure on a per-kilogram basis in infant and mother. Clinical status and behavior of these infants should be carefully observed by using standardized pediatric instruments. In this way, more definitive conclusions may be made about the clinical significance of infant serum concentrations and infant daily exposure.
 |
 |
 |
 |
Received May 30, 2000; revision received Nov. 28, 2000; accepted Dec. 15, 2000. From the Women’s Life Center, UCLA Neuropsychiatric Institute and Hospital; and the Emory University School of Medicine, Atlanta. Address reprint requests to Dr. Burt, Women’s Life Center, UCLA Neuropsychiatric Institute and Hospital, 300 UCLA Medical Plaza, Suite 2337, Los Angeles, CA 90095-6968.
1. American Academy of Pediatrics, Work Group on Breastfeeding: Breastfeeding and the use of human milk. Pediatrics 1997; 100:1035-1039Google Scholar
2. Wilson IT: Determinants and consequences of drug excretion in breast milk. Drug Metab Rev 1983; 14:619-652Crossref, Medline, Google Scholar
3. Chen Y, Yu S, Li WX: Artificial feeding and hospitalization in the first 18 months of life. Pediatrics 1988; 81:58-62Medline, Google Scholar
4. Newton N, Newton M: Physiologic aspects of lactation. N Engl J Med 1967; 277:1179-1188Google Scholar
5. Kendell RG, Chalmers JE, Platz C: Epidemiology of puerperal psychosis. Br J Psychiatry 1987; 150:662-673Crossref, Medline, Google Scholar
6. O’Hara MW: Postpartum “blues,” depression, and psychosis: a review. J Psychosom Obstet Gynaecol 1987; 7:205-227Crossref, Google Scholar
7. Cogill SR, Caplan HL, Alexandra H, Robson KM, Kumar R: Impact of maternal depression on cognitive development of young children. Br Med J 1986; 292:1165-1167Google Scholar
8. Field T, Healy B, Goldstein S, Perry S, Bendall D: Infants of depressed mothers show “depressed” behavior even with nondepressed adults. Child Dev 1988; 59:156-179Crossref, Google Scholar
9. Stein A, Gath D, Bucher J, Bond A, Sa S, Cooper PJ: The relationship between post-natal depression and mother-child interaction. Br J Psychiatry 1991; 158:46-52Crossref, Medline, Google Scholar
10. Dawson G, Klinger LG, Panagiotides H, Hill D, Spieker S: Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child Dev 1992; 63:725-737Crossref, Medline, Google Scholar
11. Murray L: The impact of postnatal depression on infant development. J Child Psychol Psychiatry 1992; 33:543-561Crossref, Medline, Google Scholar
12. Hay DF, Kumar R: Interpreting the effects of mothers’ postnatal depression on children’s intelligence: a critique and re-analysis. Child Psychiatry Hum Dev 1995; 25:165-181Crossref, Medline, Google Scholar
13. Teti DM, Messinger DS, Gelfand DM, Isabella R: Maternal depression and the quality of early attachment: an examination of infants, preschoolers, and their mothers. Dev Psychol 1995; 31:364-376Crossref, Google Scholar
14. Wilson JT, Brown RD, Cherek DR, Dailey JW, Hilman B, Jobe PC, Manno BR, Manno JE, Redetzki HM, Stewart JJ: Drug excretion in human breast milk: principles, pharmacokinetics and projected consequences. Clin Pharmacokinet 1980; 5:1-66Crossref, Medline, Google Scholar
15. Glasier A, McNeilly AS: Physiology of lactation. Baillieres Clin Endocrinol Metab 1990; 4:379-395Crossref, Medline, Google Scholar
16. Kacew S: Adverse effects of drugs and chemicals in breast milk on the nursing infant. J Clin Pharmacol 1993; 33:213-221Crossref, Medline, Google Scholar
17. Morselli PL: Clinical pharmacokinetics in newborns and infants. Clin Pharmacokinet 1980; 5:485-527Crossref, Medline, Google Scholar
18. Buist A, Norman TR, Dennerstein L: Breastfeeding and the use of psychotropic medication: a review. J Affect Disord 1990; 19:197-206Crossref, Medline, Google Scholar
19. Murray L, Segar D: Drug therapy during pregnancy and lactation. Emerg Med Clin North Am 1994; 12:129-149Medline, Google Scholar
20. Nurnberg HG: Breastfeeding and psychotropic agents (letter). Am J Psychiatry 1981; 138:120-121Medline, Google Scholar
21. Mortola J: The use of psychotropic agents in pregnancy and lactation. Psychiatr Clin North Am 1989; 12:69-87Crossref, Medline, Google Scholar
22. Rivera-Calimlin L: The significance of drugs in breast milk: pharmacokinetic considerations. Clin Perinatol 1987; 14:51-70Crossref, Medline, Google Scholar
23. Yoshida K, Smith B, Craggs M, Kumar C: Investigation of pharmacokinetics and of possible adverse effects in infants exposed to tricyclic antidepressants in breast-milk. J Affect Disord 1997; 43:225-237Crossref, Medline, Google Scholar
24. Breyer-Pfaff U, Nill K, Entenmann A, Gaertner HJ: Secretion of amitriptyline and metabolites into breast milk. Am J Psychiatry 1995; 152:812-813Medline, Google Scholar
25. Pittard WB, O’Neal W: Amitriptyline excretion in human milk. J Clin Psychopharmacol 1986; 6:383-384Crossref, Medline, Google Scholar
26. Brixen-Rasmussen L, Halgrener J, Jorgensen A: Amitriptyline and nortriptyline excretion in human breast milk. Psychopharmacology (Berl) 1982; 76:94-95Crossref, Medline, Google Scholar
27. Bader TF, Newman K: Amitriptyline in human breast milk and the nursing infant’s serum. Am J Psychiatry 1980; 137:855-856Link, Google Scholar
28. Erickson SH, Smith GH, Heidrich F: Tricyclics and breast feeding (letter). Am J Psychiatry 1979; 136:1483Link, Google Scholar
29. Wisner KL, Perel JM: Nortriptyline treatment of breast-feeding women (letter). Am J Psychiatry 1996; 153:295Medline, Google Scholar
30. Altshuler L, Burt VK, McMullen M, Hendrick V: Breastfeeding and sertraline: a 24 hour analysis. J Clin Psychiatry 1995; 56:243-245Medline, Google Scholar
31. Wisner KL, Perel JM: Serum nortriptyline levels in nursing mothers and their infants. Am J Psychiatry 1991; 148:1234-1236Google Scholar
32. Matheson I, Skaeraasen J: Milk concentrations of fluphenthixol, nortriptyline and zuclopenthixol and between-breast differences in two patients. Eur J Clin Pharmacol 1988; 35:217-222Crossref, Medline, Google Scholar
33. Sovner R, Orsulak P: Excretion of imipramine and desipramine in human breast milk. Am J Psychiatry 1979; 136:451-452Medline, Google Scholar
34. Stancer HC, Reed KL: Desipramine and 2-hydroxydesipramine in human breast milk and the nursing infant’s serum. Am J Psychiatry 1986; 143:1597-1600Google Scholar
35. Wisner K, Perel J, Foglia J: Serum clomipramine and metabolite levels in four nursing mother-infant pairs. J Clin Psychiatry 1995; 56:17-20Medline, Google Scholar
36. Schimmel MS, Katz EZ, Shaag Y, Pastuszak A, Koren G: Toxic neonatal effects following maternal clomipramine therapy. J Toxicol Clin Toxicol 1991; 29:479-484Crossref, Medline, Google Scholar
37. Birnbaum CS, Cohen LS, Bailey JW, Grush LR, Robertson LM, Stowe ZN: Serum concentrations of antidepressants and benzodiazepines in nursing infants: a case series (electronic article). Pediatrics 1999; 104:e11Google Scholar
38. Matheson I, Pande H, Altersen AR: Respiratory depression caused by N-desmethyldoxepin in breast milk (letter). Lancet 1985; 2:1124Crossref, Medline, Google Scholar
39. Kemp J, Ilett L, Booth J, Hackett L: Excretion of doxepin and N-desmethyldoxepin in human milk. Br J Clin Pharmacol 1985; 20:497-499Crossref, Medline, Google Scholar
40. Lester B, Cucca J, Lynne A, Flanagan P, Oh W: Possible association between fluoxetine hydrochloride and colic in an infant. J Am Acad Child Adolesc Psychiatry 1993; 32:1253-1255Google Scholar
41. Burch K, Wells B: Fluoxetine/norfluoxetine concentrations in human milk. Pediatrics 1992; 89:676-677Medline, Google Scholar
42. Isenberg KE: Excretion of fluoxetine in human breast milk (letter). J Clin Psychiatry 1990; 51:169Medline, Google Scholar
43. Taddio A, Ito S, Koren G: Excretion of fluoxetine and its metabolite norfluoxetine, in human breast milk. J Clin Pharmacol 1996; 36:42-47Crossref, Medline, Google Scholar
44. Yoshida K, Smith B, Craggs M, Kumar C: Fluoxetine in breast-milk and developmental outcome of breast-fed infants. Br J Psychiatry 1998; 172:175-178Crossref, Medline, Google Scholar
45. Moretti M, Sharma A, Bar-Oz B, Koren G, Ito S: Fluoxetine and its effects on the nursing infant: a prospective cohort study (abstract). Clin Pharmacol Ther 1989; 65:141Crossref, Google Scholar
46. Kristensen JH, Ilett KI, Hacketty LP, Yapp P, Paech M, Begg EJ: Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol 1999; 48:521-527Crossref, Medline, Google Scholar
47. Brent N, Wisner K: Fluoxetine and carbamazepine concentrations in a nursing mother/infant pair. Clin Pediatr 1998; 37:41-44Crossref, Medline, Google Scholar
48. Chambers CD, Anderson PO, Thomas RG, Dick LM, Felix RJ, Johnson KA, Jones KL: Weight gain in infants breastfed by mothers who take fluoxetine (electronic article). Pediatrics 1999; 105:e61Google Scholar
49. Cohen LS, Stowe Z: Mood and psychotic disorders in women during the childbearing years: an update on treatment, in 1999 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 1999, pp 260-261Google Scholar
50. Wright S, Dawling S, Ashford JJ: Excretion of fluvoxamine in breast milk (letter). Br J Clin Pharmacol 1991; 31:209Crossref, Medline, Google Scholar
51. Yoshida K, Smith B, Kumar RC: Fluvoxamine in breast-milk and infant development (letter). Br J Clin Pharmacol 1997; 44:210-211Crossref, Medline, Google Scholar
52. Stowe ZN, Cohen LS, Hostetter A, Ritchie JC, Owens MJ, Nemeroff CB: Paroxetine in human breast milk and nursing infants. Am J Psychiatry 2000; 157:185-189Link, Google Scholar
53. Spigset O, Carleborg L, Norstrom A, Sandlund M: Paroxetine level in breast milk (letter). J Clin Psychiatry 1996; 57:39Medline, Google Scholar
54. Ohman R, Staffan H, Carleborg L, Spigset O: Excretion of paroxetine into breast milk. J Clin Psychiatry 1999; 60:519-523Crossref, Medline, Google Scholar
55. Begg EJ, Duffull SB, Saunders DA, Buttimore RC, Ilett KF, Hackett LP, Yapp P, Wilson DA: Paroxetine in human milk. Br J Clin Pharmacol 1999; 48:142-147Crossref, Medline, Google Scholar
56. Hendrick C, Stowe ZN, Altshuler LL, Hostetter A, Fukuchi A: Paroxetine use during breast-feeding. J Clin Psychopharmacol 2000; 20:587-588Crossref, Medline, Google Scholar
57. Stowe ZN, Owens MJ, Landry JC, Kilts CD, Ely T, Llewellyn A, Nemeroff CB: Sertraline and desmethylsertraline in human breast milk and nursing infants. Am J Psychiatry 1997; 154:1255-1260Google Scholar
58. Mammen OK, Perel JM, Rudolph G, Foglia JP, Wheeler SB: Sertraline and norsertraline levels in three breastfed infants. J Clin Psychiatry 1997; 58:100-103Crossref, Medline, Google Scholar
59. Kristensen J, Ilett K, Dusci L, Paech M: Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol 1998; 45:453-457Crossref, Medline, Google Scholar
60. Schmidt K, Oleson OV, Jensen PN: Citalopram and breast-feeding: serum concentration and side effects in the infant. Biol Psychiatry 1999; 47:164-165Crossref, Google Scholar
61. Jensen P, Olesen O, Bertelsen A, Linnet K: Citalopram and desmethylcitalopram concentrations in breast milk and in serum of mother and infant. Ther Drug Monit 1997; 19:236-239Crossref, Medline, Google Scholar
62. Spigset O, Carleborg L, Ohman R, Norstrom A: Excretion of citalopram in breast milk. Br J Clin Pharmacol 1997; 44:236-239Google Scholar
63. Briggs G, Samson J, Ambrose P, Schroeder D: Excretion of bupropion in breast milk. Ann Pharmacother 1993; 27:431-433Crossref, Medline, Google Scholar
64. Buist A, Norman TR, Dennerstein L: Mianserin in breast milk (letter). Br J Clin Pharmacol 1993; 36:133-134Crossref, Medline, Google Scholar
65. Ilett KF, Hackett LP, Dusci LJ, Roberts MJ, Kristensen JH, Paech M, Groves A, Yapp P: Distribution and excretion of venlafaxine and O-desmethylvenlafaxine in human milk. Br J Clin Pharmacol 1998; 45:459-462Crossref, Medline, Google Scholar
66. Verbeeck RK, Ross SG, McKenna EA: Excretion of trazodone in breast milk. Br J Clin Pharmacol 1986; 22:367-370Crossref, Medline, Google Scholar
67. Pons G, Schoerlin M, Tam Y, Moran C, Pfefen JH, Francoual C, Pedarriosse A, Chavinie J, Olive G: Moclobemide excretion in human breast milk. Br J Clin Pharmacol 1990; 29:27-31Crossref, Medline, Google Scholar
68. Oo CY, Kuhn RJ, Desai N, Wright CE, McNamara PJ: Pharmacokinetics in lactating women: prediction of alprazolam transfer into milk. Br J Clin Pharmacol 1995; 40:231-236Crossref, Medline, Google Scholar
69. Anderson P, McGuire G: Neonatal alprazolam withdrawal—possible effects of breast feeding (letter). DICP 1989; 23:614Medline, Google Scholar
70. Soderman P, Matheson I: Clonazepam in breast milk. Eur J Pediatr 1988; 147:212-213Crossref, Medline, Google Scholar
71. Fisher JB, Edgren BE, Mammel MC, Coleman JM: Neonatal apnea associated with maternal clonazepam therapy: a case report. Obstet Gynecol 1985; 88:345-355Google Scholar
72. Dusci LJ, Good SM, Hall RW, Ilett KF: Excretion of diazepam and its metabolites in human milk during withdrawal from combination high dose diazepam and oxazepam. Br J Clin Pharmacol 1990; 29:123-126Crossref, Medline, Google Scholar
73. Wesson DR, Camber S, Harkey M, Smith DE: Diazepam and desmethyldiazepam in breast milk. J Psychoactive Drugs 1985; 17:55-56Crossref, Medline, Google Scholar
74. Brandt R: Passage of diazepam and desmethyldiazepam into breast milk. Arzneimittelforschung 1976; 26:454-457Medline, Google Scholar
75. Patrick MJ, Tilstone WJ, Reavey P: Diazepam and breastfeeding. Lancet 1972; 1:542-543Crossref, Medline, Google Scholar
76. Erkkola R, Kanto J: Diazepam and breast-feeding. Lancet 1972; 1:1235-1236Google Scholar
77. Summerfield RJ, Nielsen MS: Excretion of lorazepam into breast milk. Br J Anaesth 1985; 57:1042-1043Google Scholar
78. Wretland M: Excretion of oxazepam in breast milk. Eur J Clin Pharmacol 1987; 33:209-210Crossref, Medline, Google Scholar
79. Lebedevs TH, Wojnar-Horton RE, Yapp P, Roberts MJ, Dusci LJ, Hackett LP, Ilett KF: Excretion of temazepam in breast milk. Br J Clin Pharmacol 1992; 33:204-205Crossref, Medline, Google Scholar
80. Pons G, Francoual C, Guillet P, Moran C, Hermann P, Bianchetti G, Thurcelin JF, Thenot JP, Olive G: Zolpidem excretion in breast milk. Eur J Clin Pharmacol 1989; 37:245-248Crossref, Medline, Google Scholar
81. Yoshida K, Smith B, Craggs M, Kumar R: Neuroleptic drugs in breast-milk: a study of pharmacokinetics and of possible adverse effects in breast-fed infants. Psychol Med 1998; 28:81-91Crossref, Medline, Google Scholar
82. Blacker KH, Weinstein BJ, Ellman G: Mother’s milk and chlorpromazine. Am J Psychiatry 1962; 119:178-179Link, Google Scholar
83. Wiles DH, Orr MW, Kolakowska T: Chlorpromazine levels in plasma and milk of nursing mothers (letter). Br J Clin Pharmacol 1978; 5:272-273Crossref, Medline, Google Scholar
84. Kris E, Carmichael D: Chlorpromazine maintenance therapy during pregnancy and confinement. Psychiatry 1957; 31:690-695Google Scholar
85. Matheson I, Evang A, Overo F, Syverseon G: Presence of chlorprothixene and its metabolites in breast milk. Eur J Clin Pharmacol 1984; 27:611-613Crossref, Medline, Google Scholar
86. Oleson OV, Bartels U, Poulsen JH: Perphenazine in breast milk and serum (letter). Am J Psychiatry 1990; 147:1378-1379Google Scholar
87. Whalley LJ, Blain PG, Prime JK: Haloperidol secreted in breast milk. Br Med J 1981; 282:1746-1747Google Scholar
88. Stewart RB, Karas B, Springer PK: Haloperidol excretion in human milk (letter). Am J Psychiatry 1980; 137:849-850Link, Google Scholar
89. Barnas C, Bergant A, Hummer M, Saria A, Fleischhacker WW: Clozapine concentrations in maternal and fetal plasma, amniotic fluid, and breast milk (letter). Am J Psychiatry 1994; 151:945Link, Google Scholar
90. Goldstein DJ, Corbin LA, Fung MC: Olanzapine-exposed pregnancies and lactation: early experience. J Clin Psychopharmacol 2000; 20:399-403Crossref, Medline, Google Scholar
91. Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ: Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179-184Link, Google Scholar
92. Wisner KL, Perel JM: Serum levels of valproate and carbamazepine in breastfeeding mother-infant pairs. J Clin Psychopharmacol 1998; 18:167-169Crossref, Medline, Google Scholar
93. Merlob P, Mor M, Litwin A: Transient hepatic dysfunction in an infant of an epileptic mother treated with carbamazepine during pregnancy and breastfeeding. Ann Pharmacother 1992; 26:1563-1565Google Scholar
94. Frey B, Schubiger G, Musy JP: Transient cholestatic hepatitis in a neonate associated with carbamazepine exposure during pregnancy and breast-feeding. Eur J Pediatr 1990; 150:136-138Crossref, Medline, Google Scholar
95. Froescher W, Eichelbaum M, Niesen M, Dietrich K, Rausch P: Carbamazepine levels in breast milk. Ther Drug Monit 1984; 6:266-271Crossref, Medline, Google Scholar
96. Pynnonen S, Kanto J, Sillanpaa M, Erkkola R: Carbamazepine: placental transport, tissue concentrations in foetus and newborn, and level in milk. Acta Pharmacol Toxicol (Copenh) 1977; 41:244-253Crossref, Medline, Google Scholar
97. Pynnonen S, Sillanpaa M: Carbamazepine and mother’s milk (letter). Lancet 1975; 2:563Crossref, Medline, Google Scholar
98. Niebyl J, Blake D, Freeman J, Loff AD: Carbamazepine levels in pregnancy and lactation. Obstet Gynecol 1979; 53:30-40Google Scholar
99. Kok TH, Taitz LS, Bennett MJ, Holt DW: Drowsiness due to clemastine transmitted in breast milk. Lancet 1982; 1:914-915Crossref, Medline, Google Scholar
100. Kuhnz W, Jager-Romun E, Rating D, Deichel A, Kunze J, Holge H, Nau H: Carbamazepine and carbamazepine-10,11-epoxide during pregnancy and postnatal period in epileptic mothers and their nursed infants: pharmacokinetics and clinical effects. Pediatr Pharmacol 1983; 3:199-208Medline, Google Scholar
101. Von Unruh GE, Froescher W, Hoffman F, Niesen M: Valproic acid in breast milk: how much is really there? Ther Drug Monit 1984; 6:272-276Crossref, Medline, Google Scholar
102. Alexander FW: Sodium valproate and pregnancy. Arch Dis Child 1979; 54:240-245Crossref, Medline, Google Scholar
103. Dickinson RG, Harland RC, Lynn RK, Smith WB, Gerber N: Transmission of valproic acid (Depakene) across the placenta: half-life of the drug in mother and baby. J Pediatr 1979; 94:832-835Crossref, Medline, Google Scholar
104. Nau H, Rating D, Koch S, Hauser I, Helge H: Valproic acid and its metabolites: placental transfer, neonatal pharmacokinetics, transfer via mother’s milk and clinical status in neonates of epileptic mothers. J Pharmacol Exp Ther 1981; 219:768-777Medline, Google Scholar
105. Tsuru N, Maeda T, Tsuruoka M: Three cases of delivery under sodium valproate—placental transfer, milk transfer and probable teratogenicity of sodium valproate. Jpn J Psychiatry Neurol 1988; 42:89-96Medline, Google Scholar
106. Stahl MM, Neiderud J, Vinge E: Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr 1997; 130:1001-1003Google Scholar
107. Bardy AH, Granstrrom ML, Hiilesmaa VK: Valproic acid and breast-feeding, in Epilepsy, Pregnancy and the Child. Edited by Janz D, Dam M, Richens A. New York, Raven Press, 1982, pp 359-360Google Scholar
108. Philbert A, Pedersen B, Dam M: Concentration of valproate during pregnancy, in the newborn and in breast milk. Acta Neurol Scand 1985; 72:460-463Crossref, Medline, Google Scholar
109. Piontek CM, Baab S, Peindl KS, Wisner KL: Serum valproate levels in 6 breastfeeding mother-infant pairs. J Clin Psychiatry 2000; 61:170-172Crossref, Medline, Google Scholar
110. Sykes PA, Quarrie J, Alexander FW: Lithium carbonate and breast-feeding. Br Med J 1976; 2:1299Crossref, Medline, Google Scholar
111. Schou M, Amdisen A: Lithium and pregnancy, III: lithium ingestion by children breastfed by women on lithium treatment. Br Med J 1973; 2:138Crossref, Medline, Google Scholar
112. Tunnessen WW, Hert CG: Toxic effects of lithium in newborn infants: a commentary. J Pediatr 1972; 81:804-807Crossref, Medline, Google Scholar
113. Weinstein MR, Goldfield M: Lithium carbonate treatment during pregnancy. Dis Nerv Syst 1969; 30:828-832Medline, Google Scholar
114. Fries H: Lithium in pregnancy. Lancet 1970; 1:1233Crossref, Medline, Google Scholar
115. Skausig OB, Schou M: [Breast-feeding during lithium therapy.] Ugeskr Laeger 1977; 139:400-401 (Danish)Medline, Google Scholar
116. Tomson T, Ohman I, Vitols S: Lamotrigine in pregnancy and lactation: a case report. Epilepsia 1997; 38:1039-1041Google Scholar
117. Rambeck B, Kurlemann G, Stodieck S, May T, Jurgens U: Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol 1997; 51:481-484Crossref, Medline, Google Scholar
118. Ohman I, Tomson T, Vitols S: Lamotrigine levels in plasma and breast milk in nursing women and their infants (abstract). Epilepsia 1998; 39(suppl 2):21Google Scholar
119. Wisner KL, Perel JM, Findling RL: Antidepressant treatment during breast-feeding. Am J Psychiatry 1996; 153:1132-1137Google Scholar
120. Epperson CN, Anderson GM, McDougle CJ: Sertraline and breast-feeding. N Engl J Med 1997; 336:1189-1190Google Scholar
121. Dreifuss FE, Santilli N, Langer DH, Sweeney KP, Moline KA, Menander KB: Valproic acid hepatic fatalities: a retrospective review. Neurology 1987; 37:379-385Crossref, Medline, Google Scholar
122. Trimble MR: Anticonvulsants in children and adolescents. J Child Adolesc Psychopharmacol 1990; 1:107-124Crossref, Medline, Google Scholar
123. Ananth J: Side effects in the neonate from psychotropic agents excreted through breast-feeding. Am J Psychiatry 1978; 135:801-805Link, Google Scholar
124. American Academy of Pediatrics Committee on Drugs: Transfer of drugs and other chemicals into human milk. Pediatrics 1989; 84:924-936Medline, Google Scholar



