The Human Genome: Genetic Testing and Animal Models
The availability of animal models for human diseases, especially for mental illness, is critical for progress in developing therapeutics for mood disorders and schizophrenia. Because mental diseases currently have no described pathophysiology, verifying animal models is particularly difficult. However, a strategy is emerging in the area of molecular genetics to examine putative animal models to see how well they fit a human condition. The assumptions of this strategy are that a good animal model ought to use some of the same molecular mechanisms as the human disease. Human diseases, even mental diseases, now have identified gene loci that are associated with the illness. If an animal model can show that its molecular changes are similar to the illness changes, then the veracity of the model is supported.
The illustration (see figure) shows a scheme for testing methamphetamine in the rat as a model for mania with psychosis. Methamphetamine can cause symptoms in humans similar to those of manic psychosis and has been proposed to be a model for this human illness. If methamphetamine can be shown to produce the same changes in the rat brain as those associated with manic psychosis, then the usefulness of this as an animal model of the illness is enhanced.
Rat RNA from treated animals was applied to an Affymetrix GeneChip (Santa Clara, Calif.) with gene probes for more than 8,000 genes. The results showed that the gene whose expression was stimulated to the greatest degree by the methamphetamine was a G protein receptor kinase, called GRK3. This protein participates in G protein signaling by terminating the signal at a G protein receptor after stimulation. The location of the gene for GRK3 on the rat genome was identified. Then the analogous location on the human genome was found. GRK3 in the rat genome is analogous to the region 22q11 on the human genome, the area where several laboratories, including ours, have identified an association with both bipolar illness and schizophrenia. This close proximity of a susceptibility gene for bipolar disorder/schizophrenia with a gene that is activated by methamphetamine in rat brain strengthens the claim that this preparation is a model for manic psychosis. Verification of an association between the RNA expression patterns of other putative models for mood disorders and schizophrenia with the human genes associated with the illnesses themselves can also, in similar fashion, rule in or out their closeness as a model for the human disease.
Address reprint requests to Dr. Tamminga, Maryland Psychiatric Research Center, University of Maryland, P.O. Box 21247, Baltimore, MD 21228; [email protected] (e-mail). The image is courtesy of Drs. Niculescu and Kelsoe.
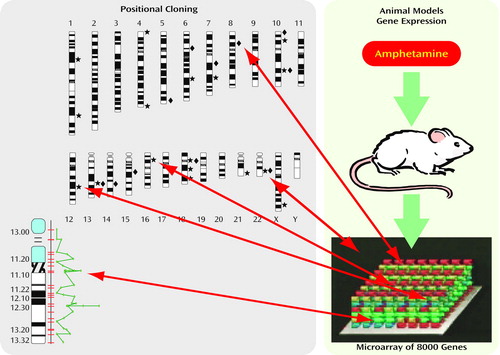
Figure Convergent functional genomics: genes whose expression is changed in an animal model and map to chromosomal loci identified by linkage are high-probability candidates. The first step is to identify more likely loci in the genome; the second is to identify specific genes within linkage peaks.



