Right Frontal Hypergyria Differentiation in Affected and Unaffected Siblings From Families Multiply Affected With Schizophrenia: A Morphometric MRI Study
Abstract
OBJECTIVE: The authors used magnetic resonance imaging to corroborate the postmortem finding of right frontal hypergyria associated with schizophrenia. METHOD: Twelve affected-unaffected sibling pairs from families multiply affected with schizophrenia were studied. Bilateral measurement of the gyrification index, the ratio of the inner and outer surface contours, was performed on three different slices of the prefrontal region. RESULTS: The mean gyrification index on the right side was significantly higher in siblings with schizophrenia or schizoaffective disorder than in the unaffected siblings. CONCLUSIONS: In this family cohort study, the postmortem finding of right-sided hypergyria in subjects with schizophrenia was replicated in vivo with magnetic resonance imaging. This observation provides further support for a neurodevelopmental mechanism in the pathogenesis of schizophrenia.
Structural brain abnormalities in the prefrontal region of patients with schizophrenia may contribute to a dysconnectivity syndrome in frontotemporolimbic circuits (1, 2). With respect to neurodevelopment, formation of the prefrontal region gyral surface begins at mid-gestation (3). The gyrification index can be calculated as the ratio of inner to outer perimeter of the gyral surface on a coronal brain slice (4). The gyrification index increases during prenatal development, is maximal during childhood, and declines during adolescence to a stable adult value (5). We recently reported in a postmortem study significant right-sided prefrontal region hypergyria associated with schizophrenia (6). The aim of the present family cohort study was to use magnetic resonance imaging (MRI) to replicate this finding in vivo.
Method
Twelve sibling pairs from families multiply affected with schizophrenia participated in the study. Written informed consent was obtained from all subjects. Each subject was interviewed by a psychiatrist, and clinical records were obtained for affected subjects. Consensus diagnoses were made according to DSM-IV criteria. Six affected subjects were diagnosed with schizophrenia and six with schizoaffective disorder. None of the unaffected siblings had diagnoses genetically associated with schizophrenia (e.g., nonaffective psychosis or schizotypal or paranoid personality disorder). Hand preference was determined with a questionnaire; a score of 1 indicated that the subject was strongly right-handed, and a score of 5 indicated a strongly left-handed subject (7).
MRI scanning was performed with a 1.5-T superconductive magnet and a circularly polarized head coil (Siemens MAGNETOM, Erlangen, Germany). A T1-weighted, fast gradient echo sequence (40 msec TR, 5 msec TE, 40° angle, 1 excitation, 25-cm field of view, 15-cm thickness of the excited volume) was used. This provided 128 consecutive slices with 1.17-mm thickness, pixel size of 1 × 1 mm, and adequate gray matter-white matter contrast.
Before analysis, scans were realigned so that the coronal plane was orthogonal to the anterior commissure-posterior commissure line. Slices were chosen for gyrification index measurements of the frontal lobe by the same approach as in the postmortem study (6). The scan in which the anterior border of the corpus callosum was visualized was chosen as the index slice for each subject. Two additional slices, 10 and 20 mm anterior to the index slice, were also used. All measurements were made independently by two raters. To calculate the gyrification index, the inner contour was measured by tracing a line along the pial surface, and the outer contour was measured by tracing a line connecting gyri crests (Figure 1). The gyrification index was calculated as the ratio of the length of the inner to the outer contours. Acceptable interrater reliability was obtained (intraclass correlation coefficient [ICC]=0.84).
Demographic variables for the affected and unaffected siblings were compared by using t tests for continuous variables and chi-square analyses for categorical variables. Gyrification index scores of the affected and unaffected siblings were compared by using paired t tests.
Results
The mean age of the subjects (30.8 years, SD=7.3, range=19–45) did not differ between affected and unaffected siblings. The affected group consisted of seven men and five women, and the unaffected group consisted of five men and seven women. Lateralization of handedness scores did not differ between the groups (affected: mean=1.3, SD=0.7; unaffected: mean=1.8, SD=1.2). For affected subjects, mean age at first hospitalization was 22.6 years (SD=8.4).
Total intracranial volumes did not differ between the groups. As seen in Figure 1, the mean gyrification index on the right side was significantly higher in the affected siblings than in the unaffected siblings (mean=2.30 [SD=0.11] versus 2.19 [SD=0.14], respectively). The mean gyrification index on the left side for the two groups was not significantly different (affected: mean=2.22, SD=0.14; unaffected: mean=2.21, SD=0.14) (paired t=0.20, df=11, p=0.84). When the analysis was limited to sibling pairs in which both members scored 1 or 2 on the handedness inventory (right hand preference), the mean gyrification index on the right side remained significantly higher (affected: mean=2.30, SD=0.12; unaffected: mean=2.22, SD=0.12) (paired t=2.34, df=8, p<0.05).
Discussion
The MRI results demonstrated significant right-sided prefrontal region hypergyria in subjects with schizophrenia and replicated our previous postmortem finding (6). The magnitude of the mean difference was approximately 5%. To provide a context, the difference in gyrification index between the rostral and intermediate thirds of the cortex is approximately 10%. Of interest, gyrification index values during childhood are greater than those found during adulthood and decline approximately 5% every 2 years during adolescence (5). The hypergyrification of the right prefrontal region in patients with schizophrenia could represent a developmental arrest. Further studies are required, since an MRI study of monozygotic twins discordant for schizophrenia did not show differences in gyrification (8). In a study limited to the left side only, the gyrification index in the left hemisphere was reported to be higher in patients with schizophrenia (9).
Methodologically, manual tracing to determine the gyrification index is more difficult with MR images, since the spatial resolution is poorer (Figure 1). As a consequence, the interrater reliability with MRI (ICC=0.84) was not as high as that obtained in the postmortem study (ICC=0.95). However, the mean values for gyrification index obtained with MRI were very similar to those obtained in the postmortem study (6), indicating that results obtained with the technique are quite robust.
Focal microgyria or hypergyria may be a consequence of disturbed fetal blood supply, intrauterine infection, or prenatal toxic events (10). Animal experiments have demonstrated wide-ranging misconnections in the neighborhood of focal hypergyrification (11). Disturbed gyrification in schizophrenia is consistent with a model of illness in which neural connectivity develops or matures abnormally.
Received Nov. 8, 1999; revision received Aug. 14, 2000; accepted Sept. 29, 2000. From the Department of Psychiatry, Friedrich Wilhelms University of Bonn. Address reprint requests to Dr. Vogeley, Department of Psychiatry, Friedrich Wilhelms University of Bonn, Sigmund-Freud-Strasse 25, 53105 Bonn, Germany; [email protected] (e-mail). Supported by grants from the Stanley Foundation and the German Research Society and a Scientist award from the Canadian Institutes for Health Research (Dr. Honer).
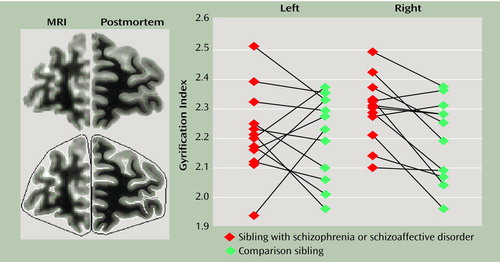
Figure 1. Gyrification Index Measurement and Data for 12 Affected-Unaffected Sibling Pairs From Families Multiply Affected With Schizophreniaa
aThe left panel shows a coronal brain slice from the prefrontal region and the difference in image quality obtained through MRI relative to postmortem analysis. Images on the bottom of the left panel show the manual tracing of the inner (white line) and outer (black line) contours used to determine the gyrification index (ratio of the length of the inner to the outer contour). The right panel shows data points for the gyrification index of the individual siblings; a significant gyrification index difference was observed only for the right frontal lobe (paired t=3.16, df=11, p=0.009).
1. Vogeley K, Falkai P: The cortical dysconnectivity hypothesis of schizophrenia. Neurol Psychiatry Brain Res 1998; 6:113–122Google Scholar
2. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:453–458Crossref, Google Scholar
3. Sidman RL, Rakic P: Development of the human central nervous system, in Histology and Histopathology of the Nervous System, vol 1. By Haymaker W, Adams RD. Springfield, Ill, Charles C Thomas, 1982, pp 3–145Google Scholar
4. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ: The human pattern of gyrification in the cerebral cortex. Anat Embryol 1988; 179:174–179Crossref, Google Scholar
5. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K: The ontogeny of human gyrification. Cereb Cortex 1995; 1:56–63Crossref, Google Scholar
6. Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P: Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiatry 2000; 157:34–39Link, Google Scholar
7. Annett M: A classification of hand preference by association analysis. Br J Psychol 1970; 61:303–321Crossref, Medline, Google Scholar
8. Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR: Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res 1996; 22:27–40Crossref, Medline, Google Scholar
9. Kulynych JJ, Luevano LF, Jones DW, Weinberger DR: Cortical abnormalities in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry 1997; 41:995–999Crossref, Medline, Google Scholar
10. Jellinger K, Rett A: Agyria-pachygyria (lissencephaly syndrome). Neuropadiatrie 1976; 7:66–91Crossref, Medline, Google Scholar
11. Goldman-Rakic PS: Morphological consequences of prenatal injury in the primate brain. Prog Brain Res 1980; 53:3–19Crossref, Google Scholar



