Occupancy of Serotonin Transporters by Paroxetine and Citalopram During Treatment of Depression: A [11C]DASB PET Imaging Study
Abstract
OBJECTIVE: Selective serotonin reuptake inhibitors are commonly used to treat major depression; however, the percentage of serotonin (5-HT) transporter (5-HTT) sites occupied during clinical dosing is unknown. This study measured the proportion of 5-HTT sites blocked during paroxetine and citalopram treatment of depression and assessed the relationship between serum paroxetine levels and the proportion of 5-HTT sites blocked. METHOD: Twelve medication-free depressed patients completed a 6-week trial of either paroxetine (N=8) or citalopram (N=4). Striatal 5-HTT binding potential was measured with [11C]DASB and positron emission tomography, before and after 4 weeks of treatment. The binding potential is proportional to receptor density. Striatal 5-HTT binding potential was measured twice in six healthy subjects and once in 11 healthy subjects. RESULTS: A significant decrease in striatal 5-HTT binding potential was found after either treatment, compared to changes found over a 4-week period in healthy subjects. For patients treated with 20 mg/day of paroxetine (N=7), the mean proportion of 5-HTT sites occupied was 83%. For patients treated with 20 mg/day of citalopram (N=4), the mean 5-HTT occupancy was 77%. 5-HTT occupancy increased in a nonlinear relationship with serum levels of paroxetine such that a plateau of occupancy around 85% occurred for serum paroxetine levels greater than 28 μg/liter. CONCLUSIONS: During treatment with clinical doses of paroxetine or citalopram, approximately 80% of 5-HTT receptors are occupied. This change in 5-HTT binding potential is greater than the known physiological range of changes in 5-HTT binding potential but may be necessary for some therapeutic effects.
Antidepressants have high affinity for a number of receptors, including subtypes of serotonin, norepinephrine, acetylcholine, and dopamine receptors (1, 2). The majority of antidepressants have high affinity for the serotonin (5-HT) transporter (5-HTT) (1, 2), and antidepressants selective for 5-HTT are the most common treatments for depression. Binding to the 5-HTT site is a well-proven therapeutic property of antidepressants.
Even though selective serotonin reuptake inhibitors (SSRIs) are often used to treat major depression, the percentage of 5-HTT sites blocked during a typical course of SSRI treatment is unknown. One [11C]McN5652 positron emission tomography (PET) study found that a single intravenous dose of citalopram (6 mg/kg) or a single oral dose of paroxetine (60–80 mg) reduced the 5-HTT binding potential in baboons and healthy humans, respectively (3). The binding potential is proportional to receptor density and affinity. One single photon emission computed tomography study using [123I]2-beta-carbomethoxy-3-beta-(4-iodophenyl)-tropane (β-CIT) reported that, compared to healthy subjects, depressed patients treated with 20–60 mg/day of citalopram had a 50% reduction in the binding potential in the combined thalamus and brainstem regions (4). This study had two potentially important confounds: 1) a within-subject design was not used and 2) between-group differences unrelated to the effect of citalopram, such as the presence of major depression, could have biased the measure of drug effect. The β-CIT binding potential in the brainstem is lower during major depression (5), and the β-CIT binding potential in the thalamus is lower during seasonal affective disorder (6). Because β-CIT has equal affinity for 5-HTT and dopamine transporters (7), unblocked dopamine transporters in the ventral tegmentum and the substantia nigra within the brainstem (8) could lead to an underestimate of the drug effect.
[11C](N,N-Dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine (DASB) is a new PET radiotracer that is highly selective, showing nanomolar affinity for 5-HTT and negligible affinity for other monoamine transporters (9). Available data suggest that [11C]DASB is superior to other PET radioligands for 5-HTT. In humans, binding potential values found with [11C]DASB PET are approximately two- to threefold greater than those found with [11C](+)McN5652 PET (9–12). With [11C](+)McN5652 PET, 5-HTT binding potential values are not detectable in the frontal cortex and are modestly detectable in only the basal ganglia and the thalamus (3). With [11C]DASB PET, 5-HTT binding potential values are detectable in the frontal cortex and are reasonably high in the basal ganglia and the thalamus (11, 12).
The main purpose of this study was to measure the occupancy of 5-HTT sites the treatment of depressed patients with paroxetine and citalopram by using [11C]DASB PET and a within-subject design. Occupancy is the percent reduction in binding potential after drug administration. A secondary aim was to assess the relationship between serum paroxetine levels and 5-HTT occupancy.
Method
Subjects
This study was approved by the University of Toronto Human Subjects Review Committee. Thirteen depressed subjects aged 21–50 years (five women and eight men; mean age=37 years, SD=8) and 17 healthy subjects aged 22–51 years (nine women and eight men; mean age 32 years, SD=9) were recruited. Twelve depressed subjects completed the full protocol. For each subject, written consent was obtained after the procedures had been fully explained.
Healthy subjects were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (13). For patients, a diagnosis of a major depressive episode secondary to major depressive disorder was confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) (14), which was administered by a trained research assistant. Each patient also received a psychiatric consultation (J.H.M.) to verify the SCID-P diagnosis. A score of 16 or greater on the 17-item Hamilton Depression Rating Scale (15) was required for entry into the study (mean=19, SD=4). Patients with psychotic symptoms, bipolar disorder (type I or II), borderline personality disorder, long-term self-harm behavior outside of major depressive episodes, or comorbid axis I diagnoses were excluded, as were subjects with a history of alcohol or drug abuse or dependence. In addition, history of all drug use was recorded. No subject had a history of exposure to 3,4-methylenedioxymethamphetamine or other drugs suspected to have neurotoxic effects on neurons expressing 5-HTT (16). Subjects with a history of substance use that did not meet criteria for the SCID-P diagnosis of substance abuse received a urine drug screening and were included in the study only if the result was negative. Two subjects had previously completed an antidepressant trial (with paroxetine or imipramine) of greater than 6 weeks’ duration, but neither had received any antidepressant treatment within 2 months. Each depressed subject had routine tests to rule out common medical causes of depression (tests of thyroid function and electrolytes, CBC).
Imaging and Treatment Protocol
Subjects with major depression were scanned with [11C]DASB PET before and after 4 weeks of treatment. Seven subjects received 20 mg/day of paroxetine, one subject received 10 mg/day of paroxetine, and four subjects received 20 mg/day of citalopram. One person discontinued participation in the study after the baseline scan. The one subject who received 10 mg/day of paroxetine initially started with 20 mg/day, but after several days this dose was reduced because of severe insomnia. For this patient, the follow-up [11C]DASB PET scan took place 4 weeks after the dose was reduced to 10 mg/day. Before treatment, patients were informed that their serum SSRI levels would be sampled on the day of the second [11C]DASB PET scan. The serum sampling and second [11C]DASB PET scan took place 6–13 hours after the last dose. Samples were frozen at –20°C and subsequently assayed by using high-performance liquid chromatography with fluorescence detection (Medical Toxicology Unit, Guy’s & St. Thomas’ Hospital Trust, London) (17).
More subjects were assigned to receive paroxetine treatment because we wanted to investigate the relationship between serum levels and occupancy for this antidepressant. We chose to investigate this relationship for the 20 mg/day dose of paroxetine because long-term dosing of paroxetine at 20 mg/day produces a wide range of drug serum levels (18).
Treatment response was determined by the Hamilton depression scale score at the end of 6 weeks of treatment. A final Hamilton depression scale score of ≥16 indicated nonresponse, 9–15 indicated a partial response, and ≤8 was considered a full response (19).
Seventeen healthy subjects were scanned with [11C]DASB PET. Six of these subjects were scanned again with [11C]DASB PET after a 4-week interval. To rule out baseline influences of major depression on the striatal 5-HTT binding potential, 13 of the healthy subjects were age-matched within 2 years to each depressed patient. Age matching was done because it has been reported that the 5-HTT binding potential declines in some brain regions with age (20).
[11C]DASB was synthesized as described previously (9, 21). Briefly, [11C]-CH3I was trapped in a high-performance liquid chromatography sample loop coated with a solution of the N-normethyl precursor (1 mg) in dimethylformamide (80 μl). After 5 minutes at ambient temperature, the contents of the sample loop were injected onto a reverse-phase high-performance liquid chromatography column, and the fraction containing the product was collected, evaporated to dryness, formulated in saline, and filtered through a 0.2-μ filter.
Imaging was based on the approach described by Houle et al. (11). An intravenous bolus of 370 MBq of [11C]DASB was injected. The [11C]DASB was of high radiochemical purity (>95%) and high specific activity (40 GBq/mol, SD=17, at the time of injection). PET images were obtained by using a GEMS 2048-15B camera (Scanditronix Medical, Uppsala, Sweden) (voxel dimensions=2, 2, and 6.5 mm in x, y, and z axes). Images were obtained in 15 1-minute frames, followed by 15 5-minute frames. The images were corrected for attenuation by using a 68Ge transmission scan and reconstructed by filtered back projection (Hanning filter, 5 mm full width at half maximum).
Data Analysis
To obtain a measure of the 5-HTT binding potential with region-of-interest data, we used multilinear regression analysis as described by Ichise et al. (23, 24), implemented within a standard software package (24). This model assumes that there is a region of interest that contains specifically bound radioligand and that there is a reference region that does not contain specifically bound radioligand. For [11C]DASB, the cerebellum is suitable as a reference region since either undetectable (25) or extremely low 5-HTT density in that region has been reported (26, 27). The bilateral striatum was chosen as the region with specific binding for primary analyses. Several other regions were also studied. The bilateral striatum was chosen as the primary region for occupancy estimates because [11C]DASB uptake is high in the striatum (11), absolute test-retest differences in 5-HTT binding potential for this region in preliminary analyses were low (10%), and we know of no reports that depression itself influences 5-HTT receptor density or affinity in this region. The binding potential is proportional to Bmax/Kd (Mintun et al. [28] were among the first to discuss this relationship. For further explanation, see the review by Meyer and Ichise [29]), where Bmax represents receptor density and Kd is the dissociation constant and is inversely proportional to affinity. This application of multilinear regression analysis uses a reference region as a substitute for arterial sampling (22, 23). It does not require arterial sampling.
Previous work at our center demonstrated that the 5-HTT binding potential found by using methods based on reference tissue is highly correlated with the ratio of the kinetically determined distribution volumes between the regions with specific binding and the reference region (12, 30). In our data, we found distribution volumes in the thalamus, striatum, frontal cortex, and cerebellum in five healthy subjects by using an arterial input function and a single tissue compartment model. Ratios of distribution volume in regions with specific binding to the distribution volume of the cerebellum were found for each brain region. This ratio was highly correlated (r=0.98, p<0.001) with the binding potential found by using multilinear regression analysis (30).
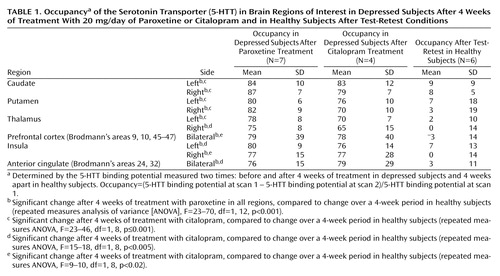
Each subject had a magnetic resonance imaging (MRI) scan (Signa 1.5-T scanner [General Electric, Milwaukee], spin-echo sequence proton density weighted image; x, y, and z voxel dimensions=0.78, 0.78, and 3 mm, respectively). MRI scans were coregistered to each PET image by using an entropy-based measure, Normalized Mutual Information (31), which is insensitive to biases of image overlap. For the region-of-interest analysis, the caudate, putamen, thalamus, prefrontal cortex, insula, anterior cingulate, and cerebellum regions of interest were drawn on summated [11C]DASB PET scans with reference to a coregistered MRI by a rater who was blind to the identity of the subjects associated with the scans. The method of drawing regions of interest in the striatum and the cerebellum to measure occupancy has been described previously (32). Regions of interest were drawn in contiguous transverse planes: two for the thalamus and anterior cingulate, four for the insula, and seven for the prefrontal cortex. Brodmann’s areas within the regions of the prefrontal cortex and the anterior cingulate are described in Table 1.
Occupancy in the striatum was defined as ([5-HTT binding potential in scan 1 – 5-HTT binding potential in scan 2]/5-HTT binding potential in scan 1). Occupancy expressed as a percentage was calculated for each subject. The variability of the occupancy measure is based on the variability of the 5-HTT binding potential at scan 1 as well as the variability of 5-HTT binding potential at scan 2.
Results
Effect of Major Depression on Striatal 5-HTT Binding Potential
If major depression were to have a considerable effect on striatal 5-HTT binding potential and if such an effect were reversed independently of occupancy, then, in theory, a bias in occupancy determination could occur. To assess this possibility, we compared the striatal 5-HTT binding potential between healthy and depressed subjects. No significant differences in striatal 5-HTT binding potential were found between the healthy and depressed groups at the first [11C]DASB PET scan, although there was a significant decline with age (analysis of covariance [ANCOVA] with age as covariate, effect of age alone, F=4.90, df=1, 18, p<0.04; ANCOVA with age as covariate, effect of diagnosis, F<0.06, df=1, 27, p=0.82). We examined the effect of depression in the striatum because it was chosen as the primary region for analyses of occupancy (see Method).
Effects of Treatment
All patients scanned before and after treatment had detectable serum paroxetine or citalopram levels and were included in the analysis (paroxetine: mean=26 μg/liter, SD=17; citalopram: mean=65 μg/liter, SD=14).
The test-retest data were obtained from healthy subjects. In the test-retest data, among regions, the mean difference, expressed as a percent of the initial binding potential was –3.7% (SD=3.7%) and ranged from –8% to 2%. The mean absolute difference, expressed as a percent of the initial binding potential was 10.9 (SD=2.9) and ranged from 7% to 15%.
There was an obvious effect of the SSRIs on the summated [11C]DASB PET images (Figure 1). The absolute decline in striatal 5-HTT binding potential was significantly greater during paroxetine and citalopram treatment than in the test-retest data for the healthy subjects (repeated measures analysis of variance [ANOVA], effect of paroxetine treatment, F=58.96, df=1, 12, p<0.001; effect of citalopram treatment, F=32.16, df=1, 8, p<0.001). For this repeated measures ANOVA, the 5-HTT binding potential was the repeated measure and effect of the test-retest condition versus drug treatment was compared.
For patients treated with 20 mg/day of paroxetine (N=7), the mean occupancy in the bilateral striatum ([5-HTT binding potential pretreatment – 5-HTT binding potential post treatment]/5-HTT binding potential pretreatment) was 83% (SD=5%). For patients treated with 20 mg/day of citalopram (N=4), the mean occupancy was 77% (SD=10%). See Table 1 for a comparison of occupancies among test-retest and drug treatments.
There was no association between the final Hamilton depression scale score after 6 weeks and occupancy in the striatum (r=0.07, df=10, p=0.84, N=11). In addition, there was no association between the final Hamilton depression scale score after 6 weeks and occupancy in any other brain region, after correcting for multiple comparisons (r=–0.31 to 0.61, df=10, p=0.047 to 0.88 [before correcting for multiple comparisons], N=11, 10 correlations, regions listed in Table 1).
Relationship Between Serum Paroxetine and Occupancy
Occupancy increased as serum levels of paroxetine increased. Previous studies of serum level and occupancy typically have described a hyperbolic relationship of the form f(x)=a*x/(b+x) (32–34). Thus, we chose this model to describe our data, and the fit was significant (F=41.00, df=1, 6, p=0.0007). Visual examination of the curve revealed that approximately 85% occupancy is reached at serum paroxetine levels of 28 μg/liter and that a considerable increase in serum levels is needed to obtain greater occupancy. No significant change in occupancy occurred for paroxetine levels greater than 28 μg/liter (linear regression, slope=–0.09% μg/liter, F=0.50, df=1, 3, p=0.53). Figure 2 shows a plot of the relationship between occupancy and paroxetine serum levels.
Discussion
To our knowledge, this is the first study of 5-HTT occupancy during SSRI treatment of depression to use a within-subject design and a PET radioligand that is highly selective for 5-HTT. We found, on average, an 80% decrease in 5-HTT binding potential in the basal ganglia after 4 weeks of treatment. We also found that occupancy increased as the serum levels of paroxetine increased, reaching a plateau for serum concentrations greater than 28 μg/liter.
The 80% decrease in 5-HTT binding potential during treatment is best explained by the high affinity of paroxetine and citalopram for 5-HTT (1, 2). As a result of drug binding to 5-HTT, fewer 5-HTT sites are available for [11C]DASB binding, decreasing the detected 5-HTT binding potential. A second mechanism by which 5-HTT binding potential may decrease is by down-regulation of 5-HTT. It is reported that 5-HTT down-regulates and that 5-HTT mRNA is reduced in animals after long-term administration of SSRIs by minipump (35–37). Occupancy is a useful measure because it can reflect both blockade and down-regulation, and it is possible that both could reduce 5-HT clearance by means of 5-HTT and raise cortex 5-HT levels (35). Raising cortex 5-HT levels is thought to be a key step in the therapeutic mechanism of SSRIs (18, 38, 39).
The 80% decrease in 5-HTT binding potential appears to be lower than what would be predicted by the in vitro affinities of these SSRIs for 5-HTT (1, 2). If SSRI serum levels and brain levels (near the 5-HTT site) were identical, one would expect greater than 99% occupancy (for a Kd for the 5-HTT of approximately 1 nM and 0.1 nM for citalopram and paroxetine, respectively [1, 2]). The most plausible explanation for the discrepancy is that serum paroxetine and citalopram concentrations are different from the concentrations of these SSRIs near 5-HTT in the brain. This in not unexpected because other factors such as lipophilicity and degree of nonspecific binding may influence brain drug concentrations. These factors would affect serum concentrations differently.
Eighty percent occupancy of 5-HTT sites reduces the proportion of functioning 5-HTT sites to a lower level than would occur under physiological conditions. In animal studies of long-term serotonin depletion, the largest reductions in 5-HTT binding potential (estimated by changes in receptor density × affinity) are about 30% (40, 41). It may be that an 80% occupancy is required to increase cortex serotonin levels to the degree that most therapeutic effects can occur. Virtually no investigations have been done to show that doses of citalopram and paroxetine below 20 mg/day effectively treat depression (42). In fact, only a few clinical trials of low-dose SSRI treatment of depression have been done (42–44). Further studies with low-dose SSRIs would be needed to understand how occupancy levels specifically relate to therapeutic effects.
The relationship of increased occupancy with increased serum levels for very low drug serum levels and an occupancy plateau for higher serum levels has also been reported for neuroleptic medications (32–34). To our knowledge, the relationship between occupancy and serum levels has not been previously reported for any SSRI.
It has become common clinical practice to raise paroxetine dosing higher than 20 mg/day in the event of nonresponse or partial response. The data of the current study suggest that for the majority of patients, increasing paroxetine beyond 20 mg/day has minimal effects on 5-HTT blockade. However, it is still possible that higher dosing of paroxetine has therapeutic benefit either by inducing minimal increases in 5-HTT blockade or by increasing binding to sites other than 5-HTT.
What level of occupancy is optimal for clinical response is an interesting question. In the treatment of depression, multiple mechanisms of SSRI effect are possible, and it is known that placebo has a significant therapeutic influence. Therefore, it would be unrealistic to expect an association between occupancy and clinical response in our sample. However, findings of strong relationships between drug serum level and occupancy will eventually contribute to answering this question. By using large samples of data from clinical trials in which serum levels of antidepressants and clinical response were measured, occupancy estimates could be derived from serum antidepressant levels. This approach should provide good power to determine the relationship between occupancy and clinical response.
This study had several methodological limitations. The binding potential reflects Bmax/Kd, and we were unable to discern between these two parameters. However, the combined measure may be more relevant to 5-HTT function. With respect to the SSRI treatment, we took many precautions to enhance compliance, such as informing patients that their serum levels were to be assayed at the time of the second PET scan as part of the study. We also took serum measurements and detected paroxetine and citalopram levels in every patient. Even so, it is possible that some patients may have temporarily interrupted their treatment at an earlier point, and this interruption would not be detected in our protocol.
In conclusion, by using a new, more selective PET radioligand for 5-HTT, we found that 80% of 5-HTT receptors were occupied during treatment with clinical doses of paroxetine or citalopram. Although this change in 5-HTT binding potential was greater than what has been observed under physiological conditions, this level of change might be necessary for some therapeutic effects. The plateau found in the relationship between occupancy and serum paroxetine level indicates that raising paroxetine doses beyond 20 mg/day is unlikely to have much effect on 5-HTT.
 |
Received Jan. 30, 2001; revision received May 30, 2001; accepted June 6, 2001. From the Centre for Addiction and Mental Health and the Department of Psychiatry, University of Toronto. Address reprint requests to Dr. Meyer, PET Centre, Centre for Addiction and Mental Health, Department of Psychiatry, University of Toronto, 250 College St., Toronto, Ontario, Canada M5T 1R8; [email protected] (e-mail). Supported by Eli Lilly and the National Alliance for Research on Schizophrenia and Depression. Dr. Meyer acknowledges salary support from the Canadian Institutes of Health Research. The authors thank Alex Kecojevic, Kevin Cheung, Armando Garcia, Li Jin, and Ruiping Guo for their assistance.
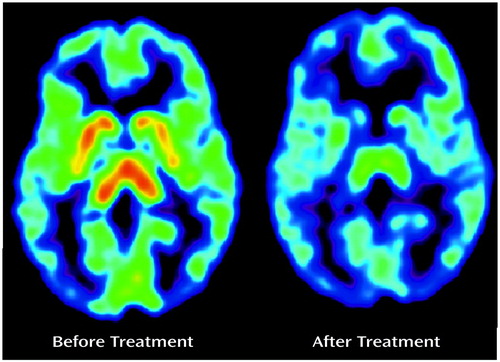
Figure 1. Effect of Citalopram;a on [11C]DASB PET Scan of the Serotonin Transporter in a Depressed Subject
aTreatment was with 20 mg/day of citalopram for 4 weeks. Images represent summated frames normalized to mean summated cerebellum values.
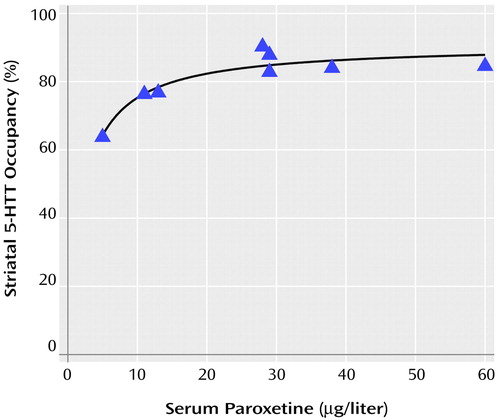
Figure 2. Relationship Between Striatal Serotonin Transporter (5-HTT) Occupancy and Serum Paroxetine Levels in Eight Depressed Patientsa
aOccupancy is defined as the percent decrease in 5-HTT binding potential after 4 weeks of treatment with paroxetine. Occupancy=(5-HTT binding potential at scan 1 – 5-HTT binding potential at scan 2)/5-HTT binding potential at scan 1. One patient (serum level, 5 μg/liter) received 10 mg/day of paroxetine, and the remaining patients received 20 mg/day of paroxetine. In nonlinear regression analysis, a hyperbolic curve of the form f(x)=a*x/(b+x) significantly fit the data (F=41.00, df=1, 6, p=0.0007).
1. Tatsumi M, Groshan K, Blakely RD, Richelson E: Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol 1997; 340:249-258Crossref, Medline, Google Scholar
2. Owens MJ, Morgan WN, Plott SJ, Nemeroff CB: Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 1997; 283:1305-1322Medline, Google Scholar
3. Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M: In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 2000; 41:1465-1477Medline, Google Scholar
4. Pirker W, Asenbaum S, Kasper S, Walter H, Angelberger P, Koch G, Pozzera A, Deecke L, Podreka I, Brucke T: β-CIT SPECT demonstrates blockade of 5HT-uptake sites by citalopram in the human brain in vivo. J Neural Transm Gen Sect 1995; 100:247-256Crossref, Medline, Google Scholar
5. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 β-carbomethoxy-3 β-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090-1098Crossref, Medline, Google Scholar
6. Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brucke T, Kasper S: [123I]-β-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry 2000; 47:482-489Crossref, Medline, Google Scholar
7. Laruelle M, Giddings SS, Zea-Ponce Y, Charney DS, Neumeyer JL, Baldwin RM, Innis RB: Methyl 3 β-(4-[125I]iodophenyl)tropane-2 β-carboxylate in vitro binding to dopamine and serotonin transporters under “physiological” conditions. J Neurochem 1994; 62:978-986Crossref, Medline, Google Scholar
8. Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI: Immunocytochemical localization of the dopamine transporter in human brain. J Comp Neurol 1999; 409:38-56Crossref, Medline, Google Scholar
9. Wilson A, Schmidt M, Ginovart N, Meyer J, Houle S: Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, in vitro and ex vivo evaluation of [11C]-labelled 2-(phenylthio) araalkylamines. J Med Chem 2000; 43:3103-3110Crossref, Medline, Google Scholar
10. Szabo Z, Kao P, Scheffel U, Suehiro M, Mathews W, Ravert H, Musachio J, Marenco S, Kim S, Ricaurte G, Wong D, Wagner H, Dannals R: Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse 1995; 20:37-43Crossref, Medline, Google Scholar
11. Houle S, Ginovart N, Hussey D, Meyer J, Wilson A: Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med 2000; 27:1719-1722Crossref, Medline, Google Scholar
12. Ginovart N, Wilson A, Meyer J, Hussey D, Houle S: PET quantification of [11C]DASB binding for in vivo visualization of the serotonin transporter in healthy human volunteers. J Cereb Blood Flow Metab (in press)Google Scholar
13. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
14. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
15. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
16. Lesch KP, Heils A, Riederer P: The role of neurotransporters in excitotoxicity, neuronal cell death, and other neurodegenerative processes. J Mol Med 1996; 74:365-378Crossref, Medline, Google Scholar
17. Spencer E: An improved method for the measurement of paroxetine in human/serum plasma by HPLC with fluorescence detection (abstract). Ther Drug Monit 1999; 21:483Crossref, Google Scholar
18. Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH: The effect of paroxetine upon 5-HT2A receptors in depression: an [18F] setoperone PET imaging study. Am J Psychiatry 2001; 158:78-85Link, Google Scholar
19. Mowbray RM: The Hamilton Rating Scale for depression: a factor analysis. Psychol Med 1972; 2:272-280Crossref, Medline, Google Scholar
20. van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, Baldwin RM, Innis RB: Age-related decline in central serotonin transporter availability with [123I]β-CIT SPECT. Neurobiol Aging 2000; 21:497-501Crossref, Medline, Google Scholar
21. Wilson A, Garcia A, Jin L, Houle S: Radiotracer synthesis from [11C]-iodomethane: a remarkably simple captive solvent method. Nucl Med Biol 2000; 27:529-532Crossref, Medline, Google Scholar
22. Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, Kung HF: Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med 1996; 37:513-520Medline, Google Scholar
23. Ichise M, Ballinger JR, Vines D, Tsai S, Kung HF: Simplified quantification and reproducibility studies of dopamine D2-receptor binding with iodine-123IBF SPECT in healthy subjects. J Nucl Med 1997; 38:31-37Medline, Google Scholar
24. Berger C, Buck A: Requirements and implementation of a flexible kinetic modelling tool. J Nucl Med 1997; 38:1818-1823Medline, Google Scholar
25. Cortes R, Soriano E, Pazos A, Probst A, Palacios J: Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience 1988; 27:473-496Crossref, Medline, Google Scholar
26. Backstrom I, Bergstrom M, Marcusson J: High affinity [3H] paroxetine binding to serotonin uptake sites in human brain tissue. Brain Res 1989; 486:261-268Crossref, Medline, Google Scholar
27. Laruelle M, Vanisberg M-A, Maloteaux J-M: Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry 1988; 24:299-309Crossref, Medline, Google Scholar
28. Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ: A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984; 15:217-227Crossref, Medline, Google Scholar
29. Meyer J, Ichise M: Modelling of receptor ligand data in PET and SPECT imaging: a review of major approaches. J Neuroimaging 2001; 11:30-39Crossref, Medline, Google Scholar
30. Ginovart N, Wilson A, Meyer J, Hussey D, Houle S: PET Quantification of [11C]-DASB binding to the serotonin transporter in humans, in Abstracts of the 48th Annual Meeting of the Society of Nuclear Medicine. Reston, Va, SNM, 2001, p 62Google Scholar
31. Studholme C, Hill D, Hawkes D: An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognition 1999; 32:71-86Crossref, Google Scholar
32. Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, Houle S: 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 1998; 155:921-928Link, Google Scholar
33. Kapur S, Cho R, Jones C, McKay G, Zipursky RB: Is amoxapine an atypical antipsychotic? positron-emission tomography investigation of its dopamine2 and serotonin2 occupancy. Biol Psychiatry 1999; 45:1217-1220Crossref, Medline, Google Scholar
34. Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L: Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry 1999; 156:869-875Link, Google Scholar
35. Benmansour S, Cecchi M, Morilak D, Gerhardt G, Javors M, Gould G, Frazer A: Effects of chronic antidepressant treatments on serotonin transporter function, density and mRNA level. J Neurosci 1999; 19:10494-10501Crossref, Medline, Google Scholar
36. Lesch KP, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL: Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Brain Res Mol Brain Res 1993; 17:31-35Crossref, Medline, Google Scholar
37. Pineyro G, Blier P, Dennis T, de Montigny C: Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci 1994; 14(5, part 2):3036-3047Google Scholar
38. Bel N, Artigas F: Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol 1992; 229:101-103Crossref, Medline, Google Scholar
39. Moret C, Briley M: Effects of acute and repeated administration of citalopram on extracellular levels of serotonin in rat brain. Eur J Pharmacol 1996; 295:189-197Crossref, Medline, Google Scholar
40. Dewar KM, Grondin L, Carli M, Lima L, Reader TA: [3H]Paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem 1992; 58:250-257Crossref, Medline, Google Scholar
41. Rattray M, Baldessari S, Gobbi M, Mennini T, Samanin R, Bendotti C: p-Chlorphenylalanine changes serotonin transporter mRNA levels and expression of the gene product. J Neurochem 1996; 67:463-472Crossref, Medline, Google Scholar
42. Feighner JP, Overo K: Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. J Clin Psychiatry 1999; 60:824-830Crossref, Medline, Google Scholar
43. Wernicke JF, Dunlop SR, Dornseif BE, Bosomworth JC, Humbert M: Low-dose fluoxetine therapy for depression. Psychopharmacol Bull 1988; 24:183-188Medline, Google Scholar
44. Schmidt ME, Fava M, Robinson JM, Judge R: The efficacy and safety of a new enteric-coated formulation of fluoxetine given once weekly during the continuation treatment of major depressive disorder. J Clin Psychiatry 2000; 61:851-857Crossref, Medline, Google Scholar



