Functional Imaging Studies: Linking Mind and Basic Neuroscience
Abstract
OBJECTIVE: The imaging of brain activity with positron emission tomography (PET) and functional magnetic resonance imaging has assumed a central position in psychiatry. Functional imaging signals arise from changes in the neurophysiological parameters of glucose and oxygen consumption mediated by blood flow. METHOD: Recent in vivo 13C nuclear magnetic resonance (NMR) neurochemical studies have established a quantitative coupling between the rates of glucose oxidation and glutamate neurotransmitter flux in rats and humans, thereby linking measured neurophysiological parameters to brain function. RESULTS: These results show that in the awake, resting, and unstimulated states, 70%–80% of brain energy consumption is devoted to the same glutamate/glutamine neurotransmitter signaling as are the small percentages stimulated by tasks. Furthermore, in anesthetized animals, in which unstimulated activity is reduced, the total signal rather than a particular increment is required for a response. CONCLUSIONS: On this basis, the total signal, as well as the difference in the signal, measures cortical neurotransmitter flux. The total signal in a region therefore contains valuable information about required brain activity. Although signal change is often more easily measured, certain PET and 13C NMR methods can quantify total regional signal activity and thereby provide another measure of neurotransmitter activity.
The human mind commands our attention. We are justly fascinated by the workings of our own mind and of others. We should be; all of our culture reveals the luxuriant complexities of the mind. Studies of the mind from neuroscience and psychiatry have risen to the richness of the subject with brilliant descriptions of mental phenomena and insights into mechanisms. It is not surprising, therefore, that scientific studies of the mind start with observations of brain responses during mental activities in an effort to explore down to a level where, as Francis Crick urged us, “our minds—the behavior of our brains—can be explained by the interactions of nerve cells (and other cells) and the molecules associated with them” (1).
Much of recent optimism about being able to bridge the brain and the mind derives from functional positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). These methods are often represented as directly mapping neuronal activity in the functioning human brain. Impressive two- and three-dimensional maps of “neuronal activity” in the functioning brain have been embraced by scientists and the popular media as providing new insights into the biological basis of how the mind works. In functional imaging studies of sensory perception, recent progress is heartening, with impressive identifications and localizations of sensory functions, such as color or edge detection in the extra striate cortex. When we combine the results of these studies on humans with electrophysiological and cellular studies of animal models, we gain important insights into how complex sensory functions are supported by the brain’s molecular and cellular activity. But when functional imaging studies have approached conceptual activities of the mind, progress has taken on a different complexion. Functional imaging studies of mental processes are interpreted almost exclusively within an established paradigmatic view of “mind”—an interpretation that exposes both the strengths and limitations of the methods.
At present the dominant paradigm for interpreting functional imaging derives from cognitive neuroscience. This field is broad and includes many different conceptualizations of mind. These different theories share the common assumption that the functions of mind may be represented as information processing. As defined by Herbert A. Simon, one of its founders, “Cognitive science is the study of intelligence and intelligent systems, with particular reference to intelligent behavior as computation” (2). Representations of information processing are most often located in the brain by functional imaging methods that localize changes in signals assigned to neuronal activities. Cognitive science has thereby provided a congenial conceptual framework for the planning and interpretation of functional imaging experiments by means of PET or fMRI.
The original application of cognitive neuroscience to functional imaging was in pioneering PET studies by Posner and Raichle (3), in which the methods for planning and interpreting these studies were developed. In this paradigm complex mental processes such as speech are assumed to be decomposable into simpler subprocesses such as verb generation and grammar. Functional imaging is then used to identify the anatomical location of these subprocesses on the basis of changes in the magnitude of the imaging signal produced when a subject performs experimental tasks. These changes give rise to the difference signal that is characteristic of all functional imaging experiments (3, 4; see, e.g., 5). There is a general awareness of the uncertainties involved in inserting a new task component and assuming that the difference signal specifically reflects the consequences of the inserted component (the insertion assumption). The possibility that all aspects of the insertion have not been identified is sometimes characterized as parallel processing (6). Considerable ingenuity and effort has been devoted to designing control conditions to minimize parallel processes and to validate any difference found. Although in the first experiments, the resting brain was used as the control condition, subsequent studies of more complex brain functions have tried to maintain differences between control and test conditions by refined and thoughtful procedures. These include symmetrically lateralized tasks (7) or similar tasks with graded differences of one component (8). Evaluations of the effects of modality on the response have been made to provide a basis for minimizing extraneous factors (9). Maps of these difference signals handily present experimental results and summarize the increased, or decreased, activations associated with different functional activities. Results are tested against other localization experiments, theories of mind, and the wide range of information available from behavioral tests and lesion studies (4; see, e.g., 5). Agreement between observable differences has often been found and provides the basis for the prevailing optimism about uncovering the links between mind and brain.
The ability to generate the profusion of functional images of brain activity and to interpret localized signals has popularized the model of cognitive neuroscience in which complex functions are localized to small, discrete regions of the brain, often referred to as modules, to the point in which other theories of mind have been abandoned and are only now slowly reviving. Has, in fact, this particular interpretation of functional imaging experiments finally resolved the disconnection between mind and brain most prominently offered by Descartes? Is the mind finally to be understood by this modern view of brain activity, as claimed by many? While welcoming the systematic parameterizing of considerable data, criticisms question the completeness and assumptions of this formulation (10–15). These concerns have been expressed by psychiatrists (10), scientists (11, 14, 15), and philosophers (12, 13)—as they should be—because the assumptions of the strictly modular view of cognitive neuroscience represent highly controversial philosophical and psychological views of mind. After being cast into Crick’s formulation of the goals of neuroscience, the questions facing the field center on whether, in fact, the computational model of cognitive neuroscience, as applied to imaging, has identified representations of mind that, when reduced to molecules and cells, are the most helpful basis for answering mind/brain questions (12). Or must the limitations of neuroscience, which start with the difficulties of including consciousness and subjectivity within a model of localized computational modules, be addressed before the reductionist progress sought by neuroscientists is possible?
A problem even more fundamental in functional imaging than questions about proper interpretation is the quantification of the signal. Although almost always represented as a measure of neuronal activity, the imaging signal is actually a measure—often indirect—of blood flow and energy consumption. The relationship between neuroenergetics and neuronal activity is controversial and has been generally ignored in the interpretation of functional imaging data. Instead, the formulations of cognitive neuroscience used in imaging experiments are increasingly interpreted as displaying the mutual reinforcement of theory and experiment found in mature, well-tested scientific fields such as thermodynamics. However, when we consider the complexities and uncertainties from a historical perspective, this agreement may in fact resemble more closely the explanatory powers of Ptolemaic epicycles or phrenology.
I propose an escape from this circularity through an independent determination of the functional neuronal activities represented in the imaging signal on the basis of recent neurochemical results from in vivo 13C magnetic resonance spectroscopy (MRS) (16–18). These experiments have established quantitative relations between the cerebral metabolic rate (CMR) of glucose oxidation and the CMR of glutamate neurotransmitter flux. Hence, functional imaging signals, which can be converted to changes in glucose oxidation, measure changes in neurotransmitter flux. Furthermore, absolute values of neurotransmitter activity on this basis, in the absence of stimulation, are shown to be larger than the changes induced by tasks, and the absolute rather than relative values are shown to be required for function. Implications of large neuronal activity for other neuroscientific models of mind will be explored in this article.
In Vivo 13C Nuclear Magnetic Resonance (NMR)
Cortical communication between neurons is fundamental to the neurobiological view of brain function. Most such information is carried across synapses by small molecules acting as neurotransmitters. Dozens of neurotransmitters have been identified and characterized. Two neurotransmitters, glutamate and γ-aminobutyric acid (GABA), stand out because of their high concentrations (in the millimolar range). Furthermore, in the mammalian cerebral cortex, 80%–90% of the synapses serve these two neurotransmitters. Glutamate is excitatory, serving to transmit activation, while GABA, in the minority, serves an inhibitory function.
High concentrations of glutamate and GABA in the brain have enabled us recently to observe their high-resolution NMR signals in vivo. The first observations by 13C NMR (sometimes called MRS) of cerebral glutamate were made by means of indirect detection at the Magnetic Resonance Center at Yale in 1983 (19, 20). Since 1.1% of carbon nuclei are the NMR-visible 13C, this stable, nonradioactive nucleus has provided opportunities to measure cerebral metabolic rates when the injected material, usually glucose, was enriched. These 13C MRS experiments followed in time the flow of 13C isotopic labels from glucose into pools of metabolites. Advanced NMR techniques and equipment improvements now enable flux to be measured in several cubic centimeters of human brain by means of 13C NMR (21, 22). This provides valuable, quantitative measurements of metabolic rates in vivo that are unobtainable by any other means.
The oldest established 13C MRS measurement is of the flow from 1-13C glucose to 4-13C glutamate, which was used to determine flux through the tricarboxylic acid cycle 21, 23, 24). The CMR of oxygen consumption was calculated from the tricarboxylic acid cycle flux, which, by basic biochemistry, is directly coupled to oxidation. Values of the CMR of oxygen consumption determined by this method in experiments on rats and humans agreed well with literature values, so that by 1995 this in vivo method of measuring oxygen consumption and therefore energy consumption was well established.
At that time, because of technical improvements, it became possible to measure in the human brain the isotope label flow from glutamate and GABA into glutamine (21, 24). Glutamine, although not a neurotransmitter, had been proposed as an intermediate substance in the glutamate neurotransmitter cycle (Figure 1). The ability to follow in time, in vivo, the isotope label flow into glutamate and GABA and subsequently into glutamine by means of 13C MRS has opened this neurotransmitter cycling to quantitation. The cycle was originally proposed by Berl and colleagues (25) and Reubi et al. (26) from 14C and 15N studies of rat brains in the 1960s and 1970s. Additional evidence arose from enzyme and vesicle localization studies and isolated cell studies in the 1980s and 1990s (27). However, the importance of this pathway in vivo was not established because of conflicting evidence from studies of brain slices and other in vitro preparations. In the glutamate/glutamine cycle, shown in Figure 1, glutamate, stored in vesicles in the presynaptic neuron, is released into the synaptic cleft by the action potential (17, 18; for a review, see reference 28). Once released it diffuses across the gap until it is recognized by a glutamate receptor on the postsynaptic surface. There it triggers subsequent postsynaptic potential changes. The bulk of the released glutamate is not retaken up by the presynaptic terminal but instead diffuses to the membranes of the surrounding astrocyte, into which it is cotransported down the Na+ gradient. Astrocytic glutamine synthetase then converts glutamate to glutamine by consuming one adenosine triphosphate molecule and one ammonia molecule. Glutamine is transported across the astrocytic membrane, through the intracellular space, and into the neuron, where the enzyme glutaminase reconverts it to glutamate. The glutamate is repackaged into vesicles, where it is once again ready to begin the cycle.
Soon after these first experiments on human subjects, a comprehensive study of these rates of flux in the rat brain examined these results and conclusions in the more easily manipulated animal model (28, 29). After a few years of rat experiments, we and other laboratories returned to conduct additional human experiments (22, 30). As the data have accumulated, it has become clear that the cycling model is equally valid in rat and human brains and that the relative rates of cycling and glucose oxidation are similar in both species (30). These relative rates of oxidation and cycling have been studied over a wide range of brain activities in the rat, taking the animals from deep pentobarbital anesthesia, with a flat EEG indicating no neuronal firing, through lighter stages of anesthesia, and ending with a lightly anesthetized state in which neuronal firing has been enhanced by nicotine. Simultaneous measurements of the 13C flow into glutamate and of the subsequent flow into glutamine were used to calculate rates of the tricarboxylic acid cycle (VTCA) and the glutamate-to-glutamine neurotransmitter cycle (Vcycle), respectively. Cycling flux (Vcycle) plotted against energy consumption CMRglucose(ox) in Figure 2 fit a linear equation of CMRglucose(ox)=1.04Vcycle + 0.10 (Equation 1; all in units of μmol/min/g).
The plot in Figure 2 is significant in several respects. First, a quantitative relationship was established between cortical oxidative energy production expressed as glucose oxidation and a particular neurotransmitter activity. A second finding is shown at the intercept in Figure 2, where cycling flux falls to 0. This corresponds to the state of flat EEG and was brought about by deep pentobarbital anesthesia. At this point the value of oxidative glucose consumption in the nonfiring brain dropped to 15%–25% of the value at rest (0.8 μmol/min/g). This shows that in the awake, resting state of the rat cerebral cortex, 75%–85% of energy consumption supports functional neuronal activity. This conclusion is further supported by the finding that the specific functional process of the glutamate/glutamine cycle increases linearly with energy consumption. This high level of brain activity in the absence of specific stimulation is a novel neuroscientific finding from these experiments.
Another unexpected feature of the data plot in Figure 2 is that the slope is approximately 1 when the cycling flux is plotted against the rate of glucose oxidation above the intercept. The slope of unity means that for every additional glucose molecule oxidized, one glutamate molecule is released as a neurotransmitter that cycles through into glutamine. On the basis of 13C MRS studies, we believe that the contribution from GABA is approximately 10% of this cycling flux (31). Future developments in MRS should allow the simultaneous measurements of glutamate and GABA contributions to this pathway. This 1:1 stoichiometry allows changes in neurotransmitter flux (which can only be measured directly by means of this sort of 13C NMR experiment) to be determined from measured changes in oxygen consumption. We have previously shown that a molecular and cellular model proposed by Pellerin and Magistretti (32) from in vitro studies, in which astrocytic uptake of one glutamate molecule requires glycolysis of one glucose molecule, accounts for in vivo NMR stoichiometries.
The Interpretation of Signals in Imaging Studies
In applying modern functional imaging methods to understanding mind, one assumes that the imaging signal measures neuronal activity. However, the signal is not a direct measure of neuronal activity. Rather, it derives from changes in blood flow, glucose consumption, and glucose oxidation, which are physiological measures of brain energy consumption. To interpret these energy signals in terms of neuronal activity, one must proceed carefully since, as we shall show, the present interpretations of the neuronal basis of the signal are based on assumptions about mental processes. Consideration of the neuronal level as derived from the signal, in fact, depends on the definition of a mental process. Hence, before functional imaging methods may be used to study “the mental apparatus and its functions,” as is generally assumed (33), these definitions must be examined. A schematic of the various interactions at play in functional imaging experiments is shown in Figure 3. The imaging signal can be related to specific neuronal processes, as indicated in the lower pathway of Figure 2, by results derived from 13C MRS. To make it so, first one must establish the relationships between the intensity of the imaging signal and the rates of neurophysiological energy processes, such as the CMRs of glucose and of oxygen. The second connection, between the neurophysiological processes and the activity of neuronal processes, is provided by the in vivo 13C MRS studies (17). It is necessary to understand these relationships before using functional imaging signals to answer questions about the neural basis of mental processes.
Imaging signals obtained from different PET experiments are related to the regional rate of specific neurophysiological processes such as the CMRs of glucose or oxygen or CBF. The fMRI blood-oxygen-level dependent signal is an indirect measure of the changes in the CMR of oxygen and of the rate of CBF during stimulation. For fMRI, methodological issues remain as to how accurately the imaging signal may be deconvoluted to measure the separate changes in the CMR of oxygen and the rate of CBF (34–37). These methodological issues are being resolved by ongoing research; the imaging signals are able, in principle, to show changes in the CMRs of oxygen and of glucose and, in some PET experiments, measurements of their absolute values (38). Symbolically, the imaging experiments can be interpreted as providing a relation between the signal (S) and the neurophysiological parameters (NP): S=NP (Equation 2), where the equals sign indicates that the variables are unique functions of each other.
In the standard assumptions of neuroscience, mental activity (M) is supported by neuronal activity (N), or N=M (Equation 3). In order to relate the imaging signal to mental processes, it has been necessary to find a relationship between neuronal activities that is relevant to mental processes, such as spike activity or neurotransmitter flux, and neurophysiological processes. Then a relationship could be made between the signal (S) and the neuronal activity induced by mental processes (M). Symbolically, the desired relationship may be expressed as S=NP=N=M (Equation 4).
13C MRS experimental results have coupled neuronal activity (N: neurotransmitter flux) and the physiological processes of energy consumption (NP) and have thereby completed the coupling between S and N in Equation 4. The problem in directly inferring neuronal activity from measurements of neurophysiological energetic processes has been that there are many brain activities not directly involved in function, such as protein synthesis and membrane turnover, which require energy. Previous estimates of the amount of energy devoted to these nonfunctional processes have ranged from 30% to 50% (39) to almost all of brain energy consumption at rest (40). More recent 13C MRS results in humans indicate that in the resting human occipital/parietal cortex, the rate of glutamate/glutamine cycling is 70%–80% of oxidative glucose consumption (17, 24). When one compares this human result with the relation of glucose oxidation and the neurotransmitter cycle determined in rats, 70%–80% of the energy in the resting human brain is devoted to functional neuronal activity.
For technical reasons, and on the basis of psychological assumptions, functional imaging experiments are generally set up to measure differences in the signal observed in the brain image between two behavioral tasks. In the control task, the subject is usually “at rest,” in the absence of the stimulation being evaluated. Images collected during rest are subtracted from those collected during performance of a task (Figure 4). Difference images, the usual presentation of results, are plots of regions in which changes in the signal are statistically significant. This presentation does not mean that other imaging signals are nonexistent; it merely means that they do not appear on the image because their magnitudes do not change significantly between the task and control images. The prevailing interpretation in functional imaging is that change in the signal (ΔS) measures the neuronal activity (ΔN) associated with the mental processes (M) involved in the task. In symbolic form, ΔS=ΔN=M (Equation 5).
It is important to note that Equation 5 differs from Equation 4, which reflects the traditional neuroscientific view of the neuronal support of mental processes. In Equation 5, the mental process is associated with the difference in neuronal activity, as opposed to the total activity, of the region.
Experimental evidence that the entire neuronal activity of a region is required for mental processing, in support of Equation 4, comes from rat studies in which sensory stimulations were performed with the animal receiving different degrees of anesthesia that corresponded to different neuronal activities involved in glutamate/glutamine cycling (41). With certain anesthetics, animals will respond to sensory stimulations such as forepaw electrical stimulation or vibratory sensory stimulation. Electrical recording studies and deoxyglucose autoradiography have indicated that the same regions of the cerebral cortex are activated by a stimulus during an anesthetized state as during an unanesthetized state. If the neuronal activity induced by a stimulus were simply incremental, it should add the same incremental change in the signal during the anesthetized and awake states (Figure 4). If, on the other hand, full neuronal activity was needed to perform the task, the final state during the task should have the same absolute activity level, regardless of the initial state. In this case, the increment of neuronal activity during the task would be larger in the deeply anesthetized state. Animal studies have shown that change in the signal increases with the depth of anesthesia, suggesting that the full magnitude of neuronal activity in a region is needed during a sensory process (41). These conclusions are supported by recent experiments in which the same animal was stimulated from two different depths of anesthesia and signals obtained from both the fMRI and the change in CBF were much larger when measured during the deeper anesthesia (42). The total signal and the total neuronal activities reached absolute values above the resting values for the region examined.
Implications of Measured Brain Activity
Several conclusions from the 13C MRS experiments are relevant for the interpretation of functional imaging experiments.
Brain energy consumption above a basal level of 15%–25% is a quantitative measure of neurotransmitter activity since glucose oxidation is stoichiometric with glutamate neurotransmitter flux. Functional imaging signals, when converted to energy, measure differences in cortical neurotransmitter flux, which is predominantly glutamate cycling.
The rate of glutamate/glutamine cycling at rest in the human cerebral cortex is 70%–80% of the rate of glucose oxidation. Hence, the resting brain has a higher level of functional neuronal activity than the incremental activity induced by stimulation.
Functional imaging experiments have shown that at stimulation from rest, brain energy consumption, and the accompanying neurotransmitter flux, increases by several percent over resting values. In stimulated anesthetized animals, the regional energy is once again higher than resting values, showing that the total functional energy consumption rather than the increment is recruited by the stimulus.
Psychiatrically oriented neuroscientists have, of course, recognized a central role for this unstimulated state. To avoid confusion, Andreasen et al. (7) proposed that “we refer to this particular state (lying with eyes closed and thinking about whatever comes to mind) with a simple descriptor: random episodic silent thinking (REST). The acronym is intentionally ironic, indicating that the ‘resting brain’ is both active and interesting.” Our data consolidate this important role for the REST state by quantitatively converting the higher rates of glucose oxidation at rest into higher rates of neurotransmitter flux. This conversion, as confirmed by direct measurement, shows that the degree of glutamate neurotransmitter flux in the REST state is between one and two orders of magnitude greater than the incremental activity that serves to locate activity.
The higher level of functional neuronal activity in the absence of stimulation has implications for the interpretation of functional imaging. Consider the experiments on the effects of practice on noun-verb pairings by Petersen et al. (43). PET blood flow measurements, taken during repeated exposure to the same noun list, showed that the signal change intensity, originally strong in the anterior cingulate and other prefrontal regions, decreased with practice, whereas signal strength from the sylvian–insular cortex increased. The authors stated, “These results indicate that two distinct circuits can be used for verbal response selection and normal subjects can change the brain circuits used during task performance following less than 15 minutes of practice.”
However, an equally valid interpretation would be that the activity remained in the original region but it simply became more efficient with practice so that the incremental activity decreased. In the absence of information about the size of the total signal associated with functional activity, one cannot favor one or the other of these interpretations. However, consider two different possible approximations of the total functional signal: first, in which the incremental signal is similar in magnitude to the total functional signal, i.e., ΔS~S, and second, in which it is much larger, i.e., S>>ΔS. In the first instance, it would not be possible to claim that when ΔS→0, the processing has become more efficient, because there would be little activity left to be efficient. However, in the second case (S>>ΔS), a possible explanation would be that the disappearance of ΔS would make little difference in the total activity and a small improvement in efficiency would support the function. Hopes are high for understanding mental activity by tracing this kind of interregional, connected brain activity; therefore, an erroneous interpretation about localization has significant consequences for connectivity between brain regions.
Regional decreases observed in the imaging signal relative to that of the resting control state during performance of a task have been considered noteworthy (44). These decreases are paradoxical (45) to the extent that it is implicitly assumed that the mental and neural processes during the REST state require minimal neuronal activity compared with the activity occurring during the task state. The negative signal has been proposed to “represent an important contribution to our understanding of cortical function” (45). The higher neuronal activity found at rest by means of 13C NMR provides an interpretation in which the negative signal simply reflects a reduction in neural activity during the task relative to activity in the resting state. An analogous interpretation would explain why certain regions do not change in signal activity during tasks but are known from lesion and electrophysiological studies to be important for mental processing. In the incremental view, the absence of statistically significant changes in signal activity increments shows negligible activity in those regions. From the standpoint of a higher resting level of neural activity, the absence of signal change may simply reflect a similar involvement of the regional mental processes in the task and at rest.
Another effect of these findings is on quantitation of the neuronal activity associated with a mental process. The involvement of brain regions in a mental process is often based on the relative size of the incremental signal. The activity of neuronal processes in the resting state significantly affects this quantitation. For example, consider a comparison of the signals of CMR of oxygen between two tasks that activate the same mental processing. Suppose that in the first task, the signal increases above the resting value by 1% and in the second task by 2%. An interpretation based on the incremental signal would be that the mental process in the second task requires twice the neural activity of the first. However, if a larger fraction of the energy consumption than the incremental activity is related to the mental process, the difference in activity between the tasks would be only about 1%. In seeking a neuronal basis for function, we find that different magnitudes of dedicated neuronal activity are suggested by these two interpretations.
This example is also relevant to studies assigning a neural basis for impaired performance of mental processes in subjects with psychiatric or neurological disease. In these studies a smaller degree of change in signal increment has been used as evidence that a region is impaired, or even completely inactive, relative to control subjects (46). Although the smaller change in signal increment may be evidence of an impairment, unless the total neuronal activity is known, the degree of impairment cannot be known.
Experiments Invoking Additional Activations
As would be expected, some admirable efforts have investigated the effects of different states of consciousness on modular brain responses. Consciousness, as represented by its component—attention—has been studied by functional imaging by observing its effect on simpler activations, usually sensory. Some particularly clear experiments were based on electrophysiological studies of the monkey brain, which had demonstrated a direct effect of the animal’s state of attention to nearby regions on the firing rate of neurons in the V2 and V4 regions of the visual cortex (47). In analogous fMRI experiments on the human brain, increased neuronal activity in anticipation of a stimulus was observed, followed by an increased response to stimulation during attention to the stimulus. In fMRI studies in humans, visual attention to stimuli in the same visual field reduced the response, while directing attention to nearby stimuli counteracted the reductions of signal change exercised by these stimuli (48). In another fMRI study, selective attention enhanced the baseline measurements in the extra striate cortex and increased response to the stimulus (49). This was done by means of event-related fMRI (50), which enables one to measure baseline values in the absence of stimulation as well as the increment of signal change. In these cases, in which parallel processing was being evaluated, the results were consistent with a higher degree of ongoing brain activity beyond the specific module being tested. We suggest that the higher neurotransmitter activity we have shown to be required for a task measures the energy needs for these and other parallel processes.
Incorporating the Resting Brain Into Theories of Mind
A view of the mind that includes subjective and unconscious activities such as those described by the REST state would be consistent with the higher neuronal activity we have measured in the absence of specific external stimuli. Our findings of higher neurotransmitter activity during REST are more compatible with such a view than with the computational module, in which unstimulated activity is ignored or at least de-emphasized. This view that the totality of mind activity is required for mental processes is shared with several major philosophical and psychological theories of mind. These theories (including Freud’s) accept the existence of subjective unconscious activities, claiming for them an important role in mental processes and having them contribute meaningfully to information processing.
Within cognitive neuroscience one finds an active core of research aiming to integrate the subjective, conscious, and unconscious activities with the testing methods of functional imaging. As Kihlstrom has stated (51), cognitive psychology has early roots in the study of attention, which has been coupled to consciousness. In the preceding summary of the studies of Luck et al. (47), Kastner et al. (48), Friston (4), and Chawla et al. (49) of the effects of attention on functional imaging, we see how such interactions did not agree with the insertion assumption. It was obviously the goal and achievement of these experiments to reach beyond the limiting assumptions of computational modules and to include attentional aspects of consciousness, thereby opening the way to more inclusive studies of mind. Although these attempts to include subjectivity represent a laudable, sensible extension of simple computational models, it remains to be seen how accommodating a theory based on computational modules can be to such efforts.
Our quantitation of high REST activity can help evaluate recent experimentally based theories of mind. Recent experiments have led to the hypothesis that imaging scientists are not neglecting resting brain activity but are assuming that neuronal activity at rest is relatively “random.” Two experiments support such a hypothesis. The need for substantial unfocused neuronal activity for the service of even sensory responses was suggested by the brilliant experiment of Tsodyks and colleagues (52). Starting with the recognition that “cortical neurons are spontaneously active in the absence of external input even in primary sensory areas,” they studied the correlations between single-unit recordings and real-time optical imaging. While interpreting their data, they concluded by suggesting that “in the absence of stimulation the cortical network wanders through various states represented by coherent firing of different neuronal assemblies” and that a stimulus pushes the network into a particular activated assembly that is the response to the stimulus.
Analogously, the elegant temporal synchronization of neuronal responses on which Singer’s conclusions are based (53) has led to the conclusion, “Of the many responses of V1 those that become synchronized best will be particularly effective in influencing neurons in higher areas.” This hypothesis recognizes the large neuronal activity in the absence of stimulation and includes that activity in proposals about brain function. Our 13C NMR results, in agreement with their assumptions, provide measures of the amounts of such unstimulated activity and thereby provide a quantitative basis for analysis.
Functional imaging experiments have played a key role in locating activation of the brain, and lately these regional studies have been directed toward coordinated activations in different brain regions. These might be considered as horizontal linkages on the surface map of the brain. Many relatively recent experimental approaches, such as those of Tsodyks et al. (52) and Singer (53), have explored the interconnections within localized regions, as well as between regions, and can be considered to address a vertical dimension of activity. Our 13C NMR results led us to propose how total regional neuronal activity can be measured by functional imaging and thereby provide quantitative measures of these vertical activities, which we have shown to be necessary for function (41, 42). Difference measurements are most easily made by functional imaging methods. However, as discussed previously, recent biophysical research is increasing the opportunities to obtain absolute or total values for energetic parameters from imaging results.
These alternative measurements allow us to face the proposed reductionist goal sketched in Crick’s quote (1). We want an explanation of mind in terms of the chemistry and connectivity of neurons. Our results show that all the cells and molecules in a brain region contribute to function—not just an incremental portion. Molecules and cells provide structures for the organization of the brain and the explanations of mind. On those structures, however, glutamate neurotransmitter fluxes and their coupled energetics superimpose another level of function, a level in which dynamic chemical reactions couple energy consumption to the needs of neuronal signaling. At this level, which is based on structure and morphology and includes the complex interactions between neurons and astrocytes in the brain, these fluxes have allowed causality to be established between functional imaging and neurochemistry. Functional imaging, interpreted in terms of neurotransmitter flux, can serve as a way station on the explanatory paths connecting mind and brain.
Received Nov. 22, 1999; revisions received April 18 and June 5, 2000; accepted June 7, 2000. From the Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine. Address reprint requests to Dr. Shulman, Department of Molecular Biophysics and Biochemistry, Yale University School of Medicine, P.O. Box 208024, New Haven, CT 06520-8024; [email protected] (e-mail).Supported by grant DK-27121 from the National Institute of Diabetes and Digestive and Kidney Diseases.The author thanks D.L. Rothman for formative discussions.
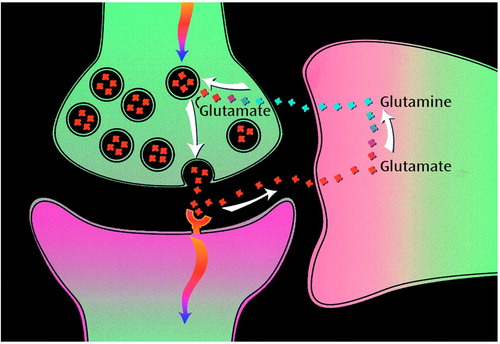
Figure 1. Proposed Pathway of Glutamate/Glutamine Neurotransmitter Cycling Between Neurons and Gliaa
aAction potentials reaching the presynaptic neuron cause release of vesicular glutamate into the synaptic cleft, where it is recognized by glutamate receptors postsynaptically and cleared by Na+-coupled transport into the glia. There it is enzymatically converted to glutamine, which passively diffuses back to the neuron; after reconversion to glutamate, it is repackaged into vesicles. The rate of glutamate-to-glutamine cycling has been derived by means of 13C magnetic resonance spectroscopy.
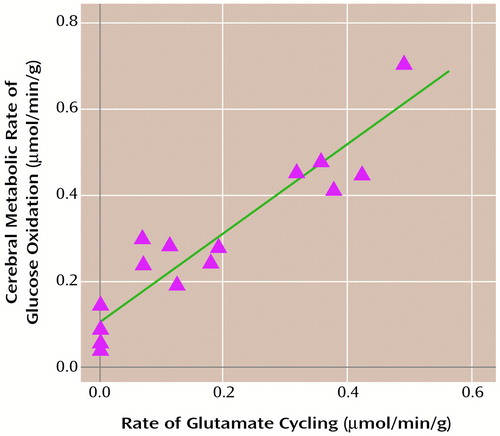
Figure 2. Rate of Glutamate Cycling (Vcycle) in Relation to Cerebral Metabolic Rate of Glucose Oxidation (CMR glucose(ox)) Measured Simultaneously in Rats During Graded Anesthesiaa
aGraded anesthesia comprised morphine sulfate, α-chloralose, then high-dose sodium pentobarbital (least active). The best fit was obtained by means of CMRglucose(ox)=1.04V cycle + 0.10. The slope shows that each mole of neurotransmitter glutamate cycling requires oxidation of one mole of glucose. The awake, resting state under anesthesia is given as CMRglucose(ox)≈0.8 μmol/min/g, which means that under this condition, approximately 80% of brain energy consumption is dedicated to glutamate cycling.
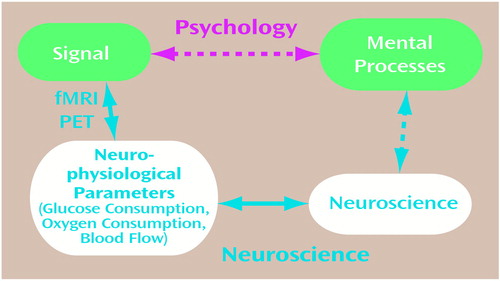
Figure 3. Relations of the Signal Obtained From Functional Magnetic Resonance Imaging (fMRI) or Positron Emission Tomography (PET) to Mental Processes a, b
aIn the usual experimental plan and interpretation, on the basis of psychology, a direct relationship between the signal and mental processes is assumed, as represented by the upper pathway. The definition of mental processes comes from psychology, while the imaging experiment serves to localize and quantitate the brain activity identified with the process.
bThe lower pathway assumes that mental processes have a molecular and cellular basis, which is broken into three steps leading to the signal. The signal in fMRI or PET experiments is primarily a measure of the neurophysiological parameters of the cerebral metabolic rate of glucose consumption, the cerebral metabolic rate of oxygen consumption, or cerebral blood flow (CBF). PET methods have been developed for measuring each of these three parameters separately, while fMRI signals respond to differences in the changes in CBF and the cerebral metabolic rate of oxygen consumption, whose quantitative relationships are being investigated. The cerebral metabolic rate of glucose consumption and the cerebral metabolic rate of oxygen consumption measure cerebral energy consumption, while the change in each measures its increment. The relation between the neurophysiological parameters of the neurophysiological measure of energy consumption and neuronal activity has been clarified by means of 13C magnetic resonance spectroscopy experiments. These findings allow measurements of the signal to be converted into measures of neuronal activity, which places us squarely facing the unsolved “hard” problem of neuroscience, i.e., what is the relationship between mental processes and neuronal activity?
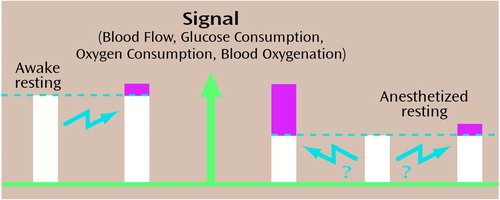
Figure 4. Change in Energy Consumption During Stimulation of Anesthetized Animala
aAn absolute amount of energy consumption, not an incremental amount, is used. The unstimulated blood flow and cerebral metabolic rate of oxygen consumption are much lower than in the unanesthetized state. Usually only the difference in signal change is used to localize and quantitate the task activation, as shown on the left pair of bars, where the baseline would be subtracted from the signal during activation.
1. Crick FHC: The Astonishing Hypothesis: The Scientific Search for the Soul. New York, Charles Scribner’s Sons, 1994, p 7Google Scholar
2. Simon HA, Kaplan CA: Foundations of cognitive science, in Foundations of Cognitive Science. Edited by Posner MI. Cambridge, Mass, MIT Press, 1989, pp 1–47Google Scholar
3. Posner MI, Raichle ME: Images of Mind. New York, WH Freeman, 1997Google Scholar
4. Friston KJ: Analyzing brain images: principles and overview, in Human Brain Function. Edited by Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta SC. San Diego, Calif, Academic Press, 1997, pp 25–41Google Scholar
5. Proc Natl Acad Sci USA 1995(3):763–929Google Scholar
6. Goldman-Rakic P: Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci 1988; 11:137–156Crossref, Medline, Google Scholar
7. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 1995; 152:1576–1585Google Scholar
8. Smith EE, Jonides J, Marsuetz C, Koeppe RA: Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA 1998; 95:876–882Crossref, Medline, Google Scholar
9. Jennings JM, McIntosh AR, Kapur S, Tulving E, Houle S: Cognitive subtractions may not add up: the interaction between semantic processing and response mode. Neuroimage 1997; 5:229–239Crossref, Medline, Google Scholar
10. Nemeroff CB, Kilts CD, Berns CS: Functional brain imaging: twenty-first century phrenology or psychobiological advance for the millennium? (editorial). Am J Psychiatry 1999; 156:671–673Abstract, Google Scholar
11. McCrone J: Going Inside. London, Faber & Faber, 1999Google Scholar
12. Churchland PS: Neurophilosophy: Toward a Unified Science of the Mind-Brain. Cambridge, Mass, MIT Press, 1986Google Scholar
13. Searle JR: The Rediscovery of the Mind. Cambridge, Mass, MIT Press, 1992, p 3Google Scholar
14. Interview With Robert G Shulman. J Cogn Neurosci 1996; 8(suppl 5):474–480Google Scholar
15. Shulman RG, Rothman DL: Freud’s theory of the mental and modern functional imaging experiments, in Changing Ideas in a Changing World: The Revolution in Psychoanalysis—Essays in Honour of Arnold Cooper. Edited by Sandler J. London, Karnac Books, 2000, pp 163–169Google Scholar
16. Shulman RG, Rothman DL: Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA 1998; 95:11993–11998Medline, Google Scholar
17. Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG: Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA 1998; 95:316–321Crossref, Medline, Google Scholar
18. Magistretti P, Pellerin L, Rothman DL, Shulman RG: Perspective: neuroscience “energy on demand.” Science 1999; 283:496–497Crossref, Medline, Google Scholar
19. Rothman DL, Behar KL, Hetherington HP, den Hollander JA, Bendall MR, Petroff OAC, Shulman RG: 1H-Observe/and 13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci USA 1985; 82:1633–1637Google Scholar
20. Behar KL, Petroff OAC, Prichard JW, Alger JR, Shulman RG: Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C] glucose. Magn Res Med 1986; 3:911–920Crossref, Medline, Google Scholar
21. Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, Shulman GI, Prichard JW, Shulman RG: Localized 13C NMR spectroscopy in the human brain of amino acid labeling from [1-13C] D-glucose. J Neurochem 1994; 63:1377–1385Google Scholar
22. Gruetter R, Seaquist ER, Kim S, Ugurbil K: Localized in vivo 13C-NMR of glutamate metabolism in the human brain: initial results at 4 tesla. Dev Neurosci 1998; 20:380–388Crossref, Medline, Google Scholar
23. Fitzpatrick SM, Hetherington HP, Behar KL, Shulman RG: The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C edited NMR spectroscopy. J Cereb Blood Flow Metab 1990; 10:170–179Crossref, Medline, Google Scholar
24. Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ: Simultaneous determination of rate of TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab 1995; 15:12–25Crossref, Medline, Google Scholar
25. Berl S, Takagaki G, Clarke DD, Waelsch H: Metabolic compartments in vivo: ammonia and glutamic acid metabolism in brain and liver. J Biol Chem 1962; 237:2562–2569Google Scholar
26. Reubi JC, Van den Berg CJ, Cuenod M: Glutamine as precursor for the GABA and glutamate transmitter pools. Neurosci Lett 1978; 10:171–174Crossref, Medline, Google Scholar
27. Erecinska M, Silver IA: Metabolism and role of glutamate in mammalian brain. Prog Neurobiol 1990; 35:245–296Crossref, Medline, Google Scholar
28. Sibson NR, Shen J, Mason GF, Rothman DL, Behar KL, Shulman RG: Functional energy metabolism: in vivo 13C NMR evidence for coupling of cerebral glucose consumption and glutamatergic neuronal activity. Dev Neurosci 1998; 20:321–330Crossref, Medline, Google Scholar
29. Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG: In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA 1997; 94:2699–2704Google Scholar
30. Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OAC, Shulman GI, Shulman RG: Determination of the rate of the glutamate-glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA 1999; 96:8235–8240Google Scholar
31. Manor D, Rothman DL, Mason GF, Hyder F, Petroff OAC, Behar KL: The rate of turnover of cortical GABA from [1-13C] glucose is reduced in rats treated with the GABA-transaminase inhibitor vigabatrin (γ-vinyl GABA). Neurochem Res 1996; 12:1031–1041Google Scholar
32. Pellerin L, Magistretti PJ: Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 1994; 91:10625–10629Google Scholar
33. Solms M: What is consciousness? J Am Psychoanal Assoc 1997; 45:681–703Crossref, Google Scholar
34. Hyder F, Shulman RG, Rothman DL: A model for the regulation of cerebral oxygen delivery. J Appl Physiol 1998; 85:554–564Crossref, Medline, Google Scholar
35. Buxton RB, Frank LR: A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 1997; 17:64–72Crossref, Medline, Google Scholar
36. Kim S-G, Ugurbil K: Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn Reson Med 1997; 38:59–65Crossref, Medline, Google Scholar
37. Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB: Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 1999; 96:9403–9408Google Scholar
38. Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Hoffman E, Alavi A, Sokoloff L: The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res 1979; 44:127–137Crossref, Medline, Google Scholar
39. Gjedde A: The energy cost of neuronal depolarization, in Functional Organisation of the Human Visual Cortex: Wenner Gren International Symposium 61. Edited by Gulyas B, Ottoson D, Roland PE. Oxford, UK, Pergamon Press, 1993, pp 291–306Google Scholar
40. Creutzfeldt OD: Neurophysiological correlates of different functional states of the brain, in Brain Work: The Coupling of Function, Metabolism and Blood Flow in the Brain: Alfred Benzon Symposium VIII. Edited by Ingvar DH, Lassen NA. Copenhagen, Munksgaard, 1975, pp 21–46Google Scholar
41. Shulman RG, Rothman DL, Hyder F: Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA 1999; 96:3245–3250Google Scholar
42. Hyder F, Shulman RG, Rothman DL, Behar KL: Localized energetic changes with brain activation from anesthesia, I: absolute CBF changes at 7 Tesla, in Proceedings of the ISMRM 8th Annual Meeting. Denver, International Society for Magnetic Resonance in Medicine, 2000, p 444Google Scholar
43. Petersen SE, van Mier H, Fiez JA, Raichle ME: The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 1998; 95:853–860Crossref, Medline, Google Scholar
44. Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE: Top-down modulation of early sensory cortex. Cereb Cortex 1997; 7:193–206Crossref, Medline, Google Scholar
45. Raichle ME: Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 1998; 95:765–772Crossref, Medline, Google Scholar
46. Buchsbaum M, Hazlett EA: Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull 1998; 24:343–364Crossref, Medline, Google Scholar
47. Luck SJ, Chelazzi L, Hillyard SA, Desimone R: Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 1997; 77:24–42Crossref, Medline, Google Scholar
48. Kastner S, DeWeerd P, Desimone R, Ungerleider LG: Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science 1998; 282:108–111Crossref, Medline, Google Scholar
49. Chawla D, Rees G, Friston KJ: The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 1999; 2:671–676Crossref, Medline, Google Scholar
50. Blamire A, Ogawa S, Ugurbil K, Rothman D, McCarthy G, Ellerman J, Hyder F, Rattner Z, Shulman RG: Dynamic mapping of the human visual cortex by high speed magnetic resonance imaging. Proc Natl Acad Sci USA 1992; 89:11069–11073Google Scholar
51. Kihlstrom J: The rediscovery of the unconscious, in The Mind, the Brain and Complex Adaptive Systems: Proceedings of the XXII Santa Fe Institute Studies in the Sciences of Complexity. Edited by Morowitz HJ, Singer JL. Reading, Mass, Addison-Wesley, 1995, pp 123–144Google Scholar
52. Tsodyks M, Kenet T, Grinvald A, Arieli A: Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 1999; 286:1943–1946Google Scholar
53. Singer W: Putative functions of temporal correlations in neocortical processing, in Large-Scale Neuronal Theories of the Brain. Edited by Koch C, Davis JL. Cambridge, Mass, MIT Press, 1994, pp 201–238Google Scholar



