Posttraumatic Stress Disorder After Severe Traumatic Brain Injury
Abstract
OBJECTIVE: This study indexed the profile of posttraumatic stress disorder (PTSD) after severe traumatic injury to the brain. METHOD: Patients who sustained a severe traumatic brain injury (N=96) were assessed for PTSD 6 months after the injury with the PTSD Interview, a structured clinical interview based on DSM-III-R criteria. RESULTS: PTSD was diagnosed in 26 (27.1%) of the patients. While only 19.2% (N=5) of the patients with PTSD reported intrusive memories of the trauma, 96.2% (N=25) reported emotional reactivity. Intrusive memories, nightmares, and emotional reactivity had very strong positive predictive values for the presence of PTSD. CONCLUSIONS: These findings indicate that PTSD can develop after severe traumatic brain injury. The predominance of emotional reactivity and the relative absence of traumatic memories in patients with PTSD who suffered impaired consciousness during trauma suggest that traumatic experiences can mediate PTSD at an implicit level.
It has traditionally been assumed that posttraumatic stress disorder (PTSD) cannot develop after severe traumatic brain injury because the pervasive loss of consciousness that occurs after a severe traumatic brain injury precludes encoding of the traumatic experience (1). Numerous case studies, however, have described PTSD after severe traumatic brain injury (2, 3). Biological theories propose that a conditioned fear of traumatic experiences can be mediated in subcortical structures that are independent of higher cortical processes (4). This view predicts that damage to the cortex would not preclude the symptoms of trauma reexperiencing. The aim of this study was to investigate the profile of PTSD after severe traumatic brain injury. We predicted that patients who develop PTSD after severe traumatic brain injury would suffer the symptoms of trauma reexperiencing in the form of emotional and physiological reactivity rather than as intrusive memories.
METHOD
Over a 36-month period, 161 patients were admitted to a brain injury rehabilitation unit. We attempted to assess each patient 6 months after hospital discharge. Patients were excluded because of the inability to speak English (N=4), insufficient cognitive ability to understand the interview (N=22), refusal to participate (N=27), and inability to contact the patient (N=12). Thus, 96 patients (77 men and 19 women) were included in the study. The duration of posttraumatic amnesia was established by use of the Westmead Posttraumatic Amnesia Scale (5). The mean value for posttraumatic amnesia was 36.97 days (SD=30.65), with a range of 7–143 days. The mean Glasgow Coma Scale (6) score was 8.00 (SD=3.78). Age ranged from 16 to 71 years (mean=34.26, SD=12.82). Mean posttraumatic amnesia and Glasgow Coma Scale scores indicated that the average level of traumatic brain injury was very severe and that on average, these patients had no cohesive recall of events that occurred in the first month after the trauma. These assessments took place between 5 and 7 months posttrauma (mean=6.27 months, SD=1.27). After a complete description of the study was given to the patients, written informed consent was obtained.
The patients were interviewed by a rehabilitation consultant who was trained in the assessment procedures by the first author (R.A.B.). A diagnosis of PTSD was made by means of the PTSD Interview (7), which is based on the DSM-III-R criteria and has good construct validity (sensitivity=0.92) and test-retest diagnostic agreement (kappa value: r=0.61). Dissociative amnesia was excluded as a possible symptom of PTSD because of the confound between dissociative amnesia and amnesia related to traumatic train injury. Additional information was obtained from medical records—including age, duration of posttraumatic amnesia, duration of hospital stay, and Glasgow Coma Scale score.
RESULTS
The 96 patients who participated in the 6-month assessment did not differ from the 65 who did not participate in terms of age, years of education, or severity of posttraumatic amnesia. Those who did not participate had lower Glasgow Coma Scale scores (mean=5.48, SD=3.62) than those who participated (mean=8.00, SD=3.78) (t=2.96, df=124, p<0.01).
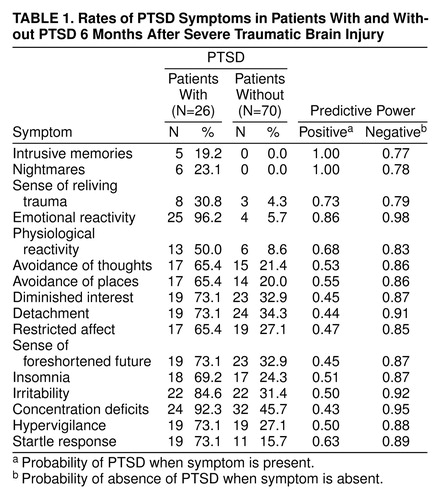
PTSD was diagnosed in 26 (27.1%) of the patientsTable 1 presents the percentage of patients who reported individual PTSD symptoms. Chi-square analyses (with Yates’s correction) of each PTSD symptom between patients with (N=26) and without (N=70) PTSD were conducted with a Bonferroni adjustment, in which the alpha level was set at 0.003 to provide an overall rejection level of 0.05 (8). More patients who met the criteria for PTSD endorsed each PTSD symptom than did those who did not meet the criteria.
The power of each PTSD symptom to predict PTSD diagnostic status was calculated (Table 1). Positive predictive power was defined as the probability of PTSD developing when a PTSD symptom was present. Negative predictive power was defined as the probability of not developing PTSD when a PTSD symptom was absent. The symptoms that had the highest positive predictive powers were intrusive memories (1.00), nightmares (1.00), and emotional reactivity (0.86).
DISCUSSION
The finding that 27.1% of our study group met the criteria for PTSD provides strong evidence against the claim that PTSD cannot occur after a severe traumatic brain injury (1). Our participants were not drawn from a consecutive patient group; therefore, the 27.1% incidence of PTSD does not reflect the rate of PTSD in populations with traumatic brain injury. The finding that only 19.2% of the patients with traumatic brain injury who met the criteria for PTSD reported intrusive memories is consistent with our prediction that trauma reexperiencing is mediated by emotional (96.2%) and physiological reactivity (50.0%). The low incidence of intrusive memories in our participants contrasts with the rate of intrusive memories in PTSD after assault (93%) (9), terrorist activity (85%) (10), and motor vehicle accidents (65%) (11). Our finding is consistent with proposals that trauma reexperiencing can be mediated by fear conditioning or perhaps by mental representations of the experience that are not accessible to conscious awareness (4, 5). It is interesting that the content of intrusive memories in the few patients who reported them was of trauma-related images that they had apparently reconstructed on the basis of information acquired after remittance of posttraumatic amnesia. For example, one patient reported that his intrusions were of images that he had seen in a photograph of his wrecked car.
The presence of PTSD was very strongly indicated by the presence of intrusive memories, nightmares, or emotional reactivity. These findings contrast with those in previous reports in which the symptoms of trauma reexperiencing had only moderate positive predictive power (9). It is possible that the deficient coping skills associated with severe traumatic brain injury resulted in patients who suffered trauma reexperiencing being unable to manage the distress caused by the symptoms. Alternately, the numerous problems associated with severe traumatic brain injury may have compounded the anxiety caused by trauma reexperiencing, and this may have contributed to PTSD.
We recognize a number of limitations in this study. First, our patients were not selected consecutively, and their responses may not be representative of populations with severe traumatic brain injury. Second, we did not obtain neuropsychological information on each patient. The role of cognitive deficits in the mediation of the symptoms of PTSD would have been clarified by relating symptoms to documented cognitive deficits. Third, the diagnosticians (R.A.B. and J.C.) were aware that all patients had sustained a traumatic brain injury. These limitations notwithstanding, these findings indicate that patients with a severe traumatic brain injury are a useful population in which to study implicit memories of traumatic experiences. Furthermore, these findings indicate that assessments of and interventions in PTSD after severe traumatic brain injury may need to address the specific symptom profile displayed by these patients.
Received Sept. 9, 1998; revisions received March 25 and Aug. 25, 1999; accepted Sept. 25, 1999. From the School of Psychology, University of New South Wales; and the Department of Rehabilitation Medicine, Westmead Hospital, Sydney, Australia. Address reprint requests to Dr. Bryant, School of Psychology, University of New South Wales, Sydney 2052, Australia; [email protected] (e-mail). Supported by the National Health and Medical Research Council and the Motor Accident Authority of New South Wales, Australia.
 |
1. Sbordone RJ, Liter JC: Mild traumatic brain injury does not produce post-traumatic stress disorder. Brain Inj 1995; 9:405–412Crossref, Medline, Google Scholar
2. Bryant RA: Posttraumatic stress disorder, flashbacks, and pseudomemories in closed head injury. J Trauma Stress 1996; 9:621–629Crossref, Medline, Google Scholar
3. Bryant RA, Harvey AG: A comparison of traumatic memories and pseudomemories in posttraumatic stress disorder. Applied Cognitive Psychol 1998; 12:81–88Crossref, Google Scholar
4. van der Kolk BA: The psychobiology of PTSD, in Traumatic Stress: The Effects of Overwhelming Experience on Mind, Body, and Society. Edited by van der Kolk BA, McFarlane AC, Weisaeth L. New York, Guilford Press, 1996, pp 214–241Google Scholar
5. Shores EA, Marosszeky JE, Sandanam J, Batchelor J: Preliminary validation of a clinical scale for measuring the duration of post-traumatic amnesia. Med J Aust 1986; 144:569–572Crossref, Medline, Google Scholar
6. Teasdale G, Jennett B: Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–84Crossref, Medline, Google Scholar
7. Watson CG, Juba MP, Manifold V, Kucala T, Anderson PE: The PTSD Interview: rationale, description, reliability, and concurrent validity of a DSM-III-based technique. J Clin Psychol 1991; 47:179–188Crossref, Medline, Google Scholar
8. Fleiss JL: Statistical Methods for Rates and Proportions, 2nd ed. New York, John Wiley & Sons, 1981Google Scholar
9. Foa EB, Riggs DS, Gershuny BS: Arousal, numbing, and intrusion: symptom structure of PTSD following assault. Am J Psychiatry 1995; 152:116–120Link, Google Scholar
10. Loughrey GC, Curran PS, Bell P: Posttraumatic stress disorder and civil violence in Northern Ireland, in International Handbook of Traumatic Stress Syndromes. Edited by Wilson JP, Raphael B. New York, Plenum, 1993, pp 377–383Google Scholar
11. Blanchard EB, Hickling EJ, Taylor AE, Loose WR, Gerard RJ: Psychological morbidity associated with motor vehicle accidents. Behav Res Ther 1994; 32:283–290Crossref, Medline, Google Scholar



