Characteristic Regional Cerebral Blood Flow Patterns in Anorexia Nervosa Patients With Binge/Purge Behavior
Abstract
OBJECTIVE: The authors’ goal was to investigate the effect of imagining food on the regional cerebral blood flow (rCBF) of anorexia nervosa patients with and without habitual binge/purge behavior. METHOD: The subjects included seven female patients with purely restrictive anorexia, seven female patients with anorexia and habitual binge/purge behavior, and seven healthy women. Single photon emission computed tomography examination was performed before and after the subjects were asked to imagine food. Changes in rCBF count ratios (percent change) were then calculated and compared. The subjects were also asked to assess their degree of fear regarding their control of food intake. RESULTS: The anorexia nervosa patients with habitual binge/purge behavior had a significantly higher percent change in the inferior, superior, prefrontal, and parietal regions of the right brain than the patients with purely restrictive anorexia and the healthy volunteers. The patients with habitual binge/purge behavior also had the highest level of apprehension in regard to food intake. CONCLUSIONS: Specific activation in cortical regions suggests an association between habitual binge/purge behavior and the food recognition process linked to anxiety in patients with anorexia nervosa.
Research into the effects of binge/purge behavior on appetite control mechanisms is assuming increasing importance. Some investigators have classified eating disorders on the basis of the presence or absence of binge/purge behavior (1, 2). Researchers have reported that binge/purge behavior may produce physiological factors that promote the binge/purge cycle (3), probably related to a dysfunction or particular hypersensitivity of some cortical region (4).
We grouped patients with anorexia nervosa according to presence or absence of habitual binge/purge behavior. Using single photon emission computed tomography (SPECT), we measured the regional cerebral blood flow (rCBF) before and after the subjects were asked to imagine food and examined the data in relation to the patients’ self-evaluated anxiety levels.
Method
Fourteen female patients meeting DSM-IV diagnostic criteria for anorexia nervosa and seven healthy female volunteers were evaluated. Seven of the patients had purely restrictive anorexia (mean age=21.7 years, SD=8.0), and seven had habitual binge/purge behavior (mean age=25.6, SD=2.8). The mean age of the healthy volunteers was 21.9 (SD=2.3). The mean body mass indexes of the subjects were as follows: 12.8 (SD=2.1) for patients with purely restrictive anorexia, 14.5 (SD=1.3) for patients with anorexia and habitual binge/purge behavior, and 20.0 (SD=1.4) for healthy subjects. Written informed consent was obtained from all participating subjects. All subjects were right-handed and had no abnormal neurological findings or history of alcohol or drug abuse. X-ray computed tomography scans revealed no major structural differences among the subjects.
We performed SPECT examination before breakfast. After antecubital vein acquisition, we instructed each subject to close her eyes and relax during the examination. Fifteen minutes later we injected a 15 mCi dose of HM-PAO, and, 5 minutes later, we started recording raw counts of rCBF using a triple-head rotating gamma camera with fan-beam high-resolution collimators set at 140 KeV ±10% of the photo window in 90 projections with 360° rotation (128 × 128 matrix). After the first scan (16 minutes), we asked each subject to visualize a piece of custard cake for 10 seconds and then asked her to imagine eating the cake for 5 minutes. At the beginning of imagining, we injected another 25-mCi dose of HM-PAO and obtained a second set of raw counts just after imagining.
The raw projection data were prefiltered with a Butterworth filter (cutoff frequency=0.13 cycle/pixel, power factor=8). We estimated the data after stimulation by subtracting the first projection data (obtained before imagining) with correction of decay from the second projection data. SPECT images were reconstructed by using a filtered back-projection algorithm. Attenuation correction was performed by assuming an elliptical outline of the head slice and uniform attenuation in the head. The 7.1-mm-thick transaxial images before and after stimulation were displayed. The slices were parallel to the orbitocanthal-meatal line. This yielded an average of 35 slices for every brain scanned. Three slices, 14.2 mm, 42.6 mm, and 71.0 mm from the orbitocanthal-meatal line, were selected from each brain scan. According to the brain atlas (5), these areas correspond to the following cortical lobes: slice 1 encompassed the cerebellum; slice 2 encompassed the inferior prefrontal, temporal, and occipital lobes, the thalamus, and the basal ganglion; and slice 3 included the superior prefrontal and parietal lobes. We placed rectangular regions of interest of 25 pixels on both central sides of the cerebellum in slice 1 and in the cortex of the lobes in slices 2 and 3.
The cerebellar regions of interest were used to standardize the regions of interest on slices 2 and 3, and the percent change was obtained by computing the following ratios: R′=region of interest count of each region / mean cerebellum region of interest count; corrected R=kR′/(1 + k – R′), where k=2; percent change=(R after imagining – R before imagining)/R before imagining × 100%. The details of the data analysis have been described elsewhere (6).
In addition, emotional responses to the imaging stimulus were evaluated by asking each subject to assess her perceived degree of difficulty in controlling food intake as either none, mild, or severe.
The percent change in rCBF was compared among the three groups by one-way analysis of variance and Tukey’s honestly significant difference test for multiple comparisons. Sense of inability to control food intake among the groups was compared by chi-square test and the highest post hoc percent cell contribution test. For all analyses, the significance level was set at <0.05.
Results
The patients with anorexia and habitual binge/purge behavior showed prominent increases in the right-side areas of the brain. These differences were significant in three specific regions of interest: 1) the right inferior prefrontal lobe, 2) the right superior prefrontal lobe, and 3) the right parietal region (Figure 1). In the right inferior prefrontal lobe the patients with anorexia and habitual binge/purge behavior showed more changes than the patients with purely restrictive anorexia and the healthy subjects. In the right superior prefrontal lobe, the patients with anorexia and habitual binge/purge behavior had more changes than the healthy subjects. In the right parietal region, the patients with anorexia and habitual binge/purge behavior had more changes than patients with purely restrictive anorexia and the healthy subjects.
The degree of perceived inability to control food intake differed significantly among the three groups (χ2=23.18, df=4, p<0.01); the highest post hoc percent cell contribution test value of 3.07 (severe level of apprehension) was assessed in the patients with anorexia and habitual binge/purge behavior.
Discussion
We found significant increases in the rCBF of the right cortices after imagining food only in the anorexia nervosa patients with habitual binge/purge behavior. Karhunen et al. (7) reported that higher rCBF in the right parietal and temporal cortices was detected by SPECT analysis in obese women looking at food than in normal-weight women looking at food. Thus, the cortical responses elicited by food-related stimuli may have a significant connection with characteristics of food perception in patients with eating disorders.
With respect to the right prefrontal activation, we think that neural pathways involved in the recall of events in the memory process may have an important role (8). Blood flow increases in the right prefrontal lobe may reflect differences between right and left frontal lobe function with respect to memory. Our findings also suggest a close association between neural network activation in episodic memory retrieval (which involves an area of the brain from the right superior and inferior prefrontal lobes to the right parietal lobe) and habitual binge eating and purging in the patients with anorexia and habitual binge/purge behavior.
Finally, the specific activation in cortical regions of the brain that play an important role in perception and memory suggests an interesting association between habitual binge/purge behavior and the cerebral recognition process. This is associated with heightened anxiety levels of the patients with anorexia and habitual binge/purge behavior. The effects of anxiolytic compounds on imagining as detected by SPECT analysis will be the subject of future study.
Presented in part at the 40th annual meeting of the Japanese Society of Psychosomatic Medicine, Hirosaki, Japan, June 3–5, 1999. Received July 28, 1998; revisions received Oct. 28, 1999, and March 2, 2000; accepted March 8, 2000. From the Department of Psychosomatic Medicine, Kagoshima University Hospital; and the Department of Radiology, Faculty of Medicine, Kagoshima University. Address reprint requests to Dr. Naruo, Department of Psychosomatic Medicine, Kagoshima University Hospital, 8-35-1, Sakuragaoka, Kagoshima-City, 890-8520, Japan; [email protected] (e-mail). Supported by a research grant from the Japanese Ministry of Health and Welfare. The authors thank the staff of the Department of Radiology, especially Mr. Toyotsugu Kiku and Mr. Noboru Hukushima, for their help in performing the SPECT examination.
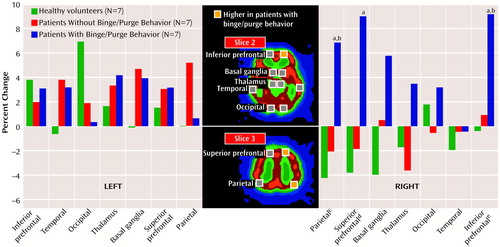
Figure 1. Mean Percent Change in Regional Cerebral Blood Flow After Imagining Food in Seven Healthy Female Volunteers and 14 Female Patients With Anorexia Nervosa With and Without Habitual Binge/Purge Behavior
aSignificantly different from healthy subjects (p <005, Tukey’s honestly significant difference).
bSignificantly different from patients without binge/purge behavior (p<0.05, Tukey’s honestly significant difference).
cSignificant group effect (F=7.04, df=2, 18, p<0.01).
dSignificant group effect (F=5.10, df=2, 18, p<0.05).
eSignificant group effect (F=9.09, df=2, 18, p<0.01).
1. Garfinkel P, Kennedy SH, Kaplan AS: Views on classification and diagnosis of eating disorders. Can J Psychiatry 1995; 40:445–456Medline, Google Scholar
2. Beebe DW: Bulimia nervosa and depression: a theoretical and clinical appraisal in light of the binge-purge cycle. Br J Clin Psychol 1994; 33:259–276Crossref, Medline, Google Scholar
3. Johnson WG, Jarrel MP, Chupurdia KM, Williamson DA: Repeated binge/purge cycles in bulimia nervosa: role of glucose and insulin. Int J Eat Disord 1994; 5:331–341Google Scholar
4. Delvenne V, Goldman S, De-Maertelaer V, Lotstra F: Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disord 1999; 25:29–37Crossref, Medline, Google Scholar
5. Matsui T, Hirano A: An Atlas of the Human Brain for Computerized Tomography. Tokyo, Igaku-Shoin, 1978Google Scholar
6. Nozoe S, Naruo T, Yonekura R, Nakabeppu Y, Soejima Y, Nagai N, Nakajo M, Tanaka H: Comparison of regional cerebral blood flow in patients with eating disorders. Brain Res Bull 1995; 36:251–255Crossref, Medline, Google Scholar
7. Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI: Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain 1997; 120:1675–1684Google Scholar
8. Nyberg L, McIntosh AR, Cabeza R, Habib R, Houle S, Tulving E: General and specific brain regions involved in encoding and retrieval of events: what, where, and when. Proc Natl Acad Sci USA 1996; 93:11280–11285Google Scholar



