Efficacy of Extended-Release Venlafaxine in Nondepressed Outpatients With Generalized Anxiety Disorder
Abstract
OBJECTIVE: This study evaluated the efficacy and safety of fixed doses of once-daily extended-release (XR) venlafaxine in outpatients with generalized anxiety disorder without concomitant major depressive disorder.METHOD: Adult outpatients with generalized anxiety disorder but not major depressive disorder with total scores of 18 or higher on the Hamilton Rating Scale for Anxiety and scores of 2 or higher on its anxious mood and tension factors were eligible. Patients were randomly assigned to receive placebo or venlafaxine XR (75, 150, or 225 mg/day) for 8 weeks. Primary efficacy variables were final total and psychic anxiety factor scores on the Hamilton anxiety scale and final severity and global improvement item scores on the Clinical Global Impression (CGI) scale.RESULTS: Of the 377 patients entering the study, 370 were included in a safety analysis and 349 in an efficacy analysis. Adjusted mean scores at 8 weeks (last-observation-carried-forward analysis) were significantly lower for one or more of the venlafaxine XR groups in four of four primary and three of four secondary outcome measures than for the placebo group. These included a change of 1.7 (versus 1.3) from baseline on CGI severity item scores and a final score of 2.2 (versus 2.6) on the CGI global improvement item. All doses of venlafaxine XR were well tolerated.CONCLUSIONS: Venlafaxine XR is an effective and well-tolerated option for the short-term treatment of generalized anxiety disorder in outpatients without major depressive disorder.
Generalized anxiety disorder is one of the most common anxiety disorders, with an estimated lifetime prevalence of 5.1% (1). As is typical of other anxiety disorders, generalized anxiety disorder appears to be more common in women than in men and occurs more frequently in patients with a family history of anxiety and depression (2). Generalized anxiety disorder usually begins when patients are in their early 20s, is often chronic (average illness lengths of 20 years have been reported [3, 4]), and has a low rate of spontaneous remission after illness onset (only 25% at 2 years [4]). As might be predicted from the prolonged course of this disease, but contrary to the popular view that generalized anxiety disorder is mild, epidemiological surveys have confirmed that generalized anxiety disorder causes substantial interference in the lives of affected patients, many of whom seek professional help followed by extensive use of medication to treat its symptoms (5).
The cardinal feature of generalized anxiety disorder is unrealistic or excessive anxiety and worry about a number of events or activities in the absence of panic or phobic symptoms (see DSM-IV). A person with generalized anxiety disorder has difficulty controlling these worries, and they persist for at least 6 months. Anxiety or increased arousal is accompanied by difficulty sleeping, irritability, or poor concentration. Somatic complaints include tremor, sweating, and gastrointestinal disturbances (3).
The psychiatric community recognizes generalized anxiety disorder as a condition that often produces significant impairment in daily functioning and requires prolonged psychosocial and pharmacologic intervention. In addition, epidemiological surveys indicate that other psychiatric disorders frequently coexist in patients with generalized anxiety disorder (6, 7). Reported comorbidity rates range from 3% to 27% for panic disorder, 16% to 59% for social phobia, and 8% to 39% for major depression (8, 9). Because of the substantial comorbidity of generalized anxiety disorder, there has been much disagreement about its validity as a separate diagnostic entity (7). However, at least one-third of the people with current generalized anxiety disorder, according to the National Comorbidity Survey, have no other recent or current diagnosis (7). Also, available data now suggest genetic factors may play a modest role in the etiology of generalized anxiety disorder (6). Current findings indicate that generalized anxiety disorder is a serious illness, needs prolonged treatment, and requires an effective treatment agent that demonstrates a robust efficacy.
Although the emotional impact of generalized anxiety disorder on the individual is obvious, generalized anxiety disorder also carries a significant financial burden. Total costs for the treatment of anxiety disorders, encompassing generalized anxiety disorder, represented a significant proportion of health care expenditures for mental illness in 1990 ($46.6 billion, or 31.5%); direct costs (medical treatment) accounted for 23.1% of this total, and indirect costs (e.g., lost productivity and suicide) for 76.1% (10).
In the United States, benzodiazepines and buspirone are the most commonly used antianxiety agents in the treatment of anxiety disorders, including generalized anxiety disorder (6, 11). Although these agents successfully mitigate anxiety, buspirone has a relatively delayed onset of action, and the safety limitations associated with the use of benzodiazepines warrant development of alternative agents. Antidepressants such as the tricyclics, selective serotonin reuptake inhibitors, trazodone, and nefazodone have been evaluated in generalized anxiety disorder (12–16), but data are extremely limited and, in some cases, complicated by the inclusion of patients with major depression (17). Only imipramine’s efficacy in generalized anxiety disorder without major depressive disorder has been demonstrated in placebo-controlled trials (15, 16). Since venlafaxine, which is similar to imipramine, exerts its antidepressant efficacy via serotonergic and noradrenergic pathways, anxiolytic effects were hypothesized for venlafaxine. Therefore, the current study was undertaken to test this hypothesis, using the extended-release (XR) formulation of venlafaxine.
Method
Study Design
This study was a multicenter (involving 15 U.S. centers), randomized, double-blind, parallel-group, placebo-controlled trial using outpatients with generalized anxiety disorder. Patients who satisfied the selection criteria were randomly assigned to receive either placebo or venlafaxine XR at one of three dose levels (75, 150, or 225 mg/day) for up to 8 weeks. All study medication was taken in the morning with food. During the first week of treatment all patients who were taking venlafaxine XR received 75 mg/day; on days 8–14 patients randomly assigned to 150 or 225 mg/day received 150 mg/day; beginning on day 15 patients in the high-dose group received 225 mg/day. After day 15 all patients were maintained at their respective venlafaxine XR doses for the remainder of the study. Patients were evaluated at screening and baseline visits; at days 8, 15, 22, 29, 43, and 57 during the double-blind phase; and then 4–10 days after the drug was tapered.
Patient Selection
Male and female outpatients 18 years and older who met the DSM-IV criteria for generalized anxiety disorder but not for major depressive disorder and who were sufficiently symptomatic to require treatment were eligible. Patients were also required to have screening and baseline total scores on the Hamilton Rating Scale for Anxiety (18) of 18 or higher and scores of 2 or higher on item 1 (anxious mood) and item 2 (tension). Furthermore, patients who had a decrease of more than 20% on total scores of the Hamilton anxiety scale between the prestudy and baseline measurements were excluded from the study. Finally, patients were required to have total scores of 9 or lower on the Raskin Depression Scale (19) and scores at screening and baseline on the Covi Anxiety Scale (20) that were greater than their total score on the Raskin Depression Scale. The protocol was approved by the institutional review boards of all study centers, and written informed consent was obtained from each patient before study enrollment.
To ensure that only the effect of venlafaxine XR on generalized anxiety disorder was being evaluated in the current study, patients were excluded from participation if they had a diagnosis of major depressive disorder (according to DSM-IV criteria) within 6 months of study entry or a current diagnosis based on a structured interview at screening. However, 26 (6.9%) of the 377 patients in the study had a history of major depressive disorder and two (0.5%) had a history of dysthymia. Other exclusionary criteria included a history or presence of any psychotic illness, bipolar disorder, antisocial personality disorder or other severe axis II disorder, or a clinically significant psychiatric disorder besides generalized anxiety disorder. Patients were excluded if they had a score on the Raskin Depression Scale of higher than 3 on any single item or a score on the Covi Anxiety Scale higher than 4 on “somatic complaints.”
Other reasons for exclusion were the regular use of a benzodiazepine within 30 days of study day 1, the use of any other investigational drug or procedure, any antipsychotic drug or fluoxetine, or any nonpsychopharmacologic drug or substance with psychotropic effects within 7 days of double-blind treatment, unless a stable dose had been maintained for at least 3 months before that phase. Women who were pregnant or lactating were not eligible for the study, nor were women of childbearing potential who were not using a medically acceptable form of contraception.
The screening evaluation included a medical and psychiatric history, a complete physical examination, laboratory determinations, a 12-lead ECG, and efficacy evaluations using the Covi Anxiety Scale, the Raskin Depression Scale, and the Hamilton anxiety scale. Patients then entered a washout period for 7±3 days, after which they returned for a baseline visit, which included a repeat assessment with the Covi Anxiety Scale, the Raskin Depression Scale, and the Hamilton anxiety scale. Patients were required to meet entry criteria for these scales at both the prestudy and baseline visits. Patients were allowed to take chloral hydrate (up to 1000 mg at bedtime no more than four times per week) through study day 21 if needed for sleep.
Measurements
Adverse events, compliance, efficacy, and vital signs were evaluated at each visit. A physical examination, laboratory determinations, and an ECG were performed at the screening visit and repeated at the final visit during the double-blind phase. Primary efficacy variables were the final on-therapy total and psychic anxiety factor scores on the Hamilton anxiety scale and item scores for the Clinical Global Impression (CGI) scale (21) (severity of illness and global improvement). A patient was considered a responder to treatment if he or she had a score of 1 (very much improved) or 2 (much improved) on the CGI global improvement item. Secondary efficacy variables were scores on the Hamilton anxiety scale’s somatic anxiety factor and the anxiety subscale of the Hospital Anxiety and Depression Scale (22). Additional key parameters analyzed included scores on the anxious mood and tension factors of the Hamilton anxiety scale.
Safety evaluations were based on patients’ reports of adverse events and routine physical examinations, laboratory determinations, and ECGs. Efficacy analyses, which were completed on an intent-to-treat basis, included all patients who had a baseline evaluation and at least one evaluation during the double-blind treatment phase on at least one of the primary efficacy variables within 3 days of terminating treatment with the study drug. The study was designed to enroll approximately 90 intent-to-treat patients per treatment group.
Data Analysis
Aspects of the data analysis that are described in this section were specified in the protocol before the data were unblinded. The primary variables were change from baseline for total and psychic anxiety factor scores on the Hamilton anxiety scale and on the CGI severity and improvement items. The primary time point was week 8. If a patient dropped out before week 8, the last available on-therapy score for that patient was used in the analysis. Secondary efficacy variables included change from baseline for scores on the somatic, anxious mood, and tension factors of the Hamilton anxiety scale and on the anxiety subscale of the Hospital Anxiety and Depression Scale. The statistical model used to analyze the variables was an analysis of covariance, with treatment and investigator as main effects and baseline score as the covariate. The CGI severity item was analyzed by using an analysis of variance with investigator, treatment, and baseline score as main effects. The CGI improvement item was also analyzed by using the same model, with the exception that there was no baseline score in the model.
To provide an overall test of efficacy for each variable at week 8, a linear trend test was done (23). The null hypothesis for this test was that venlafaxine XR had no effect on the efficacy variable used in the analysis. The linear trend test was designed to be sensitive to rejecting this null hypothesis when venlafaxine XR causes increasing response with increasing doses (with placebo included as a zero dose). The test was set up by forming a statistical contrast in which the adjusted mean response in the placebo group was multiplied by –3, the 75-mg dose by –1, the 150-mg dose by 1, and the 225-mg dose by 3. The statistical null hypothesis for the test was that the contrast in the population equaled zero. Statistically significant treatment differences for the four primary efficacy variables existed if the overall test had a p value of ≤0.05, two-tailed. To adjust for multiple comparisons involving the four secondary efficacy variables, the p values had to be ≤0.01 to be considered significant. Eight-week active drug groups and placebo comparisons were based on least-squares analysis.
The efficacy results reported in this article are based on a data set in which the last observation was carried forward into all subsequent time points for patients who dropped out before the end of the study.
Results
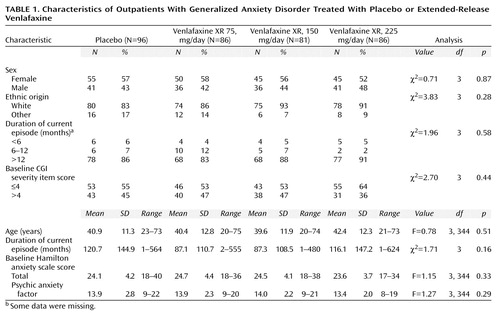
Demographic Characteristics
A total of 370 patients who completed the placebo run-in period and received the study drug during the double-blind phase were included in the safety analysis. For 21 of these patients, no primary efficacy evaluations during therapy or within 3 days of discontinuing the study drug were recorded; these patients were not included in the intent-to-treat efficacy analysis. The baseline demographic and clinical characteristics of the 349 patients with efficacy data are shown in Table 1. There were no significant differences among the groups. A total of 102 (29%) patients discontinued treatment during the double-blind phase (Table 2). Additionally, an examination of the interactions of center and treatment did not reveal significant differences.
Efficacy
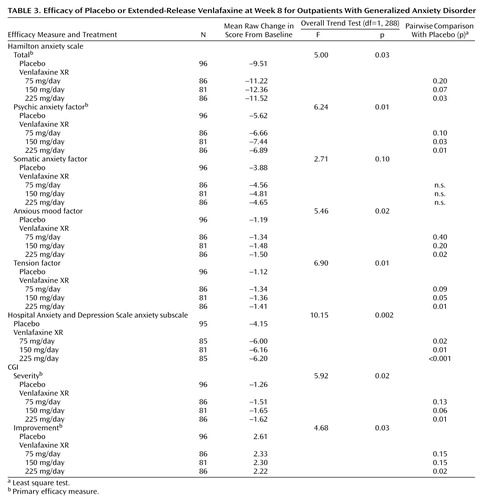
The results for the four primary and four secondary outcome measures are shown in Table 3. Selected results are presented graphically in three figures (Figure 1, Figure 2, and Figure 3), demonstrating separation from placebo for all three venlafaxine XR doses, beginning as early as week 1 and maintained throughout the entire 8 weeks of the study. Efficacy results for total and psychic anxiety factor scores on the Hamilton anxiety scale are given in Figure 1 and Figure 2 (Table 3 shows p values for the primary time point—8 weeks). Although all three venlafaxine XR doses demonstrated some efficacy, further inspection of the data shows that the most positive results were obtained in the group receiving the 225-mg dose. Scores on the CGI severity and global improvement items showed similar results (Table 3).
The results of the analyses of secondary efficacy variables support the findings of a superior response with venlafaxine XR. The mean scores for the anxiety subscale of the Hospital Anxiety and Depression Scale were significantly different from placebo at week 8 in all venlafaxine XR treatment groups (Figure 3; Table 3 shows p values for the primary time point—8 weeks). For scores on the somatic anxiety factor of the Hamilton anxiety scale, only a weak, not statistically significant, difference in favor of venlafaxine XR over placebo was found. Additionally, the anxious mood and tension factors of the Hamilton anxiety scale, considered to represent key parameters with specificity for, and sensitivity to, anxiolytic response, were analyzed and also revealed statistically significant differences from placebo at week 8 (Table 3).
Safety
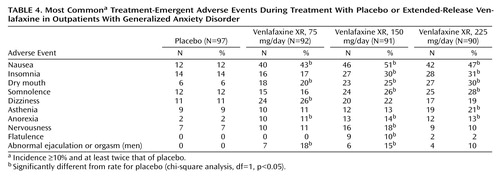
Adverse reactions were the most frequent cause of early discontinuation from the study. Most discontinuations occurred during the first week of double-blind treatment.
Table 4 shows data on treatment-emergent adverse events that occurred with an incidence of at least 10%, at least twice the incidence of those occurring with placebo. The most common treatment-emergent adverse event associated with treatment with venlafaxine XR was nausea, followed by insomnia, dry mouth, somnolence, dizziness, and asthenia. These events were generally mild to moderate and occurred early in the course of treatment; most tended to subside with continued venlafaxine XR therapy. Nausea, insomnia, and dizziness were the most common adverse events that led to discontinuation from the study; overall results indicate that venlafaxine XR was well tolerated. These results are lent support by a study by Khan et al. (24)—which was indicative of venlafaxine being tolerated well among geriatric outpatients with depression—when considering the wide age range (Table 1) of patients included in our study.
The values for ECGs, laboratory examinations, weights, and vital signs were generally similar for the active treatment group and for the placebo group. Overall, the degrees of these changes were generally small, isolated, or transient and were not considered to be of clinical relevance.
Discussion
The use of benzodiazepines for the treatment of generalized anxiety disorder has been generally safe (11, 12), but it has been limited by concerns of withdrawal or the potential for abuse in long-term use (2, 11). Buspirone is effective but has a delayed onset of action, and some practitioners prefer it for chronic generalized anxiety disorder (11). Antidepressants have long been considered alternatives to benzodiazepines in the treatment of some types of anxiety and as promising alternatives to the treatment of generalized anxiety disorder (12, 15).
A placebo-controlled, double-blind study by Laakmann et al. (25), comparing the anxiolytic properties of buspirone with those of a benzodiazepine (lorazepam) and placebo in outpatients with generalized anxiety disorder (per DSM-III criteria), showed that buspirone and lorazepam were more efficacious than placebo during treatment and taper periods. The tricyclic imipramine has been systematically studied under placebo-controlled conditions in patients in whom generalized anxiety disorder without major depressive disorder has been diagnosed (15, 16). To our knowledge, the present study is the first published double-blind, placebo-controlled report demonstrating anxiolytic effects beyond those caused by placebo for a nontricyclic antidepressant, namely venlafaxine XR, in outpatients with generalized anxiety disorder but without comorbid major depressive disorder.
Venlafaxine IR (immediate-release) carries a single indication for the treatment of depression, but it has also been studied for its efficacy in decreasing anxiety symptoms associated with depression. In a meta-analysis of six studies, Rudolph and colleagues (26) found that anxious, depressed patients who received venlafaxine IR showed significantly greater improvement in their anxiety symptoms than did patients who received placebo. An additional double-blind, placebo-controlled comparative study by Feighner et al. (17) showed that once-daily venlafaxine XR was statistically superior to placebo in treating depression with associated symptoms of anxiety. Finally, venlafaxine IR was studied in patients with generalized anxiety disorder in an open-label, 4-week pilot study with apparent beneficial results (27).
Venlafaxine XR recently was granted an additional indication for the treatment of generalized anxiety disorder, and results of the present double-blind, placebo-controlled study extend those of Rudolph et al. (26) in indicating that the once-a-day administration of venlafaxine XR provides safe and effective treatment of anxiety symptoms in outpatients diagnosed with generalized anxiety disorder without comorbid major depressive disorder.
Compared to the placebo group, one or more of the venlafaxine XR groups had statistically significantly lower adjusted mean scores on the Hamilton anxiety scale total, on the Hamilton anxiety scale psychic anxiety factor, on the CGI severity item, and on the CGI global improvement item at the week-8 treatment endpoint. In addition, all three venlafaxine XR treatment groups showed robust anxiolytic effect on the Hospital Anxiety and Depression Scale’s anxiety subscale, a patient-rated assessment, but not on the Hamilton anxiety scale’s somatic cluster. Scores on the anxiety subscale of the Hospital Anxiety and Depression Scale support the findings observed with the physician-rated primary scales and may reflect the important clinical benefit to the patient of a reduction in anxiety symptoms. None of the patients had a diagnosis of major depressive disorder within 6 months of study entry—although, as noted, 6.9% (N=26) had a history of major depressive disorder and 0.5% (N=2) a history of dysthymia—so they therefore had a low historical incidence of depression. Finally, venlafaxine XR treatment led to significant improvement in items 1 and 2 (anxious mood and tension, respectively) on the Hamilton anxiety scale, factors that represent the cardinal features of generalized anxiety disorder.
Conclusions
The results presented here demonstrate that venlafaxine XR has significant anxiolytic effects, as determined by both physician- and patient-rated outcome measures, and suggest that, in addition to its known antidepressant actions, venlafaxine XR may be a useful alternative to currently available treatments for anxiety.
Acknowledgments
The authors thank the following members of the 210-U.S. Study Group: B.D. Beitman (University of Missouri, Columbia); R.J. Bielski (Institute for Health Studies, Okemos, Mich.); M. Cooperman (Melrose Park, Pa.); J.T. Hartford (Hartford Research Group, Cincinnati); P.D. Londborg (Seattle Clinical Research Center, Seattle); D.J. Munjack (Beverly Hills, Calif.); W.M. Patterson (Birmingham Research Group, Birmingham, Ala.); S.H. Preskorn (Psychiatric Research Institute, Wichita, Kan.); R. Shrivastava (Eastside Comprehensive Medical Services, New York); J.S. Simon (The Northbrooke Research Center, Brown Deer, Wis.); W.T. Smith (Pacific Northwest Clinical Research Center, Portland, Ore.); K.L. Weihs (George Washington University Medical School, Washington, D.C.); and R.H. Weisler (Raleigh, N.C.).
 |
 |
 |
 |
Received Aug. 25, 1998; revisions received Feb. 26, Aug. 31, and Oct. 12, 1999; accepted Nov. 12, 1999. From the Psychopharmacology Unit, University Science Center, University of Pennsylvania; Massachusetts General Hospital, Boston; the Institute for Research in Psychiatry, University of South Florida, Tampa; and Wyeth-Ayerst Laboratories, Philadelphia. Address reprint requests to Dr. Rickels, University Science Center, University of Pennsylvania, 3600 Market St., Suite 803, Philadelphia, PA 19104-2649; [email protected] (e-mail). Supported by Wyeth-Ayerst Laboratories.
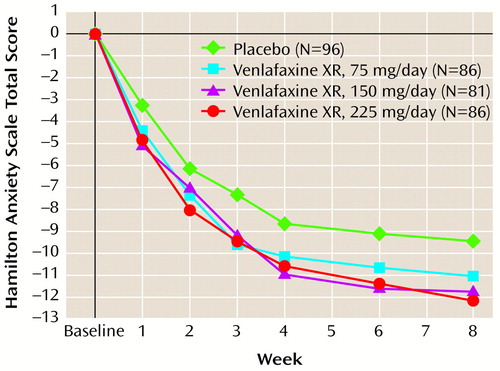
Figure 1. Mean Change From Baseline in Total Scores on the Hamilton Anxiety Scale During Treatment for Generalized Anxiety Disorder With Placebo or Extended-Release Venlafaxinea
aEfficacy analyses included all patients with a baseline evaluation and at least one evaluation during treatment, with the last observation carried forward. A two-way analysis of covariance was used.
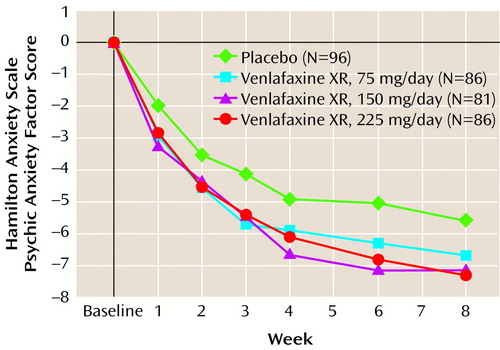
Figure 2. Mean Change From Baseline in Scores on the Psychic Anxiety Factor of the Hamilton Anxiety Scale During Treatment for Generalized Anxiety Disorder With Placebo or Extended-Release Venlafaxinea
aEfficacy analyses included all patients with a baseline evaluation and at least one evaluation during treatment, with the last observation carried forward. A two-way analysis of covariance was used.
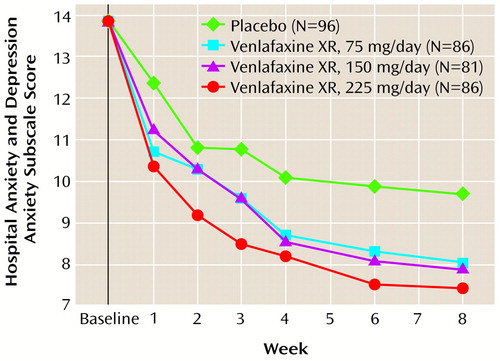
Figure 3. Mean Scores on the Anxiety Subscale of the Hospital Anxiety and Depression Scale During Treatment for Generalized Anxiety Disorder With Placebo or Extended-Release Venlafaxinea
aEfficacy analyses included all patients with a baseline evaluation and at least one evaluation during treatment, with the last observation carried forward. A two-way analysis of covariance was used.
1. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H-U, Kendler KS: Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Crossref, Medline, Google Scholar
2. Kirkwood CK, Hayes PE: Anxiety disorders, in Pharmacotherapy: A Pathophysiologic Approach, 3rd ed. Edited by DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Stamford, Conn, Appleton & Lange, 1997, pp 1443–1462Google Scholar
3. Barlow DH, Blanchard EB, Vermilyea JA, Vermilyea BB, DiNardo PA: Generalized anxiety and generalized anxiety disorder: description and reconceptualization. Am J Psychiatry 1986; 143:40–44Link, Google Scholar
4. Yonkers KA, Warshaw MG, Massion AO, Keller MB: Phenomenology and course of generalised anxiety disorder. Br J Psychiatry 1996; 168:308–313Crossref, Medline, Google Scholar
5. Wittchen H-U, Zhao S, Kessler RC, Eaton WW: DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:355–364Crossref, Medline, Google Scholar
6. Brawman-Mintzer O, Lydiard RB: Biological basis of generalized anxiety disorder. J Clin Psychiatry 1997; 58(suppl 3):16–25Google Scholar
7. Brawman-Mintzer O, Lydiard RB: Generalized anxiety disorder: issues in epidemiology. J Clin Psychiatry 1996; 57(suppl 7):3–8Google Scholar
8. Brawman-Mintzer O, Lydiard RB, Emmanuel N, Payeur R, Johnson M, Roberts J, Jarrell MP, Ballenger JC: Psychiatric comorbidity in patients with generalized anxiety disorder. Am J Psychiatry 1993; 150:1216–1218Google Scholar
9. Angst J: Comorbidity of anxiety, phobia, compulsion and depression. Int Clin Psychopharmacol 1993; 8(suppl 1):21–25Google Scholar
10. DuPont RL, Rice DP, Miller LS, Shiraki SS, Rowland CR, Harwood HJ: Economic costs of anxiety disorders. Anxiety 1996; 2:167–172Crossref, Medline, Google Scholar
11. Dubovsky SL: Generalized anxiety disorder: new concepts and psychopharmacologic therapies. J Clin Psychiatry 1990; 51(1, suppl):3–10Google Scholar
12. Rocca P, Fonzo V, Scotta M, Zanalda E, Ravissa L: Paroxetine efficacy in the treatment of generalized anxiety disorder. Acta Psychiatr Scand 1997; 95:444–450Crossref, Medline, Google Scholar
13. Hedges DW, Reimherr FW, Strong RE, Halls CH, Rust C: An open trial of nefazodone in adult patients with generalized anxiety disorder. Psychopharmacol Bull 1996; 32:671–676Medline, Google Scholar
14. Hoehn-Saric R, McLeod DR, Zimmerli WD: Differential effects of alprazolam and imipramine in generalized anxiety disorder: somatic versus psychic symptoms. J Clin Psychiatry 1988; 49:293–301Medline, Google Scholar
15. Rickels K, Downing R, Schweizer E, Hassman H: Antidepressants for the treatment of generalized anxiety disorder: a placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry 1993; 50:884–895Crossref, Medline, Google Scholar
16. Kahn RJ, McNair DM, Lipman RS, Covi L, Rickels K, Downing R, Fisher S, Frankenthaler LM: Imipramine and chlordiazepoxide in depressive and anxiety disorders, II: efficacy in anxious outpatients. Arch Gen Psychiatry 1986; 43:79–85Crossref, Medline, Google Scholar
17. Feighner JP, Entsuah AR, McPherson MK: Efficacy of once-daily venlafaxine extended release (XR) for symptoms of anxiety in depressed outpatients. J Affect Disord 1998; 47:55–62Crossref, Medline, Google Scholar
18. Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
19. Raskin A, Schulterbrant J, Reatig N, McKeon JJ: Replication of factors of psychopathology in interview, ward behavior, and self-report ratings of hospitalized depressives. J Nerv Ment Dis 1969; 148:87–98Crossref, Medline, Google Scholar
20. Lipman RS: Differentiating anxiety and depression in anxiety disorders: use of rating scales. Psychopharmacol Bull 1982; 18:69–77Medline, Google Scholar
21. National Institute of Mental Health: CGI (Clinical Global Impression) scale. Psychopharmacol Bull 1985; 21:839–843Google Scholar
22. Zigmond AS, Snaith RP: The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
23. Winer BJ: Design and analysis of single-factor experiments, in Statistical Principles in Experimental Design, 2nd ed. Edited by Winer BJ. New York, McGraw-Hill, 1971, pp 388–394Google Scholar
24. Khan A, Rudolph R, Baumel B, Ferguson J, Ryan P, Shrivastava R: Venlafaxine in depressed geriatric outpatients: an open-label clinical study. Psychopharmacol Bull 1995; 31:753–758Medline, Google Scholar
25. Laakmann G, Schule C, Lorkowski G, Baghai T, Kuhn K, Ehrentraut S: Buspirone and lorazepam in the treatment of generalized anxiety disorder in outpatients. Psychopharmacology (Berl) 1998; 136:357–366Crossref, Medline, Google Scholar
26. Rudolph RL, Entsuah R, Chitra R: A meta-analysis of the effects of venlafaxine on anxiety associated with depression. J Clin Psychopharmacol 1998; 18:136–144Crossref, Medline, Google Scholar
27. Villarreal G, Emmanuel NP, Lydiard RB, Ballenger JC: Venlafaxine in generalized anxiety disorder, in 1995 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 1995, p 85Google Scholar



