Genetic Risk, Number of Previous Depressive Episodes, and Stressful Life Events in Predicting Onset of Major Depression
Abstract
OBJECTIVE: The association between stressful life events and the onset of major depression decreases as the number of previous depressive episodes increases. How do genetic risk factors for major depression impact on this “kindling” phenomenon? In particular, do those at high genetic risk exhibit an increase in the speed of kindling, or are they “prekindled”? METHOD: Using discrete-time survival analysis, the authors examined the interaction between genetic risk, number of previous depressive episodes, and life event exposure in the prediction of episodes of major depression in female-female twin pairs from a population-based registry. The twins were interviewed four times over a 9-year period, producing 92,521 person-months of exposure. RESULTS: The decline in the association between stressful life events and risk for major depression as the number of previous depressive episodes increased was strongest in those at low genetic risk and was weak to absent in those at high genetic risk. In the absence of previous depressive episodes, those at high genetic risk frequently experienced depressive episodes without major environmental stressors. CONCLUSIONS: Genetic risk factors for depression produce a “prekindling” effect rather than increase the speed of kindling. The “kindled” state, wherein depressive episodes occur with little provocation, may be reached by two pathways: many previous depressive episodes, perhaps driven by multiple adversities, and high genetic risk.
Multiple previous reports have examined the impact of previous depressive episodes on the association between stressful life events and the onset of major depression (1–7); all have found that the proportion of individuals for whom stressful life events preceded their current episode of major depression was greater in those experiencing a first as opposed to a recurrent episode. This pattern of depressive episode onsets becoming more autonomous and progressively less linked to environmental adversity has been termed the “kindling hypothesis” (8).
We recently reexamined this phenomenon in a large, longitudinal population-based twin study. Upon ruling out several potential methodological artifacts, we showed that the association between stressful life events and onset of major depression became progressively weaker as the number of previous depressive episodes increased from zero to nine (9). Some further attenuation of the relationship was evident with additional previous depressive episodes, but the change was small.
Given this consistent evidence in support of the kindling hypothesis and the demonstrated importance of genetic factors in the etiology of major depression (10, 11), it is of considerable interest to clarify how genetic risk factors for major depression impact on the kindling process. Although there are many possible models for the interaction between genetic risk, previous depressive episodes, and stressful life events in the etiology of major depression, we would suggest that three hypotheses are particularly plausible. The first predicts that while genes impact on the overall risk for major depression, they do not influence the nature of the kindling process (no-effect model). A second hypothesis, the “speed-of-kindling model,” predicts that all individuals begin with a similar degree of association between environmental adversity and risk of depressive onset but that the speed with which kindling occurs (i.e., the increasing disassociation between stressful life events and depressive onsets with repeated depressive episodes) is positively correlated with genetic risk. Thus, those at high genetic risk are “rapid kindlers,” since high genetic liability partly manifests itself by increasing the speed with which the mind/brain learns to become depressed autonomously.
The third hypothesis, which we will refer to as the “prekindling model,” suggests that the initial strength of the association between environmental adversity and risk for major depression is a function of genetic risk. Those at low genetic risk have little propensity to develop spontaneous depressive episodes and therefore demonstrate a strong association between stressful life events and major depression. By contrast, those at high genetic risk would begin life “prekindled,” in that without previous experience with depression, they would nonetheless have a predilection to develop spontaneous depressive episodes. This model predicts two pathways to being at high risk for autonomous depressive episodes: multiple previous depressive episodes or a high level of genetic risk.
As illustrated in Figure 1, the speed-of-kindling and prekindling models predict opposite patterns of findings. In particular, the change in the relationship between stressful life events and onset of major depression as the number of previous depressive episodes increases will, for the speed-of-kindling model, be greatest among those at highest genetic risk. By contrast, the prekindling model predicts that changes in this relationship as the number of previous depressive episodes increases will be greatest among those with the lowest genetic risk.
Method
The sample and statistical methods employed in this study have been outlined in detail previously (9). In brief, subjects were members of Caucasian female-female twin pairs born during the years 1934–1974 and ascertained from the population-based Virginia Twin Registry. The total sample of 2,395 individual twins (of whom we had information about the lifetime history of major depression in their co-twin for 2,245) were interviewed up to four times between 1988 and 1997. Signed informed consent was obtained before all face-to-face interviews and verbal assent before all telephone interviews. The mean ages of the sample at the first and fourth assessment waves were 29.3 (SD=7.7) and 36.3 (SD=8.3) years, respectively. The subjects had a mean of 13.5 (SD=2.0) years of education. Subject attrition across waves ranged from 8% to 15% (12). All assessments were separated by at least 13 months.
The history of major depression in the year preceding each assessment was determined by structured psychiatric interview based on the Structured Clinical Interview for DSM-III-R (13, 14). As outlined previously (9), respondents were asked to date the onset and offset of each episode to the nearest month. DSM-III-R diagnoses were assigned by computer algorithm. At waves 1, 3, and 4, we also assessed the lifetime history of major depression before the last year, including total number of previous depressive episodes. We did not exclude the small number of patients with bipolar illness, since previous analyses showed that their exclusion had no impact on the observed patterns of kindling (9). We developed an algorithm for continuously updating the number of previous depressive episodes across the four waves (see reference 9 for details). In sections of the personal interview preceding those evaluating major depression, we assessed the occurrence over the last year of 11 “personal” events (i.e., events that occurred primarily to the informant [15, 16]) and four classes of “network” events (i.e., events that occurred primarily to, or in interaction with, an individual in the respondent’s close social network). The interviewer and respondent, referring to a calendar if necessary, then dated these events to the nearest month.
We examined the changing association between stressful life events and onset of major depression as a function of previous depressive episodes and genetic risk using an event history analysis with a discrete-time approach (17, 18). The dependent variable in these analyses is the “person-month.” As in other survival-like analytic methods, multiple person-months from the same individual, although clearly nonindependent, are included in the analysis. At each interview wave, we assessed both depressive onsets and the occurrence of stressful life events in the month of the interview and the 12 preceding months. Therefore, for each person-month in our data, we knew which, if any, stressful life events occurred and whether an episode of major depression started or ended in that month. Each observation record also included three covariates: age, the hazard rate for major depression for that month (to account for the fact that episodes of major depression were not entirely evenly distributed over the last year), and genetic risk for major depression (15). For genetic risk, twins were divided into the following four groups: lowest risk (monozygotic twin [N=927] whose co-twin had no lifetime history of major depression); low risk (dizygotic twin [N=592] whose co-twin had no lifetime history of major depression); high risk (dizygotic twin [N=325] whose co-twin had a lifetime history of major depression); and highest risk (monozygotic twin [N=401] whose co-twin had a lifetime history of major depression). The proportions of these four groups that reported one or more depressive episodes in the year before any of our interviews were 13.5%, 24.3%, 28.3%, and 37.2%, respectively.
When a twin experienced an episode of major depression, she was censored from the sample until recovery, at which time she reentered the sample with the number of previous depressive episodes increased by one. The odds ratio for major depression given stressful life events is calculated from the logistic regression coefficient controlling for all the aforementioned covariates. In these analyses, we examined only the onset of major depression in the month of event occurrence, since most of the depressogenic impact of stressful life events in these data occurred shortly after the stressful life event (15, 16). Two-tailed p values are reported.
Our major interest was in examining the interaction between genetic risk, stressful life events, and previous depressive episodes in the prediction of onset of major depression. To assess this correctly, however, we had to properly specify the relationship between previous depressive episodes and risk for major depression. As outlined previously (9), the association between previous number of depressive episodes and the pathogenic impact of stressful life events on onset of major depression was clearly biphasic. Through nine episodes, the association between stressful life event and risk for major depression progressively declined but was largely unchanged with further episodes. We originally modeled this relationship using piece-wise logistic regression so that we could examine the entire range of previous depressive episodes. In this study, we restrict our analyses to the approximately 95% of our sample of person-months with up to nine previous depressive episodes (a total of 92,521 person-months) because the strong kindling effect was seen in this range of previous depressive episodes.
Results
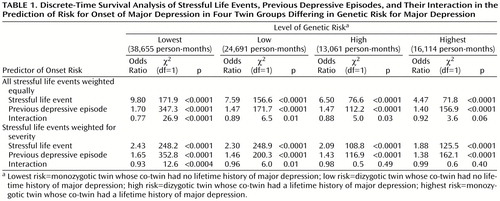
Our first analyses modeled event exposure as the presence or absence of one or more stressful life events in any given month. Across all genetic risk groups, both stressful life events and previous depressive episodes robustly predicted the risk for onset of major depression (Table 1). It is interesting to note that the impact of both of these main effects was attenuated in the high- versus low-risk groups. The interaction between previous depressive episodes and stressful life events in the prediction of risk for onset of major depression was significant in the lowest, low, and high genetic risk groups but fell short of significance in the group at highest risk. In the lowest genetic risk group, the strength of the association between the occurrence of a stressful life event and an onset of major depression declined 23% with each new previous depressive episode. In the low- and high-risk groups, the parallel figure was approximately 12%. In the highest-risk group, the strength of the association between stressful life events and onset of major depression declined only 8% with each additional previous depressive episode.
In addition to running these analyses separately for the four twin groups, we ran the entire data set in a single analysis that included main effects for stressful life event, previous depressive episodes, and genetic risk, all the appropriate two-way interactions, and the key three-way interaction. The three-way interaction (stressful life event by previous depressive episode by genetic risk) was significant (odds ratio=1.07, χ2=4.9, df=1, p=0.03), meaning that the interactions between stressful life event and previous depressive episode differed significantly across levels of genetic risk.
A limitation of these analyses was that we treated all stressful life events as equivalent despite previous evidence that they differ substantially in the strength of their association with major depression (16). Furthermore, the mean severity of reported stressful life events was significantly and positively related to level of genetic risk (t=4.1, df=2,098, p=0.009). Therefore, we repeated these analyses correcting for event severity (16) (Table 1). This was done by assigning each event category a weight that reflected the strength of the association, from our data, between occurrence of that event and the onset of major depression (16). The pattern of results was similar, with the magnitude of the stressful life event and previous depressive episode interaction declining monotonically as the level of genetic risk increased. In the analysis of the entire sample, the stressful life event by previous depressive episode by genetic risk interaction remained statistically significant (odds ratio=1.03, χ2=5.6, df=1, p=0.02).
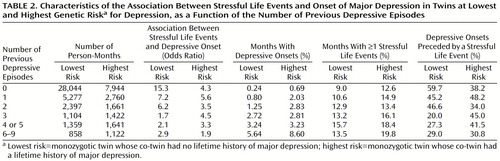
In an effort to understand these results in greater depth, we examined in more detail results from our two most contrasting groups—those at lowest and highest genetic risk (Table 2). Four results are noteworthy. First, as predicted by the prekindling model, among those with no previous depressive episodes, the association between stressful life event occurrence and depressive onset was much stronger in the group at the lowest genetic risk. Furthermore, the changes in the odds ratio for risk of depressive onset after any stressful life event as the number of previous depressive episodes increased differed greatly in the two groups. Whereas the association fell dramatically as the number of previous depressive episodes increased for those at lowest genetic risk, in the group at highest genetic risk it declined modestly if at all. Almost the entire decline in the association between stressful life events and onset of major depression occurred by the third episode of illness in those at lowest genetic risk.
Second, the monthly risk of a depressive onset increased in both groups as the number of previous depressive episodes increased. However, this increase was proportionally greater in those at lowest genetic risk. Put another way, recurrence had a greater impact on risk for major depression in those at low versus those at high genetic risk. Third, the frequency of stressful life events was consistently greater in those at highest versus those at lowest genetic risk. We examined this relationship in the entire person-month sample and found genetic risk to be a strong predictor of risk for event occurrence (odds ratio=1.35, χ2=592.0, df=1, p<0.0001). However, as the number of previous depressive episodes increased, the increase in number of stressful life events was considerably slower than the increase in risk for depressive episodes. Fourth, a first depressive onset in those with no previous depressive episodes was associated with a stressful life event occurrence in 59.7% of those at lowest genetic risk but only 38.2% of those at highest genetic risk. However, in those with three or more previous depressive episodes, the picture was quite different. The proportion of depressive episodes that were event related fell considerably in those at lowest genetic risk but changed little in those at highest risk.
Discussion
We sought in these analyses to clarify the interaction between three important risk factors for major depression: stressful life events, previous depressive episodes, and genetic risk. In particular, we wondered how genetic factors impacted on the pattern of “kindling” in which the association between stressful life events and onset of major depression declines in magnitude as the number of previous depressive episodes increases. We suggested three plausible models. Our results discriminated clearly between them. We could reject the no-effect model because the pattern of kindling differed substantially between groups with varying levels of genetic risk. Our data were also inconsistent with the speed-of-kindling model. The decline in the association between stressful life events and onset of major depression as the number of previous depressive episodes increased was not more rapid in those at highest genetic risk as would be predicted by this model. By contrast, our results were consistent with the prekindling model. Among individuals with no previous depressive episodes, those at highest genetic risk had a considerably weaker association between stressful life events and major depression than did those at low genetic risk. With additional episodes, the magnitude of the association between stressful life events and onset of major depression declined quickly in those at lowest genetic risk and hardly changed in those at highest risk. Consistent with the prekindling model, increasing genetic risk shifted the curve downward. In an individual at high genetic risk with no previous episodes, the association between stressful life events and onset of major depression was similar to that seen in an individual at low genetic risk who had had three previous depressive episodes.
These results suggest that there are potentially distinct environmental and genetic pathways to the “kindled” or “sensitized” state in which the mind/brain is predisposed to spontaneous depressive episodes. In the environmental pathway, an individual at low genetic risk may be exposed to a series of psychosocial adversities that precipitate a number of depressive episodes. The experience of these episodes reduces the threshold for the individual’s mind/brain to enter into the depressive state sufficiently that episodes can occur with little or no environmental precipitant. Alternatively, a similar “kindled” state may be reached through the genetic pathway, without the necessity for previous environmental exposures, by inheriting high levels of risk.
We have previously outlined several potential methodologic limitations with the methods used in this and our previous report (9), and here review four. First, these results are restricted to female Caucasians and may or may not extrapolate to male subjects or other ethnic groups. Second, this study contained no direct measure of “kindling.” Instead, we observed a decline, as the number of depressive episodes increased, in the strength of the association between reports of the occurrence of stressful life events and the onset of depressive episodes. We have inferred that this change reflects an increasing propensity either for spontaneous depressive episodes or for episodes precipitated by stressors too minor or too idiosyncratic to be contained in our extensive inventory of stressful life events. Our study provides no information about whether this phenomenon is best conceptualized as arising from the brain (i.e., declining thresholds for activation of mood centers) or the mind (i.e., learning to be depressed with increasing self-derogatory cognitive biases). Third, discrete-time survival analysis treats each individual person-month as an independent observation. Allison (17, 19) has shown that, under the assumption of independence of the individual observations, given one “failure” event per subject (i.e., a depressive onset), such a model produces the true maximum-likelihood estimators. However, some of our subjects reported multiple depressive onsets over the assessment periods. Any residual violations of this assumption will result in standard error estimates that are downward biased. However, given our sample size and the fact that by including previous depressive episodes as a covariate we accounted for the major source of nonindependence of individual person-months, the magnitude of bias was likely to be minimal. Fourth, our definition of genetic risk relied on the presence or absence of a single depressive episode in the co-twin. Since this may be a low threshold, we repeated the analyses using severity of stressful life events, but defining co-twins as positive only if they reported at least three lifetime episodes of major depression (20). The results were nearly identical to those presented in Table 1 with, for example, the odds ratios for the interaction between the severity of stressful life events and previous depressive episode in the prediction of major depression in the four genetic risk groups being estimated at 0.94, 0.96, 0.97, and 0.99, respectively.
 |
 |
Received March 20, 2000; revision received Sept. 19, 2000; accepted Nov. 13, 2000. From the Departments of Psychiatry and Human Genetics, Medical College of Virginia, Virginia Commonwealth University, Richmond; and the Virginia Institute for Psychiatric and Behavioral Genetics. Address reprint requests to Dr. Kendler, Virginia Institute for Psychiatric and Behavioral Genetics, P.O. Box 980126, Richmond, VA 23298-0126. Supported by grants from NIMH (MH-40828), the National Institute on Alcohol Abuse and Alcoholism (AA-09095), and a Research Scientist Award (MH-01277) to Dr. Kendler. The Mid-Atlantic Twin Registry, directed by Drs. L. Corey and L. Murrelle, has received support from NIH, the Carman Trust, and the W.M. Keck, John Templeton, and Robert Wood Johnson Foundations. The authors acknowledge the contributions of the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry, in ascertainment of subjects for this study and Patrick Sullivan, M.D., who provided advice during the preparation of this article.
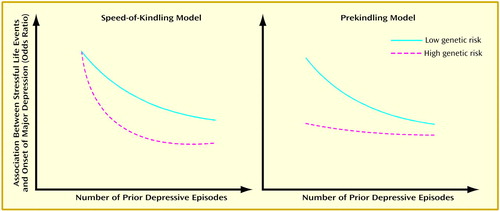
Figure 1. Two Hypotheses of How Genetic Risk Influences the Reduced Association Between Stressful Life Events and Onset of Major Depression as the Number of Prior Episodes Increases (Kindling Effect)
1. Perris H: Life events and depression, part 2: results in diagnostic subgroups, and in relation to the recurrence of depression. J Affect Disord 1984; 7:25–36Crossref, Medline, Google Scholar
2. Dolan RJ, Calloway SP, Fonagy P, De Souza FVA, Wakeling A: Life events, depression and hypothalamic-pituitary-adrenal axis function. Br J Psychiatry 1985; 147:429–433Crossref, Medline, Google Scholar
3. Ezquiaga E, Gutierrez JLA, Lopez AG: Psychosocial factors and episode number in depression. J Affect Disord 1987; 12:135–138Crossref, Medline, Google Scholar
4. Cassano GB, Akiskal HS, Musetti L, Perugi G, Soriani A, Mignani V: Psychopathology, temperament, and past course in primary major depressions. Psychopathology 1989; 22:278–288Crossref, Medline, Google Scholar
5. Ghaziuddin M, Ghaziuddin N, Stein GS: Life events and the recurrence of depression. Can J Psychiatry 1990; 35:239–242Crossref, Medline, Google Scholar
6. Brown GW, Harris TO, Hepworth C: Life events and endogenous depression. Arch Gen Psychiatry 1994; 51:525–534Crossref, Medline, Google Scholar
7. Bruce ML: Divorce and psychopathology, in Adversity, Stress, and Psychopathology. Edited by Dohrenwend BP. New York, Oxford University Press, 1997, pp 219–234Google Scholar
8. Post RM: Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992; 149:999–1010Google Scholar
9. Kendler KS, Thornton LM, Gardner CO: Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry 2000; 157:1243–1251Google Scholar
10. Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
11. Sullivan PF, Neale MC, Kendler KS: Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157:1552–1562Google Scholar
12. Kendler KS, Prescott CA: A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry 1999; 56:39–44Crossref, Medline, Google Scholar
13. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A population-based twin study of major depression in women: the impact of varying definitions of illness. Arch Gen Psychiatry 1992; 49:257–266Crossref, Medline, Google Scholar
14. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1985Google Scholar
15. Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ: Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry 1995; 152:833–842Link, Google Scholar
16. Kendler KS, Karkowski L, Prescott CA: Stressful life events and major depression: risk period, long-term contextual threat and diagnostic specificity. J Nerv Ment Dis 1998; 186:661–669Crossref, Medline, Google Scholar
17. Allison PD: Discrete-time methods for the analysis of event histories, in Sociological Methodology. Edited by Leinhardt S. San Francisco, Jossey-Bass, 1982, pp 61–98Google Scholar
18. Laird N, Olivier D: Covariance analysis of censored survival data using log-linear analysis techniques. J Am Statistical Assoc 1981; 76:231–240Crossref, Google Scholar
19. Allison PD: Survival Analysis Using the SAS System: A Practical Guide, 2nd ed. Cary, NC, SAS Institute, 1995Google Scholar
20. Kendler KS, Gardner CO, Prescott CA: Clinical characteristics of major depression that predict risk of depression in relatives. Arch Gen Psychiatry 1999; 56:322–327Crossref, Medline, Google Scholar



