Vitamin B12 Deficiency and Depression in Physically Disabled Older Women: Epidemiologic Evidence From the Women’s Health and Aging Study
Abstract
OBJECTIVE: It has been hypothesized that adequate concentrations of vitamin B12 and folate are essential to maintain the integrity of the neurological systems involved in mood regulation, but epidemiologic evidence for such a link in the general population is unavailable. This study examined whether community-dwelling older women with metabolically significant vitamin B12 or folate deficiency are particularly prone to depression. METHOD: Serum levels of vitamin B12, folate, methylmalonic acid, and total homocysteine were assayed in 700 disabled, nondemented women aged 65 years and over living in the community. Depressive symptoms were measured by means of the Geriatric Depression Scale and categorized as no depression, mild depression, and severe depression. RESULTS: Serum homocysteine levels, serum folate levels, and the prevalences of folate deficiency and anemia were not associated with depression status. The depressed subjects, especially those with severe depression, had a significantly higher serum methylmalonic acid level and a nonsignificantly lower serum vitamin B12 level than the nondepressed subjects. Metabolically significant vitamin B12 deficiency was present in 14.9% of the 478 nondepressed subjects, 17.0% of the 100 mildly depressed subjects, and 27.0% of the 122 severely depressed women. After adjustment for sociodemographic characteristics and health status, the subjects with vitamin B12 deficiency were 2.05 times as likely to be severely depressed as were nondeficient subjects. CONCLUSIONS: In community-dwelling older women, metabolically significant vitamin B12deficiency is associated with a twofold risk of severe depression.
Vitamin B12 (cobalamin) and folate are essential in several metabolic pathways in the central nervous system, and their metabolism is intimately connected (1, 2). Both are involved in single-carbon transfer (methylation) reactions necessary for the production of monoamine neurotransmitters, phospholipids, and nucleotides. A deficiency of either vitamin may cause an impaired methylation in the central nervous system and may result in neurological and/or psychiatric disease that becomes irreversible if not treated properly (3, 4). This connection is supported by findings that psychiatric patients, especially depressed patients, frequently are found to have abnormalities in vitamin B12 and folate status (5–7). Furthermore, both folate and vitamin B12 replacement therapy in patients with major depression appear to produce substantial affective improvements (8–12). However, since the studies thus far have been restricted to psychiatric patients, it is unknown whether vitamin B12 and folate deficiencies affect depressed mood in the general, community-dwelling population.
Low serum vitamin B12 and folate levels are not very specific and have low sensitivity in diagnosing tissue deficiency (13, 14). Therefore, in the present study the definitions of vitamin B12 and folate deficiency were based on both low levels of these vitamins and high levels of the specific metabolites methylmalonic acid and total homocysteine, respectively (15–17). In the present study we examined whether these assessments of vitamin B12 and folate deficiency were associated with depression in a community-dwelling, representative sample of disabled older women.
Method
Study Sample
In this study we used data from the baseline assessment of the Women’s Health and Aging Study, a prospective cohort study of the causes and course of physical disability in a representative sample of physically disabled older women without severe cognitive impairment living in the community. The study design and characteristics of the study sample have been described in detail elsewhere (18). Briefly, an age-stratified random sample of 6,521 community-dwelling women aged 65 years and older was selected from the Health Care Financing Administration’s Medicare enrollees living in the Baltimore area. After exclusion of those who had died, were institutionalized, or had moved from the area, 5,316 were eligible for screening, and 4,137 consented to a screening interview. The criteria for study eligibility were a Mini-Mental State score of 18 or higher (19) and reported difficulty in performing tasks in at least two of four domains: mobility/exercise tolerance, upper-extremity abilities, higher-function tasks of independent living, and basic self-care. Overall, 1,409 women were eligible, of whom 1,002 (71.1%) participated in the full baseline interview and, 1 to 2 weeks later, an in-home comprehensive examination. The respondents’ written informed consent to participation was obtained after complete description of the study.
At the time of the examination each participant was asked whether she was willing to give a blood sample, which was collected by a phlebotomist at the third home visit. A separate informed consent statement was signed for this procedure. Approximately 75% of the participants completed the phlebotomy at baseline. For this report we used the data on the 700 women for whom all laboratory test results needed to classify vitamin deficiency status were available. These women, as compared to those for whom blood results were not available, were significantly younger (mean age, 77.3 [SD=7.8] versus 80.7 [SD=8.2] years) (t=6.2, df=1000, p<0.001) and somewhat less disabled in self-care tasks but were not significantly different in terms of race, education, or depressive symptoms (mean score on Geriatric Depression Scale [20], 7.9 [SD=5.4] versus 8.2 [SD=5.7]) (t=0.6, df=1000, p=0.53).
Determination of Vitamin Deficiency
Low serum vitamin B12 and folate levels are not specific in diagnosing tissue deficiency. A substantial proportion of persons with low serum vitamin B12 and folate levels do not appear to have tissue deficiencies of these vitamins (21). Moreover, the serum vitamin level may be low normal despite a tissue deficiency (8). The measurement of two metabolites of vitamin-dependent conversions, in combination with serum vitamin levels, provides more valid evidence for the presence of tissue vitamin deficiency (15, 16). Vitamin B12 deficiency reduces the enzymatic conversion of l-methylmalonyl-CoA to succinyl-CoA and, consequently, increases the conversion of d-methylmalonyl-CoA to methylmalonic acid. Both folate and vitamin B12 deficiency limit the methylation of homocysteine to methionine. For this study, the presence of high levels of serum methylmalonic acid and homocysteine in combination with low serum vitamin B12 and folate levels were used to determine tissue vitamin deficiency.
The serum samples were processed and aliquoted in the Core Genetics Laboratory of the Johns Hopkins University School of Medicine. The samples were shipped on dry ice to the University of Colorado Health Sciences Center for metabolite assays. Methylmalonic acid, homocysteine, and total 2-methylcitric acid were assayed by using stable isotope dilution and gas chromatography/mass spectrometry with selected ion monitoring. The normal ranges for the serum metabolites had been previously determined from 60 normal blood donors aged 18–65 years with a male-female ratio of 1:1 and were defined as two standard deviations above and below the mean after log normalization to correct for skewness of the data. The normal ranges were as follows: homocysteine, 5.4–13.9 mmol/liter; methylmalonic acid, 73–271 nmol/liter; and total 2-methylcitric acid, 60–228 nmol/liter. Serum vitamin B12 and folate levels were measured by competitive protein binding assays in the central laboratory of Corning Clinical Laboratories in Teterboro, N.J., by using intrinsic factor and folate-binding protein according to the method of Ciba-Corning Diagnostics Corporation (Medfield, Mass.). The normal ranges specified by the laboratory for vitamin B12 and folate were 148–664 pmol/liter and 6.8–36.0 nmol/liter, respectively.
As in an earlier report (22), vitamin B12 deficiency was defined according to two classifications, using a high and a low cutoff, in order to explore the existence of a dose-response relationship. High-cutoff vitamin B12 deficiency is present when the serum vitamin B12 level is less than 258 pmol/liter, the methylmalonic acid level is higher than 271 nmol/liter, and the methylmalonic acid level is higher than the total 2-methylcitric acid level. A low-cutoff vitamin B12 deficiency is present when the serum vitamin B12 level is less than 148 pmol/liter, the methylmalonic acid level is higher than 271 nmol/liter, and the methylmalonic acid level is higher than the total 2-methylcitric acid level. Folate deficiency is present when the serum folate level is less than 11.4 nmol/liter and the homocysteine level is higher than 13.9 mmol/liter. This broad definition of folate deficiency might overlap with vitamin B12 deficiency, since elevated homocysteine levels could be from vitamin B12 deficiency alone. A second, more specific definition avoids this overlap by defining folate deficiency as a serum folate level less than 11.4 nmol/liter, a homocysteine level higher than 13.9 mmol/liter, and a methylmalonic acid level of 271 nmol/liter or less.
Measurement of Depression
Depressive symptoms were measured by means of the 30-item Geriatric Depression Scale (20). The Geriatric Depression Scale has been shown to be a reliable and valid measure for older persons (20, 23). Scores can range from 0 to 30, with high scores indicating high levels of depressive symptoms. By using generally accepted cutoff scores (23, 24), the respondents were categorized as having no depression (score≤9), mild depression (score=10–13), or severe depression (score≥14). It should be emphasized that the Geriatric Depression Scale measures depressive symptoms and does not make a clinical diagnosis of depression. Nevertheless, the criterion validity of its cutoff points for clinical depression is good (for mild and severe cutoff points, respectively: sensitivity, 89% and 78%; specificity, 73% and 86%) (23).
Covariates
The sociodemographic variables included age, race, years of education completed, and household income. Alcohol intake was classified as an average consumption of less than one versus one or more alcoholic drinks per day. Body mass index was calculated by dividing the measured weight in kilograms by the square of the height in meters. Since impaired renal function may elevate serum metabolite levels independent of vitamin level, the serum creatinine level (assayed with sodium picrate according to the Ciba-Corning creatinine procedure) was used to indicate possible presence of renal failure. The participants were requested to display the containers for all prescription and nonprescription medication (including vitamin preparations) taken in the past 2 weeks. The interviewers recorded medication name, form, strength, and dose. The presence of 17 major chronic diseases was determined by using standardized algorithms incorporating participant self-reports, physical examination findings, medication use, physicians’ reports, and review of medical records (18). Self-reported disability was categorized into three levels: receipt of help from a person to perform one or more basic activities of daily living (bathing, dressing, eating, using the toilet, getting in or out of bed or chairs), no receipt of help but difficulty with one or more activities of daily living, and moderate disability (meeting the study inclusion criteria but not involving difficulties with activities of daily living).
Data Analyses
Chi-square statistics for trend and analysis of variance were used to assess differences in proportions and means, respectively, between the nondepressed, mildly depressed, and severely depressed women. Polychotomous logistic regression models, computed with the procedure Catmod of the SAS program (25), were used to assess the association of vitamin deficiency and the three-category depression variable. Unadjusted and adjusted odds ratios and 95% confidence intervals (CIs) were calculated.
Results
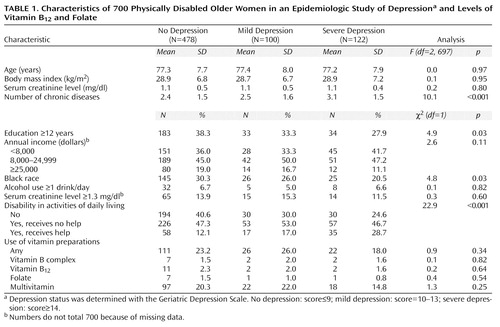
The mean age of the study sample was 77.3 years (range=65–100, SD=7.8), 28.0% were black, and 64.2% had less than 12 years of education. Of the 700 participants, 478 (68.3%) were not depressed, 100 (14.3%) were mildly depressed, and 122 (17.4%) were severely depressed. Table 1 shows the demographic characteristics, disease status, and multivitamin use of the study sample according to depression status. Compared to the nondepressed subjects, the depressed women had less education, were more likely to be white, and had more chronic diseases and disability in activities of daily living. No differences were found with respect to age, income, body mass index, alcohol use, and use of vitamin preparations.
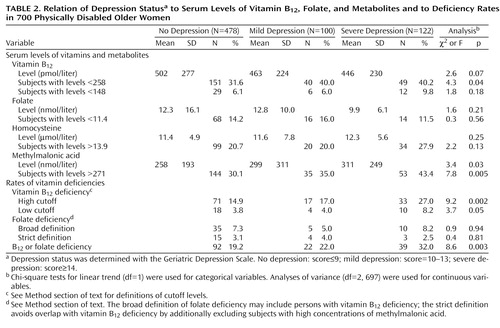
The prevalence of vitamin B12 deficiency defined according to the high cutoff (i.e., serum vitamin B12 level less than 258 pmol/liter plus an elevated serum methylmalonic acid level) was 17.3% (N=121). Of these women, 32 (4.6% of total sample) qualified for the lower, conventional cutoff for vitamin B12 deficiency, that is, a serum vitamin B12 level below 148 pmol/liter plus an elevated serum methylmalonic acid level. Folate deficiency was found in 7.1% (N=50) of the participants. According to the more specific definition (which avoids overlap with vitamin B12 deficiency), 3.1% (N=22) had folate deficiency. Only 18 (2.6 %) of the women had both vitamin B12 and folate deficiency.
Table 2 shows the serum vitamin and metabolite concentrations and the vitamin deficiency prevalences by depression status. The mildly and severely depressed women tended to have lower vitamin B12 levels than the nondepressed women, but this difference was not statistically significant. Forty percent of the depressed women scored below the vitamin B12 cutoff of 258 pmol/liter, which was significantly higher than the 31.6% among the nondepressed. Even more profound differences between the nondepressed and depressed subjects were found for the serum methylmalonic acid concentration. Of the severely depressed women, 43.4% had elevated methylmalonic acid levels, whereas the rates were 35.0% and 30.1% among the mildly depressed and nondepressed subjects, respectively. Serum folate and homocysteine concentrations did not differ according to depression status.
Vitamin B12 deficiency was present significantly more often among the severely depressed and mildly depressed subjects than among the nondepressed women (Table 2). This pattern was also observed when the low cutoff was used to define vitamin B12 deficiency. Folate deficiency, defined by either the broad or the strict criteria, was not associated with depression status. Other serum metabolites related to vitamin B12 and/or folate metabolism were assayed, and there were no significant differences in methionine, methylglycine, dimethylglycine, cystathionine, and 2-methylcitric acid across depression status.
The mean corpuscular volume did not significantly differ between the subjects with a high-cutoff vitamin B12 deficiency (93.5 fl, SD=7.5) and those without a deficiency (93.3 fl, SD=6.5). Likewise, serum hematocrit levels did not significantly differ between the subjects with vitamin B12 deficiency (mean=39.9 mg/dl, SD=4.7) and nondeficient persons (mean=39.9 mg/dl, SD=4.2). Anemia (defined as a serum hematocrit less than 35.0 mg/dl) was somewhat more common in the subjects with vitamin B12 deficiency (17.4%) than in nondeficient subjects (11.0%) (c2=3.9, df=1, p=0.05). Anemia was not associated with depression status (c2=1.7, df=1, p=0.40).
As compared to those without a high-cutoff vitamin B12 deficiency, the deficient subjects were more likely to have congestive heart failure (16.5% versus 9.3%) (c2=5.5, df=1, p=0.02) or cancer (21.5% versus 14.2%) (c2=4.1, df=1, p=0.04) but less likely to have diabetes (8.3% versus 19.2%) (c2=8.3, df=1, p=0.004). Multivariate analyses were subsequently adjusted for the presence of these three diseases. The prevalences of other diseases (e.g., disc disease, spinal stenosis, arthritis, myocardial infarction, angina, pulmonary disease, and stroke) were not significantly associated with vitamin B12 deficiency. Also, there was no association between vitamin B12 deficiency and cognitive function. The percentage of persons with scores on the Mini-Mental State between 18 (study inclusion criterion) and 23 (indicative of mild cognitive impairment) did not significantly differ (c2=0.0, df=1, p=0.84) between those with vitamin B12 deficiency (17.4%) and nondeficient persons (16.6%).
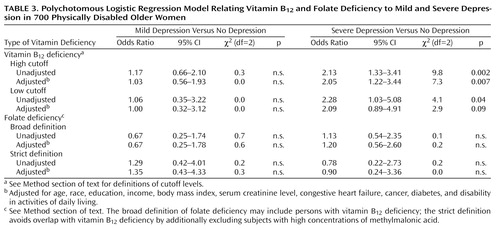
Polychotomous logistic regression models were calculated to assess the associations of vitamin B12 deficiency and folate deficiency with the three-category depression variable (Table 3). Adjustment was made for age, race, education, income, body mass index, serum creatinine level, disability in activities of daily living, and the presence of diabetes, cancer, and congestive heart failure. For mild depression, no associations with vitamin B12 or folate deficiency were found. However, for severe depression, strong associations with vitamin B12 deficiency were found. Women with a high-cutoff vitamin B12 deficiency were 2.13 times as likely to be severely depressed. After adjustment for all covariates, the odds ratio became 2.05. When we used the more stringent, low-cutoff definition of vitamin B12 deficiency, the odds ratios were somewhat higher (unadjusted odds ratio=2.28, adjusted odds ratio=2.09). Folate deficiency was not associated with severe depression.
Discussion
To our knowledge, this is the first study examining the relationship between vitamin B12 deficiency and depression in a community-based population. We found that community-dwelling older physically disabled women with metabolically significant vitamin B12 deficiency had a risk of depression that was more than twice as high as that of women without vitamin B12 deficiency. The higher risk was apparent for severe depression but not for mild depression. No association with depression was found for metabolically significant folate deficiency.
If vitamin B12 deficiency is truly associated with depression, what are the potential mechanisms? Vitamin B12 deficiency significantly reduces the reactions promoted by two B12-dependent enzymes: l-methylmalonyl-CoA mutase and methionine synthase. There is a resulting accumulation of methylmalonic acid and homocysteine, respectively. The serum vitamin B12 levels in our sample were indeed strongly, inversely correlated with serum levels of homocysteine and methylmalonic acid (21). Various neurotoxic mechanisms involving the homocysteine metabolism pathway have been suggested; these include a buildup of S-adenosylhomocysteine and an increased metabolism of homocysteic acid that may become neurotoxic through activation of N-methyl-d-aspartate receptors (26). However, the association between homocysteine and depression was not very strong in our study, whereas the association with the other metabolite, methylmalonic acid, was markedly stronger: the depressed subjects had significantly higher methylmalonic acid levels than the nondepressed subjects. Methylmalonic acid levels can be elevated in subjects with renal insufficiency, but this did not play a role in our study since the serum creatinine levels and the proportions with elevated creatinine levels were similar in the depressed and nondepressed subjects. To our knowledge, detrimental effects on mood due to actions in the methylmalonic acid metabolism pathway have not been extensively examined and described before. Our findings should encourage further research efforts in this area.
Some other psychobiological explanations for the link between vitamin B12 deficiency and depression have been suggested as well. Vitamin B12 is required for the synthesis of S-adenosylmethionine, which is the major methyl donor in many important methylation reactions in the brain. Since S-adenosylmethionine has antidepressant properties (27), inhibited synthesis may cause depression. However, in our study, N-methylglycine and methionine concentrations, which should be low in the case of S-adenosylmethionine deficiency, were normal in women who were severely depressed (mean=1.5 mmol/liter, SD=5.9, and mean=20.8 mmol/liter, SD=0.6, respectively) and were not significantly different from the levels in the subjects with no or mild depression. Consequently, it is not very likely that the effect of vitamin B12 deficiency on depression operates through inhibited S-adenosylmethionine synthesis. Another explanation may be that vitamin B12 deficiency affects serotonin and catecholamine synthesis, which may result in depressive illnesses (27, 28). Finally, vitamin B12 deficiency has been shown to cause demyelination of the spinal cord and the brain (4, 11) and consequently may result in neuropsychiatric disorders.
Some alternative, nonpsychobiological explanations should be given as well. Depression itself could cause low vitamin B12 levels through decreased appetite and resultant decreased food intake (29). This is unlikely in our sample, as body mass index and the percentage of subjects who reported weight loss during the previous year (23.8%) did not differ across depression status. Also, serum folate level, which is sensitive to dietary intake, did not differ across depression groups. However, we do not have information on specific nutrient intake in this study. In addition, malabsorption of vitamins and increased utilization of vitamin B12 among depressed patients have been suggested to play roles (30). These possibilities cannot be directly addressed by our data and require further examination. It has also been suggested that estrogen use has an effect on vitamin B12 level. However, the relation of estrogen use and vitamin B12 level was examined in a study of elderly women by Carmel et al. (31), and no link was found. Also, in our sample, estrogen use (by 9.3% of the subjects) was not associated with vitamin B12 level or with depression status, indicating that estrogen use did not play any role in the link between vitamin B12 deficiency and depression. Finally, our results could be explained by serious undiagnosed illnesses leading to both malnutrition and depression. This is unlikely, however, since the Women’s Health and Aging Study used a very rich source of information concerning diseases, disability, and health status. Adjustment for these variables did not affect the findings.
We did not find an association between depression and folate deficiency. This result is inconsistent with findings from some earlier studies of depressed psychiatric inpatients, in which there was an inverse relationship between folate status and severity of depression: depressed patients with folate in the deficient range had more severe depression than those with folate in the normal range (5, 6). The discrepancy between our and others’ results may be due to the fact that the earlier studies used only serum folate level to diagnose folate deficiency and did not use serum metabolites to confirm the true presence. Serum folate levels are rapidly affected by fluctuations in daily dietary intake and may obscure the true folate status. Consequently, associations between depression and serum folate level that are not metabolically confirmed may be more likely to be due to decreased appetite and lower resultant food intake in the most severely depressed patients. Further, the subjects of our study were members of the community-dwelling older population, whereas earlier studies all focused on depressed patients in institutions. The prevalence of folate deficiency in our community-dwelling sample was lower than prevalences found in studies of depressed psychiatric inpatients, even though those studies used lower cutoffs for serum folate to define deficiency. Although the severity of depression appears to be associated with abnormally low folate levels among patients with a psychiatric diagnosis of depression, our study suggests that an association between folate deficiency and depression is absent in the population of community-dwelling older disabled women.
Our study had some limitations. The analyses presented here are cross-sectional; thus, cause-and-effect relationships cannot be proven. Longitudinal data are necessary to further examine the causal pathway in the link between vitamin B12 deficiency and depression. Other factors—such as the duration of the vitamin deficiency, the duration of the depressive symptoms, associated metabolic disorders, and genetic predisposition—may also determine whether an affective disorder will occur. We did not have data about these aspects. Finally, our results apply to disabled women only. Exploration of possible sex differences and generalization of our findings to healthier populations will be necessary in further studies.
The prevalence of depression in our sample was high (31.7%) and higher than in similarly aged samples of (less disabled) women (around 20%) (32). Vitamin B12 deficiency is rather common among older persons. In our study sample of physically disabled older women, 17.3% had a confirmed tissue deficiency of vitamin B12, which is slightly higher than the 12% to 15% reported in other (less disabled) older populations (33, 34). In line with findings in other studies (8, 21), our data showed that only a small proportion (17.4%) of the B12-deficient subjects had megaloblastic anemia. Clinicians and other health care providers need to be aware of the high prevalence of vitamin B12 deficiency in disabled older women, and they need to screen and treat appropriately, irrespective of the presence of anemia. Our findings show, apparently for the first time, that vitamin B12 deficiency and depression are associated in a community-based population of disabled older women.
 |
 |
 |
Received March 24, 1999; revision received Oct. 5, 1999; accepted Nov. 12, 1999. From the Epidemiology, Demography, and Biometry Program, National Institute on Aging; the Institute for Research in Extramural Medicine, Vrije University, Amsterdam; the Departments of Medicine and Epidemiology, School of Medicine, Johns Hopkins Medical Institutions, Baltimore; and the Division of Hematology, Department of Medicine, University of Colorado Health Sciences Center, Denver. Address reprint requests to Dr. Guralnik, Epidemiology, Demography, and Biometry Program, National Institute on Aging, Room 3C-309, Gateway Building, 7201 Wisconsin Ave., Bethesda, MD 20892-9205; [email protected] (e-mail). The Women’s Health and Aging Study was supported by contracts AG-12112 and AG-09834 from the National Institute on Aging.
1. Chanarin I, Deacon R, Lumb M, Perry J: Cobalamin-folate interrelations. Blood Rev 1989; 3:211–215Crossref, Medline, Google Scholar
2. Shane B, Stokstad EL: Vitamin B12-folate interrelationships. Annu Rev Nutr 1985; 5:115–141Crossref, Medline, Google Scholar
3. Hector M, Burton JR: What are the psychiatric manifestations of vitamin B12 deficiency? J Am Geriatr Soc 1988; 36:1105–1112Google Scholar
4. Bottiglieri T: Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev 1996; 54:382–390Crossref, Medline, Google Scholar
5. Carney MWP: Serum folate values in 423 psychiatric patients. Br Med J 1967; 4:512–516Crossref, Medline, Google Scholar
6. Carney MWP, Chary TKN, Laundy M, Bottiglieri T, Chanarin I, Reynolds EH, Toone B: Red cell folate concentrations in psychiatric patients. J Affect Disord 1990:19:207–213Google Scholar
7. Bell IR, Edman JS, Morrow FD, Marby DW, Mirages S, Perrone G, Kayne HL, Cole JO: B complex vitamin patterns in geriatric and young adult inpatients with major depression. J Am Geriatr Soc 1991; 39:252–257Crossref, Medline, Google Scholar
8. Lindenbaum J, Healton E, Savage DG, Brust JCM, Garrett TJ, Podell ER, Marcell PD, Stabler SP, Allen RH: Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med 1988; 318:1720–1728Google Scholar
9. Carney MWP, Sheffield BF: Associations of subnormal serum folate and vitamin B12 and effects of replacement therapy. J Nerv Ment Dis 1970; 150:404–412Crossref, Medline, Google Scholar
10. Coppen A, Chaudry S, Swade C: Folic acid enhances lithium prophylaxis. J Affect Disord 1986; 10:9–13Crossref, Medline, Google Scholar
11. Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J: Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991; 70:229–245Crossref, Medline, Google Scholar
12. Godfrey PSA, Toone BK, Carney MWP, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH: Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990; 336:392–395Crossref, Medline, Google Scholar
13. Stabler SP: Screening the older population for cobalamin (vitamin B12) deficiency. J Am Geriatr Soc 1995; 43:1290–1297Google Scholar
14. Stabler SP, Lindenbaum J, Allen RH: Vitamin B12 deficiency in the elderly: current dilemmas. Am J Clin Nutr 1997; 66:741–749Crossref, Medline, Google Scholar
15. Savage DG, Lindenbaum J, Stabler SP, Allen RH: Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 1994; 96:239–246Crossref, Medline, Google Scholar
16. Allen RH, Stabler SP, Savage DG, Lindenbaum J: Diagnosis of cobalamin deficiency, I: usefulness of serum methylmalonic and total homocysteine concentrations. Am J Hematol 1990; 34:90–98Crossref, Medline, Google Scholar
17. Joosten E, van den Berg A, Riezler R, Naurath HJ, Lindenbaum J, Stabler SP, Allen RH: Metabolic evidence that deficiencies of vitamin B-12 (cobalamin), folate, and vitamin B-6 occur commonly in elderly people. Am J Clin Nutr 1993; 58:468–476Crossref, Medline, Google Scholar
18. Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME: The Women’s Health and Aging Study: Health and Social Characteristics of Older Women With Disability: NIH Publication 95-4009. Bethesda, Md, National Institute on Aging, 1995Google Scholar
19. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
20. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO: Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982–1983; 17:37–49Google Scholar
21. Stabler SP, Allen RH, Savage DG, Lindenbaum J: Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990; 76:871–881Crossref, Medline, Google Scholar
22. Stabler SP, Allen RH, Fried LP, Pahor M, Kittner SJ, Penninx BW, Guralnik JM: Racial differences in prevalence of cobalamin and folate deficiencies in disabled elderly women. Am J Clin Nutr 1999; 70:911–919Crossref, Medline, Google Scholar
23. Norris JT, Gallagher D, Wilson A, Winograd CH: Assessment of depression in geriatric medical outpatients: the validity of two screening measures. J Am Geriatr Soc 1987; 35:989–995Crossref, Medline, Google Scholar
24. Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED: Screening for depression in elderly primary care patients. Arch Intern Med 1997; 157:449–454Crossref, Medline, Google Scholar
25. Rosner B: Multivariate methods for clustered binary data with multiple subclasses, with application to binary longitudinal data. Biometrics 1992; 48:721–731Crossref, Medline, Google Scholar
26. Parnetti L, Bottiglieri T, Lowenthal D: Role of homocysteine in age-related vascular and non-vascular diseases. Aging (Milano) 1997; 9:241–257Medline, Google Scholar
27. Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ: Depression and tetrahydrobiopterin: the folate connection. J Affect Disord 1989; 16:103–107Crossref, Medline, Google Scholar
28. Bottiglieri T, Hyland K, Laundy M, Godfrey P, Carney MW, Toone BK, Reynolds EH: Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med 1992; 22:871–876Crossref, Medline, Google Scholar
29. Abou-Saleh MT, Chuing-A-On KO: Folate and vitamin B12 in eating disorders (letter). Br J Psychiatry 1987; 150:133Crossref, Medline, Google Scholar
30. Abou-Saleh MT, Coppen A: The biology of folate in depression: implications for nutritional hypotheses of psychoses. J Psychiatr Res 1986; 20:91–101Crossref, Medline, Google Scholar
31. Carmel R, Howard JM, Green R, Jacobsen DW, Azen C: Hormone replacement therapy and cobalamin status in elderly women. Am J Clin Nutr 1996; 64:856–859Crossref, Medline, Google Scholar
32. Beekman ATF, Copeland JRM, Prince MJ: Review of community prevalence of depression in later life. Br J Psychiatry 1999; 174:307–311Crossref, Medline, Google Scholar
33. Lindenbaum J, Rosenberg I, Wilson P, Stabler SP, Allen RH: Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994; 60:2–11Crossref, Medline, Google Scholar
34. Pennypacker LX, Allen RH, Kelly JP, Matthews LM, Grigsby J, Kaye K, Lindenbaum J, Stabler SP: High prevalence of cobalamin deficiency in elderly populations. J Am Geriatr Soc 1992; 40:1197–1204Google Scholar



