Low Level of Brain Dopamine D2 Receptors in Methamphetamine Abusers: Association With Metabolism in the Orbitofrontal Cortex
Abstract
OBJECTIVE: The role of dopamine in the addictive process (loss of control and compulsive drug intake) is poorly understood. A consistent finding in drug-addicted subjects is a lower level of dopamine D2 receptors. In cocaine abusers, low levels of D2 receptors are associated with a lower level of metabolism in the orbitofrontal cortex. Because the orbitofrontal cortex is associated with compulsive behaviors, its disruption may contribute to compulsive drug intake in addicted subjects. This study explored whether a similar association occurs in methamphetamine abusers. METHOD: Fifteen methamphetamine abusers and 20 non-drug-abusing comparison subjects were studied with positron emission tomography (PET) and [11C]raclopride to assess the availability of dopamine D2 receptors and with [18F]fluorodeoxyglucose to assess regional brain glucose metabolism, a marker of brain function. RESULTS: Methamphetamine abusers had a significantly lower level of D2 receptor availability than comparison subjects (a difference of 16% in the caudate and 10% in the putamen). D2 receptor availability was associated with metabolic rate in the orbitofrontal cortex in abusers and in comparison subjects. CONCLUSIONS: Lower levels of dopamine D2 receptor availability have been previously reported in cocaine abusers, alcoholics, and heroine abusers. This study extends this finding to methamphetamine abusers. The association between level of dopamine D2 receptors and metabolism in the orbitofrontal cortex in methamphetamine abusers, which replicates previous findings in cocaine abusers, suggests that D2 receptor-mediated dysregulation of the orbitofrontal cortex could underlie a common mechanism for loss of control and compulsive drug intake in drug-addicted subjects.
Methamphetamine is a highly addictive drug of abuse (1) whose reinforcing effects have been equated to those of cocaine (2, 3). In humans, the reinforcing effects of methamphetamine, which are most accentuated when the drug is taken intravenously or smoked (4), can lead to rapid escalation of drug use and compulsive drug administration (5). Over the past decade methamphetamine abuse has significantly risen in several areas of the United States and the world (6–8) and has become a significant public health problem.
Although many neurotransmitter systems have been implicated in methamphetamine’s reinforcing effects, by far the most evidence points to the dopamine system (1, 9). Most studies of the effects of repeated methamphetamine administration have concentrated on its neurotoxic effects on dopamine cells. In laboratory animals, methamphetamine induces profound and long lasting damage to dopamine cells (10). Human postmortem and imaging studies in methamphetamine abusers have documented significant losses in dopamine transporters, which are structural elements of the dopamine terminals (11–13). In methamphetamine abusers, the loss of dopamine transporters has been associated with motor and cognitive impairment (13) but has not been linked to the mechanisms underlying methamphetamine addiction. Moreover, little is known about the role of dopamine in the loss of control and compulsive drug intake seen in methamphetamine-addicted subjects.
Imaging studies have shown that a common abnormality in drug-addicted subjects, including alcoholics, cocaine abusers, and heroin abusers, is a lower than normal level of dopamine D2 receptor availability (14–17). Moreover, we have shown an association in cocaine abusers between striatal dopamine D2 receptor densities and metabolic rates in the orbitofrontal cortex (18). Since disruption of the orbitofrontal cortex is associated with obsessive and compulsive behaviors (19, 20), we hypothesized that dopamine dysregulation of the orbitofrontal cortex underlies compulsive drug intake in cocaine-addicted subjects (21, 22). Here we evaluated the question of whether a similar disruption could underlie methamphetamine addiction.
Positron emission tomography (PET) was used with [11C]raclopride to measure dopamine D2 receptors and with [18F]fluorodeoxyglucose (FDG) to measure brain glucose metabolism in methamphetamine abusers and in comparison subjects. We hypothesized that methamphetamine abusers would have a lower level of dopamine D2 receptor availability than the comparison subjects and that the level of D2 receptor availability would be associated with metabolism in the orbitofrontal cortex. The results from the FDG studies in the methamphetamine abusers were published as part of a study that compared regional brain glucose metabolism between methamphetamine abusers and comparison subjects (23).
Method
Subjects
Fifteen subjects (six men, nine women; mean age=32 years, SD=7) who fulfilled DSM-IV criteria for methamphetamine dependence were enrolled in the study. Twelve subjects were evaluated within 2 weeks to 5 months of their last methamphetamine abuse, and the other three were studied between 11 and 35 months after their last methamphetamine abuse. Methamphetamine abusers were included in the study if they had an average methamphetamine use of at least 0.5 gram/day at least 5 days per week for at least 2 years, a minimum of 2 weeks of abstinence from methamphetamine, and a negative urine toxicology screen. Methamphetamine abusers were excluded from the study if they were seropositive for HIV or had a past or present history of a comorbid psychiatric illness (DSM-IV axis I or II diagnosis) and/or a neurological disorder, abnormal laboratory screening test results, a current or past history of addiction to drugs other than methamphetamine and nicotine, or a history of head trauma with loss of consciousness for more than 30 minutes. Methamphetamine abusers were recruited from several drug rehabilitation centers in the Los Angeles area and were enrolled in a California court-monitored drug rehabilitation program that required periodic drug screening to ensure lack of drug use during detoxification. After an initial telephone or on-site face-to-face screening interview, potential research subjects provided a detailed medical and drug use history and underwent physical and neuropsychiatric evaluations (L.C.). Diagnosis and exclusion criteria were corroborated by a physician from the State University of New York or from Brookhaven National Laboratory (M.S., G.-J.W., D.F.). The methamphetamine abusers had an average 10-year (SD=6) history of methamphetamine abuse, with a reported cumulative lifelong use of 13 kg (SD=20) of methamphetamine, which they consumed predominantly either by the intravenous or the smoked route of administration.
The comparison subjects were 20 healthy volunteers (14 men, six women; mean age=31 years, SD=7) recruited by local advertisement. The exclusion criteria for the comparison subjects were the same as those used for the methamphetamine abusers, except for dependence or abuse of methamphetamine. A complete medical, neurological, and psychiatric examination was performed to ensure lack of disease. Except for HIV serology, all laboratory test results obtained for the methamphetamine abusers were also obtained for the comparison subjects.
None of the subjects was taking medication at the time of the study. Prescan urine tests were done to ensure the absence of psychoactive drug use in the comparison subjects and in the methamphetamine abusers. Written informed consent was obtained from the subjects after the procedures had been fully explained. The study was approved by the institutional review boards at the Brookhaven National Laboratory, State University of New York, Stony Brook, and Harbor–University of California, Los Angeles, Medical Center.
Imaging
PET scans were performed with a CTI 931 scanner (Siemens, Knoxville, Tenn.). Subjects were scanned after intravenous injection of [11C]raclopride and of FDG. Details of the procedures for positioning, arterial and venous catetherization, and quantification of the radiotracer have been published for the [11C]raclopride scans (24) and the FDG scans (25). Briefly, the 60-minute dynamic scans with [11C]raclopride were started immediately after intravenous injection of 4–10 mCi of [11C]raclopride (specific activity >0.25 Ci/μmol at time of injection). One emission scan (20 minutes) with FDG was taken 35 minutes after intravenous injection of 4–6 mCi of FDG. During the study, the subject lay with eyes open in the PET camera, the room was dimly lit, and noise was kept to a minimum. A nurse remained with the subject throughout the procedure to ensure that the subject did not fall asleep during the study.
Image Analysis
Regions of interest in the [11C]raclopride images were obtained for the striatum (the caudate and the putamen) and for the cerebellum. The regions of interest were initially selected on an averaged scan (activity from 10 to 60 minutes) and were then projected to the dynamic scans, as previously described (24). The time-activity curves for [11C]raclopride in the striatum and the cerebellum and the time-activity curves for unchanged tracer in plasma were used to calculate distribution volumes by using a graphical analyses technique for reversible systems (Logan Plots) (26). The parameter Bmax/Kd, obtained as the ratio of the distribution volume in the striatum to that in the cerebellum minus 1, was used as the model parameter of dopamine D2 receptor availability. This parameter is insensitive to changes in cerebral blood flow (27).
The regions of interest in the metabolic images were selected by using a previously described template (25). With this template we selected the region of interest for the orbitofrontal cortex, for which we hypothesized a priori an association with dopamine D2 receptor availability; the regions of interest for the caudate and the putamen, where dopamine D2 receptors were measured; and the regions of interest in the temporal cortex and the cerebellum, which were chosen as neutral regions of interest.
Statistical Analyses
Differences between the groups in K1 (rate constant for the transport of [11C]raclopride from plasma to tissue), distribution volume, and dopamine D2 receptor availability (Bmax/Kd) were tested with one-factor analysis of variance (ANOVA). Pearson product-moment correlation analyses were used to examine the relationship between dopamine D2 receptor availability and both the absolute metabolic measure and the normalized metabolic measure (regional measure divided by the whole brain measure) separately in the methamphetamine abusers and in the comparison subjects.
Results
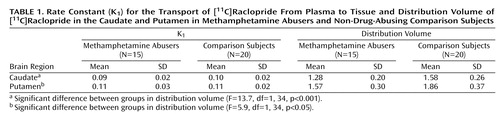
The transport rate parameters of [11C]raclopride from plasma to brain (K1) for the striatum or for the cerebellum did not differ between the methamphetamine abusers and the comparison subjects. The distribution volume in the caudate and the putamen, but not in the cerebellum, was significantly lower in the methamphetamine abusers than in the comparison subjects (Figure 1, Table 1). The estimates of dopamine D2 receptor availability (Bmax/Kd) were significantly lower in the methamphetamine abusers than in the comparison subjects in both the caudate and the putamen (Figure 2). Although the differences in Bmax/Kd between the groups appeared larger in the caudate than in the putamen (a difference of 16% in the caudate versus 10% in the putamen), these differences between the regions were not significant, according to results of a one-factor (comparison subjects versus methamphetamine abusers), repeated measures (caudate versus putamen) ANOVA (F=0.87, df=1, 33, p=0.36). No differences in Bmax/Kd in the caudate and the putamen were found between the 12 methamphetamine abusers who had last used methamphetamine within 5 months of the study and the three who had not used methamphetamine for more than 11 months (caudate: mean Bmax/Kd>=2.08, SD=0.25, and 2.09, SD=0.17, respectively [F=0.001, df=1, 14, p=0.97]; putamen: mean Bmax/Kd=2.81, SD=0.53, and 2.80, SD=0.40, respectively [F=0.002, df=1, 14, p=0.97]).
In the methamphetamine abusers, the dopamine D2 receptor availability measures in the putamen were correlated with metabolism in the orbitofrontal cortex (r=0.69, df=14, p<0.005) but not with metabolism in the caudate (r=0.40, df=14, p=0.15), putamen (r=0.39, df=14, p=0.15), temporal cortex (r=0.34 df=14, p=0.22), or cerebellum (r=0.36, df=14, p=0.19). Similar results were observed in the comparison subjects, for whom a significant correlation was found only between dopamine D2 receptor availability in the putamen and metabolism in orbitofrontal cortex (r=0.52, df=19, p<0.02). None of the correlations with dopamine D2 receptors in the caudate in the methamphetamine abusers or the comparison subjects were significant (data not shown). There were no differences in metabolism in the orbitofrontal cortex between the comparison subjects (mean=48 μmol/100 g per minute, SD=9) and the abusers (mean=52 μmol/100 g per minute, SD=13), but D2 receptor availability was lower in methamphetamine abusers. Therefore, the regression slopes appear to show that for a given estimate of dopamine D2 receptor availability, the absolute metabolism in the orbitofrontal cortex is higher in the methamphetamine abusers than in the comparison subjects (Figure 3). However, a covariate analysis to test for differences between the groups in orbitofrontal metabolism conditional on the D2 measures was not significant.
For the methamphetamine abusers, the correlations between the dopamine D2 receptor measures and the normalized metabolic measures (measure for the region of interest divided by the measure for the whole brain) showed results similar to those for the absolute metabolic measures: the only significant correlation was between D2 receptor availability in the putamen and metabolism in the orbitofrontal cortex (r=0.74, df=14, p<0.002) (Figure 3). In the comparison subjects, none of the correlations with the normalized metabolic measures was significant, including the correlation between the measure of D2 receptor availability in the putamen and metabolism in the orbitofrontal cortex (r=0.31, df=19, p=0.18).
Discussion
Imaging and postmortem studies have documented in methamphetamine abusers markedly lower levels of dopamine transporters, which serve as presynaptic markers for the dopamine terminal (11–13). However, to our knowledge, this is the first PET study to document lower levels of dopamine D2 receptors in methamphetamine abusers. PET measures of dopamine D2 receptors mostly reflect the level of postsynaptic receptors (28). Thus, these findings provide evidence that methamphetamine also affects postsynaptic dopamine elements, which in the striatum most likely reflect effects of methamphetamine on intrinsic γ-aminobutyric acid cells. These findings corroborate the few preclinical studies showing that methamphetamine, in addition to causing changes in presynaptic dopamine markers, also reduces postsynaptic dopamine D2(29, 30) and D1 receptors (31).
Lower levels of D2 receptor availability in methamphetamine abusers could reflect receptor down-regulation from exposure to a higher extracellular dopamine concentration secondary to methamphetamine’s acute pharmacological effects as well as methamphetamine-induced losses of dopamine transporters (11). Even though studies have documented decreased striatal dopamine concentration with methamphetamine administration (11, 32), the concomitant dopamine transporter losses could still result in enhanced extracellular dopamine, as shown in dopamine-transporter knockout mice (33). Alternatively, the low levels of D2 receptors could have preceded methamphetamine use and may have predisposed these subjects to drug use. In support of this possibility is a study showing that, in non-drug-abusing comparison subjects, striatal D2 receptor levels predicted responses to psychostimulant administration (34). Subjects with low D2 receptor levels experienced a “pleasurable” response, whereas subjects with high receptor levels experienced an “unpleasant” response. These findings led us to speculate that D2 receptors, by modulating pleasant versus unpleasant drug responses, may be a variable that contributes to drug abuse and addiction. However, in the study reported here, it was not possible to determine if the lower levels of dopamine D2 receptors preceded the use of methamphetamine use or reflected chronic use and, if the lower levels resulted from chronic use, whether they recover with detoxification. Although in this study we were unable to detect differences in D2 receptors between the 12 methamphetamine abusers tested within 5 months of last methamphetamine use and the three tested after 11 months of detoxification, the size of the study group was too small to determine if recovery of D2 receptors occurs with detoxification. Also, since [11C]raclopride is sensitive to endogenous dopamine, we cannot rule out the possibility that the lower levels of D2 receptor availability could reflect competition of [11C]raclopride binding with dopamine (35).
Reductions in D2 receptors have been reported in other drug abusers, including cocaine abusers (14, 18, 36), alcoholics (15, 37), and heroin abusers (17), suggesting that reductions in D2 receptors are not specific to any type of drug addiction but may underlie a common abnormality in addicted states and/or a common predisposing factor. Moreover, we recently demonstrated a lower level of D2 receptors in pathologically obese subjects, who share with drug-addicted subjects the compulsive administration of the reinforcer, which for obese subjects is not a drug but food (38).
The association between metabolic activity in the orbitofrontal cortex and measures of D2 receptors could reflect dopamine-mediated striatal regulation of orbitofrontal activity by means of striato-thalamo-cortical pathways (39). The orbitofrontal cortex receives projections both from the nucleus accumbens (40), which is the region in the striatum that is traditionally associated with the reinforcing effects of drugs of abuse (41), and from the ventral tegmental area, which is the main dopamine projection to the nucleus accumbens (42). However, the orbitofrontal cortex also sends projections to the nucleus accumbens (39), so we cannot rule out the possibility that the association reflects orbitofrontal regulation of dopamine striatal activity. The reciprocal neuroanatomical connections between the orbitofrontal cortex and the nucleus accumbens make the orbitofrontal cortex a direct target for the effects of drugs of abuse and a region that could modulate these responses. Because of the limited spatial resolution of the PET camera, dopamine D2 receptor measures were quantified in the putamen (one can not accurately measure receptor availability in nucleus accumbens). Future studies done with PET cameras with better spatial resolution and sensitivity are required to specifically evaluate the association between activity in the orbitofrontal cortex and measures of D2 receptors in the nucleus accumbens.
The relationship between metabolic activity in the orbitofrontal cortex and the availability of D2 receptors was significant both in the methamphetamine abusers and the comparison subjects. However, the association between D2 receptors and the normalized metabolic measures in the orbitofrontal cortex was significant in the abusers but not in the comparison subjects. Since normalized measures are more sensitive to regional changes than absolute measures, this association could reflect a higher sensitivity of the orbitofrontal cortex to dopamine modulation in methamphetamine abusers than in comparison subjects. However, further studies are required to determine if there is enhanced sensitivity of the orbitofrontal cortex to dopamine modulation and/or enhanced striatal dopamine regulation by the orbitofrontal cortex in drug-addicted subjects, compared with non-drug-addicted subjects.
Dopamine modulation of the orbitofrontal cortex could underlie addictive behaviors in several ways. First, the orbitofrontal cortex is involved in the regulation of “drive” (43), and thus enhanced activation secondary to drug-induced dopamine stimulation could result in an intense motivation to self-administer methamphetamine in the addicted subjects. Moreover, because the orbitofrontal cortex processes information about the rewarding properties of stimuli (44), its disruption could account for the enhanced salience of drug-related stimuli. Second, the orbitofrontal cortex has been implicated in the occurrence of compulsive behaviors (19, 20), and thus one could postulate that its inappropriate activation could induce compulsive drug administration in methamphetamine abusers. In laboratory animals, damage to the orbitofrontal cortex results in perseveration and resistance to extinction of reward-associated behaviors (45, 46). These findings are reminiscent of the reports of drug addicts who claim that once they start taking a drug of abuse they cannot stop even when the drug is no longer pleasurable. Third, the orbitofrontal cortex is involved with learning stimulus-reinforcement associations (47) and with conditioned responses (48) and could therefore participate in cues or drug-induced craving. Laboratory animals exposed to an environment where they had received a drug of abuse show orbitofrontal activation (49), and lesions of the orbitofrontal cortex interfere with drug-induced conditioned place preference (50). These findings are relevant because drug-induced conditioned responses have been implicated in the craving elicited in humans by drug-related stimuli (51), which is one of the factors that contributes to relapse in drug abusers (52). Moreover, activation of the orbitofrontal cortex has been shown in drug abusers during craving elicited by a drug (53), by viewing a video of drug paraphernalia (54), and by recalling previous drug experiences (55).
Limitations for this study are those inherent in clinical research in drug abuse populations, including inaccuracies in clinical histories and histories of drug use by the subjects, as well as confounds from differences between the groups investigated (e.g., differences in levels of consumption of nicotine or alcohol). Thus, in this study, we were unable to completely rule out the influence of comorbid factors. In interpreting these results, one also needs to consider the importance of other brain regions, other dopamine receptor subtypes, and other neurotransmitter systems in the modulation of the orbitofrontal cortex and in methamphetamine addiction.
This study shows lower levels of dopamine D2 receptor availability in methamphetamine abusers than in non-drug-abusing comparison subjects, providing evidence for the involvement of postsynaptic cells in the effects of methamphetamine on dopamine neurotransmission. The significant association between dopamine D2 receptors in the putamen and activity in the orbitofrontal cortex, a brain region involved with compulsive behaviors, suggests that dysregulation of the orbitofrontal cortex may be one of the mechanisms by which disruption of dopamine activity in methamphetamine abusers could lead to compulsive drug-taking behavior.
 |
Received Dec. 8, 2000; revision received June 13, 2001; accepted June 20, 2001. From the Medical and Chemistry Departments, Brookhaven National Laboratory; and the Department of Psychiatry, State University of New York at Stony Brook, Stony Brook, NY. Address reprint requests to Dr. Volkow, Brookhaven National Laboratory, B.S.A., P.O. Box 5000, Upton, NY 11973; [email protected] (e-mail). Supported in part by grant ACO2-98CH-10886 from the Department of Energy Office of Biological and Environmental Research, grants DA-7092-01 and DA-00280 from the National Institute on Drug Abuse, NIH General Clinical Research Center grant RR-10710, and the Office of National Drug Control Policy. The authors thank David Schlyer and Robert Carciello for Cyclotron operations; Donald Warner for PET operations; Colleen Shea, Victor Garza, Robert MacGregor, David Alexoff, and Payton King for radiotracer preparation and analysis; and Pauline Carter and Noelwah Netusil for patient care.
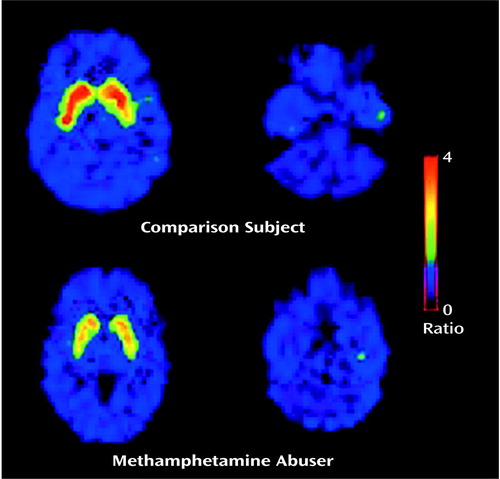
Figure 1. Ratio of the Distribution Volume of [11C]Raclopride in the Striatum Normalized to the Distribution Volume in the Cerebellum in a Non-Drug-Abusing Comparison Subject and a Methamphetamine Abuser
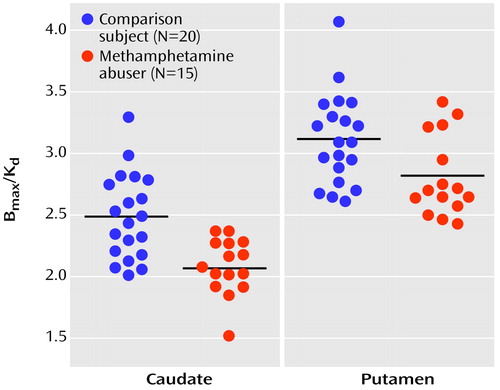
Figure 2. Estimates of Dopamine D2 Receptor Availability (Bmax/Kd) in the Caudate and the Putamen in Non-Drug-Abusing Comparison Subjects and Methamphetamine Abusersa
aBmax/Kd estimates were significantly lower in methamphetamine abusers than in the comparison subjects in the caudate (F=16.0, df=1, 34, p<0.0005) and the putamen (F=6.4, df=1, 34, p<0.02).
Figure 3. Correlation of Dopamine D2 Receptor Availability (Bmax/Kd) in the Putamen With Absolute and Normalized Measures of Metabolism in the Orbitofrontal Cortex in Methamphetamine Abusers and Non-Drug-Abusing Comparison Subjects
1. Woolverton WL, Cervo L, Johanson CE: Effects of repeated methamphetamine administration on methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav 1984; 21:737-741Crossref, Medline, Google Scholar
2. Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW: Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther 1996; 277:212-218Medline, Google Scholar
3. Shimosato K, Ohkuma S: Simultaneous monitoring of conditioned place preference and locomotor sensitization following repeated administration of cocaine and methamphetamine. Pharmacol Biochem Behav 2000; 66:285-292Crossref, Medline, Google Scholar
4. Murray JB: Psychophysiological aspects of amphetamine-methamphetamine abuse. J Psychol 1998; 132:227-237Crossref, Medline, Google Scholar
5. Cho AK: Ice: a new dosage form of an old drug. Science 1990; 249:631-634Crossref, Medline, Google Scholar
6. Lukas SE: Proceedings of the National Consensus Meeting on the Use, Abuse and Sequelae of Abuse of Methamphetamine With Implications for Prevention, Treatment and Research: DHHS Publication SMA 96-8013. Rockville, Md, US Department of Health and Human Services, 1997Google Scholar
7. National Institute on Drug Abuse: Methamphetamine Abuse Alert: NIDA Notes 13. Washington, DC, NIDA, 1999, pp 15-16Google Scholar
8. Shaw KP: Human methamphetamine-related fatalities in Taiwan during 1991-1996. J Forensic Sci 1999; 44:27-31Medline, Google Scholar
9. Munzar P, Baumann MH, Shoaib M, Goldberg SR: Effects of dopamine and serotonin-releasing agents on methamphetamine discrimination and self-administration in rats. Psychopharmacology (Berl) 1999; 141:287-296Crossref, Medline, Google Scholar
10. Preston KL, Wagner GC, Schuster CR, Seiden LS: Long-term effects of repeated methylamphetamine administration on monoamine neurons in the rhesus monkey brain. Brain Res 1985; 338:243-248Crossref, Medline, Google Scholar
11. Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ: Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 1996; 2:699-703Crossref, Medline, Google Scholar
12. McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA: Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998; 18:8417-8422Crossref, Medline, Google Scholar
13. Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler M, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377-382Google Scholar
14. Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Albert R, Dewey SL, Logan J, Bendriem B, Christman D: Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990; 147:719-724Link, Google Scholar
15. Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann RJ, Ding YS, Pappas N, Shea C, Piscani K: Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 1996; 20:1594-1598Crossref, Medline, Google Scholar
16. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Pappas N: Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997; 386:830-833Crossref, Medline, Google Scholar
17. Wang G-J, Volkow ND, Fowler JS, Logan J, Hitzemann RJ, Pappas NS, Piscani K: Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone precipitated withdrawal. Neuropsychopharmacology 1997; 16:174-182Crossref, Medline, Google Scholar
18. Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer D, Dewey S, Wolf AP: Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993; 14:169-177Crossref, Medline, Google Scholar
19. Baxter LR, Phelps ME, Mazziotta J: Local cerebral glucose metabolic rates in obsessive compulsive disorder: a comparison with rates in unipolar depression and normal controls. Arch Gen Psychiatry 1987; 44:211-218Crossref, Medline, Google Scholar
20. Insel TR: Towards a neuroanatomy of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49:739-744Crossref, Medline, Google Scholar
21. Volkow ND, Ding Y-S, Fowler JS, Wang G-J: Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis 1996; 15:55-71Crossref, Medline, Google Scholar
22. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318-325Crossref, Medline, Google Scholar
23. Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Wong C, Logan J: Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry 158:383-389Google Scholar
24. Volkow ND, Fowler JS, Wang G-J, Dewey SL, Schlyer D, MacGregor R, Logan J, Alexoff D, Shea C, Hitzemann R, Angrist B, Wolf AP: Reproducibility of repeated measures of 11C raclopride binding in the human brain. J Nucl Med 1993; 34:609-613Medline, Google Scholar
25. Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP: Functional significance of ventricular enlargement and cortical atrophy in normals and alcoholics as assessed by PET, MRI and neuropsychological testing. Radiology 1992; 186:59-65Crossref, Google Scholar
26. Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer D, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, Christman DR: Graphical analysis of reversible binding from time activity measurements. J Cereb Blood Flow Metab 1990; 10:740-747Crossref, Medline, Google Scholar
27. Logan J, Volkow ND, Fowler JS, Wang G-J, Dewey SL, MacGregor R, Schlyer D, Gatley SJ, Pappas N, King P, Hitzemman R, Vitkun S: Effects of blood flow on [11C] raclopride binding in the brain: model simulations and kinetic analysis of PET data. J Cereb Blood Flow Metab 1994; 14:995-1010Crossref, Medline, Google Scholar
28. Hume SP, Lammertsma AA, Myers R, Rajeswaran S, Bloomfield PM, Ashworth S, Fricker RA, Torres EM, Watson I, Jones T: The potential of high-resolution positron emission tomography to monitor striatal dopaminergic function in rat models of disease. J Neurosci Methods 1996; 67:103-112Medline, Google Scholar
29. Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE: Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol 1998; 361:269-275Crossref, Medline, Google Scholar
30. Gatley SJ, Volkow ND, Pyatt B, Gifford AN: Effects of methamphetamine on neurochemistry and behavior (abstract). Abstr Soc Neurosci 2000; 26:196Google Scholar
31. Cadet JL, Ladenheim B, Hirata H: Effects of toxic doses of methamphetamine (METH) on dopamine D1 receptors in the mouse brain. Brain Res 1998; 786:240-242Crossref, Medline, Google Scholar
32. Robinson TE, Yew J, Paulson PE, Camp DM: The long-term effects of neurotoxic doses of methamphetamine on the extracellular concentration of dopamine measured with microdialysis in striatum. Neurosci Lett 1990; 110:193-198Crossref, Medline, Google Scholar
33. Giros B, Jaber M, Jones SR, Wightman RM, Caron MG: Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996; 379:606-612Crossref, Medline, Google Scholar
34. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding Y-S, Pappas N: Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 1999; 156:1440-1443Link, Google Scholar
35. Volkow ND, Wang G-J, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, Burr G, Cooper T, Wolf AP: Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 1994; 16:255-262Crossref, Medline, Google Scholar
36. Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Pappas N: Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997; 386:830-833Crossref, Medline, Google Scholar
37. Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U: Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994; 116:285-290Crossref, Medline, Google Scholar
38. Wang G-J, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS: Brain dopamine and obesity. Lancet 2001; 3:354-357Crossref, Google Scholar
39. Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E: The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 1995; 15:4851-4867Crossref, Medline, Google Scholar
40. Ray JP, Price JL: The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. Comp Neurol 1993; 337:1-31Crossref, Medline, Google Scholar
41. Koob GF, Bloom FE: Cellular and molecular mechanisms of drug dependence. Science 1988; 242:715-723Crossref, Medline, Google Scholar
42. Oades RD, Halliday GM: Ventral tegmental (A10) system: neurobiology, 1: anatomy and connectivity. Brain Res 1987; 434:117-165Crossref, Medline, Google Scholar
43. Tucker DM, Luu P, Pribram KH: Social and emotional self-regulation. Ann NY Acad Sci 1995; 769:213-239Crossref, Medline, Google Scholar
44. Tremblay L, Schultz W: Relative reward preference in primate orbitofrontal cortex. Nature 1999; 398:704-708Crossref, Medline, Google Scholar
45. Butter CM, Mishkin M, Rosvold HE: Conditioning and extinction of a food rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol 1963; 7:65-67Crossref, Medline, Google Scholar
46. Johnson TN: Topographic projections in the globus pallidus and the substantia nigra of selectively placed lesions in the precommissural caudate nucleus and putamen in the monkey. Exp Neurol 1971; 33:584-596Crossref, Medline, Google Scholar
47. Schoenbaum G, Chiba AA, Gallagher M: Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci 1998; 1:155-159Crossref, Medline, Google Scholar
48. Hugdahl K, Berardi A, Thompson WL, Kosslyn SM, Macy R, Baker DP, Alpert NM, LeDoux JE: Brain mechanisms in human classical conditioning: a PET blood flow study. Neuroreport 1995; 6:1723-1728Crossref, Medline, Google Scholar
49. Brown EE, Robertson GS, Fibiger HC: Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. Neuroscience 1992; 12:4112-4121Crossref, Medline, Google Scholar
50. Isaac WL, Nonneman AJ, Neisewander J, Landers T, Bardo MT: Prefrontal cortex lesions differentially disrupt cocaine-reinforced conditioned place preference but not conditioned taste aversion. Behav Neurosci 1989; 103:345-355Crossref, Medline, Google Scholar
51. O’Brien CP, Childress AR, Ehrman R, Robbins SJ: Conditioning factors in drug abuse: can they explain compulsion? Psychopharmacology (Berl) 1998; 12:15-22Crossref, Google Scholar
52. McKay JR: Studies of factors in relapse to alcohol, drug and nicotine use: a critical review of methodologies and findings. J Stud Alcohol 1999; 60:566-576Crossref, Medline, Google Scholar
53. Volkow ND, Wang G-J, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding Y-S, Pappas N: Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 1999; 156:19-26Link, Google Scholar
54. Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A: Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 1996; 93:12040-12045Crossref, Medline, Google Scholar
55. Wang G-J, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas N, Wong CT, Felder C: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775-784Crossref, Medline, Google Scholar



