Abnormal Angular Gyrus Asymmetry in Schizophrenia
Abstract
OBJECTIVE: Few studies have evaluated the parietal lobe in schizophrenia despite the fact that it has an important role in attention, memory, and language—all functions that have been reported to be abnormal in schizophrenia. The inferior parietal lobule, in particular, is of interest because it is not only part of the heteromodal association cortex but also is part of the semantic-lexical network, which also includes the planum temporale. Both the inferior parietal lobule, particularly the angular gyrus of the inferior parietal lobule, and the planum temporale are brain regions that play a critical role as biological substrates of language and thought. The authors compared volume and asymmetry measures of the individual gyri of the parietal lobe by means of magnetic resonance imaging (MRI) scans. METHOD: MRI scans with a 1.5-Tesla magnet were obtained from 15 male chronic schizophrenic and 15 comparison subjects matched for age, gender, and parental socioeconomic status. RESULTS: Inferior parietal lobule volumes showed a leftward asymmetry (left 7.0% larger than right) in comparison subjects and a reversed asymmetry (left 6.3% smaller than right) in schizophrenic subjects. The angular gyrus accounted for this difference in asymmetry, with the left angular gyrus being significantly larger (18.7%) than the right in comparison subjects, a finding that was not observed in schizophrenic patients. A further test of angular gyrus asymmetry showed a reversal of the normal left-greater-than-right asymmetry in the schizophrenic patients. CONCLUSIONS: Patients with schizophrenia showed a reversed asymmetry in the inferior parietal lobule that was localized to the angular gyrus, a structure belonging to the heteromodal association cortex as well as being part of the semantic-lexical network. This finding contributes to a more comprehensive understanding of the neural substrates of language and thought disorder in schizophrenia.
The parietal lobe has received little attention in schizophrenia despite its recognized importance in processes that are likely disturbed in schizophrenia, such as language (1), spatial working memory, and attention (2–4). This lack of attention to parietal regions of the brain is further highlighted by the fact that only nine magnetic resonance imaging (MRI) studies of the parietal lobe, in contrast to more than 35 for both temporal and frontal lobes, have been conducted in subjects with schizophrenia (5, 6).
The parietal lobe itself consists of the postcentral gyrus, superior parietal gyrus, and inferior parietal lobule, which is further subdivided into the supramarginal gyrus (area 39) and angular gyrus (area 40). The inferior parietal lobule is part of the heteromodal association cortex, which has been proposed as the site of the key abnormality in schizophrenia (7). Further, both the supramarginal gyrus and the angular gyrus have been described as part of a semantic-lexical network that supports “word meanings” represented by a “grid of connectivity” that constitutes a “final pathway for the chunking of words into thought” (1). The role of the inferior parietal lobule, and especially the angular gyrus, in language comprehension has been further confirmed by functional MRI (fMRI) and positron emission tomography studies (8–10). Moreover, the semantic-lexical network proposed by Mesulam (1) includes both the inferior parietal lobule and the planum temporale, the latter being located on the superioposterior surface of superior temporal gyrus, which, in turn, has been correlated with symptoms of thought disorder (5, 6, 11). More anteriorly, the superior temporal gyrus has been correlated with auditory hallucinations (5, 6, 12).
Of note, the inferior parietal lobule and neighboring cortical regions often exhibit marked lateral asymmetry (13, 14) and belong to structures that support language. The presence of left-greater-than-right asymmetry also appears to be important for normal language development. For example, the absence of normal lateralization in these regions has been reported in subjects with autism (15, 16) as well as in other language disorders (17). In schizophrenia, a reversal of normal asymmetry in the superior temporal gyrus, including the planum temporale, has been associated with thought disorder (5, 6). Thus, a reversal in normal asymmetry may be importantly related to schizophrenic pathology (18–20). Consistent with these findings, we hypothesized that a reversal of normal asymmetry in the superior temporal gyrus would also be found in the inferior parietal lobule. Furthermore, since cortical asymmetries are present during fetal development (21), finding an absence or a reversal of normal asymmetry might indicate a disruption in neuronal development.
The relevance of the angular gyrus and supramarginal gyrus to schizophrenia stems not only from their functions as part of the heteromodal association cortex but also from their reciprocal neuroanatomical connections to the prefrontal (22) and temporal lobes (23)—brain regions that have been shown to have disease-related abnormalities. We examined these neuroanatomical relationships by capitalizing on the opportunity of having volumetric data on prefrontal and temporal lobe regions in the same subjects (11, 24, 25). If the hypothesis that schizophrenia is a disorder characterized primarily by heteromodal association cortex abnormalities is correct, then higher correlations between the inferior parietal lobule and other areas interconnected with the heteromodal association cortex would be predicted for schizophrenic patients but not for comparison subjects. Also, in an exploratory analysis, we examined the relationship between the heteromodal inferior parietal lobule and formal thought disorder, as well as neuropsychological measures of attention and memory, all of which are likely to contribute to thinking and judgment—functions commonly associated with the inferior parietal lobule.
METHOD
Subjects
Fifteen right-handed male subjects with chronic schizophrenia were recruited from the Veterans Affairs Medical Center in Brockton, Mass. (11). Diagnoses were determined by using DSM-III-R criteria from the Schedule for Affective Disorders and Schizophrenia (26) and from hospital chart reviews. All patients were receiving neuroleptic medication equivalent to a mean of 881 mg of chlorpromazine per day. The patients had spent a mean of 7.1 years (SD=4.6) in the hospital, with a mean duration of illness of 15.8 years (SD=8.8).
Fifteen normal comparison subjects were matched to the patient group on gender (male), handedness (right), age (20–55 years, matched within 2 years), and parental socioeconomic status. No comparison subjects had a history of mental illness nor did their first-degree relatives. The exclusion criteria for both groups included no major drug or alcohol abuse in the previous 5 years, no history of electroconvulsive therapy, no neurological illness, and no medications known to affect brain MRI scans (e.g., steroids). There were no statistical differences between the comparison and schizophrenic subjects in height, weight, head circumference, or scores on the WAIS-R information subscale (27). Written informed consent was obtained from all 30 subjects after the procedures had been fully explained.
Clinical and Neuropsychological Measures
Eleven patients were categorized as having mostly positive symptoms according to the Scale for the Assessment of Positive Symptoms (28), none had mostly negative symptoms according to the Scale for the Assessment of Negative Symptoms (29), and four were rated as having mixed negative and positive symptoms. The mean score on the Thought Disorder Index (30) for the patients was 60.4 (SD=61.8), on which normal subjects score no greater than 5. Schizophrenic patients were evaluated by means of both standardized and experimental neuropsychological procedures (31).
MRI Methods
Image acquisition.
A detailed description of the parcellation rules and anatomical definitions used in this study is included in appendix 1. The MRI images were acquired on a Signa 1.5-T system (GE Medical Systems, Milwaukee). A sagittal localizer was used to orient the images in the proper plane. Total brain volume was obtained by means of a double-echo, spin-echo acquisition with the following parameters: TR=3000 msec, TE=30 and 80 msec, field of view=24 cm, acquisition matrix=256 × 256 pixels, and voxel dimensions=0.9 mm × 0.9 mm × 3 mm, which resulted in 108 contiguous double-echo axial slices (54 levels).
For the inferior parietal lobule brain regions, a three-dimensional Fourier transform spoiled gradient recall acquisition was used with the following parameters: TR=35 msec, TE=5 msec with one repetition, flip angle=45°; field of view=24 cm; matrix=256 × 256 × 124 (192 phase-encoding steps). The dimensions for each voxel were 0.9 mm × 0.9 mm × 1.5 mm. The images were reformatted into 124 contiguous 1.5-mm coronal slices.
Image processing.
Image processing was completed on workstations (Sun Microsystems, Mountain View, Calif.) with several multistep computer algorithms. A postprocessing filter was used to reduce noise and to enhance morphologic details (32), followed by a semiautomated segmentation algorithm used to separate gray matter, white matter, and CSF (33). Brain volumes for gray matter, white matter, and CSF were then calculated by summing the voxels for each of theses tissue classes across all brain slices (11, 24).
Reliability.
Raters were blind to subject diagnosis for all measures. Both inter- and intrarater reliability were measured for each of the parietal regions by using an intraclass correlation coefficient. For interrater reliability, three judges (R.D., D.V.I., and J.L.) measured each of the parietal regions on 10 coronal slices (two sets of five contiguous slices) on three randomly selected brains, thus producing six measures for each parietal region (i.e., a left and right measure for each of three brains). For these six measures in each parietal region, interrater reliabilities were 0.96 for the inferior parietal lobule, 0.96 for the superior parietal gyrus, and 0.97 for the postcentral gyrus. Intrarater reliabilities, computed by using all of the slices from one randomly selected brain and measured by one rater (R.D.) at two separate times (approximately 1 year apart), were 0.97 for the inferior parietal lobule, 0.98 for the superior parietal gyrus, and 0.94 for the postcentral gyrus.
Statistical Analysis
All measures were corrected for total intracranial volume (unless otherwise mentioned) in order to control for variations in head size. To test for group volume differences, t tests uncorrected for multiple comparisons were conducted on the total volume of the parietal lobe, superior parietal gyrus, and the postcentral gyrus. However, to test the main hypothesis of group volume differences within the inferior parietal lobule, a repeated measures analysis of variance (ANOVA) was used with diagnosis as a between-group factor (patient versus normal comparison group) and two within-group factors of laterality (left versus right) and region (angular gyrus and supramarginal gyrus). To follow up on the laterality-by-group interaction, asymmetry scores were also computed for each region of interest by using the following formula: (left–right/left+right) × 100.
Pearson correlations for both absolute and relative values were used to examine the relationship between the volumes of parietal lobe gray matter and anatomically connected regions of the prefrontal (24, 25) and temporal gray matters (11). Because of the nonnormal distribution of clinical measures, an additional exploratory analysis was performed in which nonparametric Spearman rank order tests were used to test for significant correlations between the parietal lobe gray matter volumes and clinical and neuropsychological data. Again, we note that these correlations were exploratory in nature and not our major focus of interest.
RESULTS
MRI Volume Comparisons
For comparative purposes, table 1 provides volumes for all parietal regions studied. As predicted, no group volume differences were found for the total parietal lobe, superior parietal gyrus, or the postcentral gyrus. However, volume differences were noted between the two groups for the inferior parietal lobule, specifically the angular gyrus.
A repeated measures ANOVA showed no overall group volume differences or an overall laterality effect (table 1). However, there was a significant laterality-by-group interaction that indicated a difference in asymmetry between the two groups. As shown in figure 1, the comparison subjects had a leftward asymmetry (left inferior parietal lobule volume 7.0% larger than the right), and the schizophrenic patients showed a reversed asymmetry (left inferior parietal lobule volume 6.3% smaller than the right).
The volumes of the angular gyrus and the supramarginal gyrus for the two groups were significantly different (table 2). There was also a significant region-by-group interaction. Post hoc tests indicated that schizophrenic patients had a larger right angular gyrus than the normal comparison group (table 1). In the comparison subjects, the left angular gyrus volume was considerably larger (18.4%) than the right (paired t=2.71, df=14, p=0.02), whereas in the patients, the left angular gyrus was not significantly different (4.7% less volume) from the right (t=0.7, df=14, p>0.05).
Left-Right MRI Volume Asymmetries
To evaluate further the aforementioned laterality-by-group interaction of the inferior parietal lobule as well as the laterality of the other parietal regions, we computed asymmetry coefficients by using the formula (left–right)/(left+right) × 100 (note that the asymmetry coefficient is dimensionless). A negative value indicates a larger right than left side volume. Student’s t tests were used to compare asymmetry coefficients between the two groups for all of the regions.
There was a significant difference between the two groups in the asymmetry coefficient for the total parietal lobe (table 3). The comparison group exhibited a leftward asymmetry, with the left parietal lobe 6.0% larger than right (paired t=3.18, df=14, p=0.007), while the schizophrenic group exhibited virtually no total parietal asymmetry (t=–1.1, df=14, p>0.30).
Neither group exhibited significant asymmetry of the superior parietal gyrus, and both left and right postcentral gyrus volumes were similar in the schizophrenic and comparison groups. Both groups showed a significant leftward asymmetry, with the left postcentral gyrus being 14.8% larger than the right in the comparison subjects (paired t=2.76, df=14, p=0.02), and 13.4% larger in the schizophrenic patients (paired t=3.00, df=14, p=0.01).
A significant group difference in asymmetry coefficient was evident for the inferior parietal lobule (table 3), which was accounted for by a group difference in the angular gyrus. Note that all of the schizophrenic subjects except two had asymmetry coefficients below the mean (and median) of the subjects in the normal comparison group (figure 2). The supramarginal gyrus did not show a significant asymmetry for either group. Thus, although the post hoc tests based on the ANOVA group-by-region interaction did not show statistically significant group differences between left and right angular gyrus in the schizophrenic patients, the differences between groups in the asymmetry coefficient suggests that there is a reversal of the normal asymmetry in the schizophrenic patients for the angular gyrus.
Correlations Between Parietal Gray Matter Volumes and Anatomically Connected Regions in Prefrontal and Temporal Cortex
For all correlations, significance levels were set at p≤0.05 (two-tailed), which corresponded to r>0.51 for the 15 schizophrenic subjects and r>0.53 for 14 comparison subjects (one comparison subject was dropped because artifact in the prefrontal cortex made this region too difficult to assess the volume accurately). In addition, the values reported here are for absolute volumes, but the correlations were considered significant only if they reached p≤0.05 for both relative and absolute volumes. For all of these correlations, parietal cortex regions included the right and left inferior parietal lobules, superior parietal gyrus, and postcentral gyrus; prefrontal cortex regions included right and left superior frontal, middle frontal, inferior frontal, and orbital frontal gyri; and temporal cortex regions included right and left anterior superior temporal gyrus, posterior superior temporal gyrus, parahippocampal gyrus, and amygdala-hippocampal complex.
High correlations were found between left and right inferior parietal lobule volumes in both schizophrenic (r=0.68, p<0.005) and comparison subjects (r=0.54, p<0.04), as well as between the left and right superior parietal gyrus (schizophrenic subjects: r=0.81, p<0.001; comparison subjects: r=0.75, p<0.001). Additionally, the comparison subjects showed a significant correlation between the left and right postcentral gyrus (r=0.63, p<0.01).
As hypothesized, the schizophrenic group showed several high correlations between gray matter volumes of the inferior parietal lobule and regions of the prefrontal cortex (table 4). Figure 3 provides an illustrative summary of these correlations, with the bottom arrows depicting prefrontal correlations with the left inferior parietal lobule, and the top arrows depicting prefrontal correlations with the right inferior parietal lobule (note: arrows do not imply direction). In addition to these correlations, the left postcentral gyrus correlated significantly with the left superior frontal gyrus (r=0.72, p<0.003) and with the right inferior frontal gyrus (r=0.70, p<0.005). In contrast, the comparison subjects showed no volumetric correlations between inferior parietal lobule volumes and prefrontal volumes at p<0.05.
The differences between groups in the correlations for respective brain areas were tested by using a Fisher’s z transformation. Of note, significant group differences in correlations emerged for the left inferior parietal lobule and prefrontal structures even though the correlations between left and right inferior parietal lobules and the prefrontal lobe structures were comparably high in the schizophrenic group. This result highlights the salience of the left inferior parietal lobule correlations with prefrontal measures in the schizophrenic group.
In the comparison subjects, but not in the schizophrenic subjects, the inferior parietal lobule asymmetry coefficient correlated inversely with all of the prefrontal structures (r=–0.50 to –0.76, p=0.03 to ≤0.001). In addition, the left postcentral gyrus correlated significantly with several prefrontal structures: the left superior frontal gyrus (r=0.83, p<0.001), left orbital gyrus (r=0.70, p<0.004), right superior frontal gyrus (r=0.64, p<0.01), and the right orbital gyrus (r=0.64, p<0.01).
In the schizophrenic group, both the left and right inferior parietal lobules correlated significantly with the left amygdala (r=0.67, p<0.007) and the left anterior portion of the superior temporal gyrus (r=0.66, p<0.008 (figure 4 and table 5). In the normal comparison group, the one significant correlation was between left inferior parietal lobule and the left amygdala (r=0.53, p=0.04). The group differences in the respective, pairwise correlations, as tested with Fisher’s formula, did not reach statistical significance.
Correlations With Clinical Status and Clinical Measures and With Neuropsychological Data
Because of the exploratory nature of the correlations and the issue of multiple tests (i.e., some of the significant results might be due to chance), we report only correlations where p was below 0.01.
No correlations between regions of interest and clinical measures in schizophrenic patients reached the significance level of p<0.01. Exploratory analyses of correlations between neuropsychological measures and brain volume in schizophrenia patients indicated that reduced right inferior parietal lobule volume correlated with lower scores on tests of visual attention and visual memory (Trails B: rs=–0.76, p<0.002; visual reproduction I: rs=0.71, p<0.003; visual reproduction II: rs=0.84, p<0.001). Additionally, reduced inferior parietal lobule asymmetry was related to poor performance on Trails A (rs=–0.70, p<0.005) and Trails B (rs=–0.69, N=14, p<0.004).
DISCUSSION
The present study examined gray matter volume in individual gyri of the parietal lobe by using high resolution MRI (1.5-mm thick slices) and neuroanatomically based boundary definitions. The major findings from this study were that schizophrenic patients, in contrast to a normal comparison group, showed 1) a reversal of the normal left-greater-than-right asymmetry in the inferior parietal lobule that was localized to the angular gyrus and 2) significant volumetric correlations between parietal lobe regions and regions of the frontal and temporal cortex.
More specifically, gray matter abnormalities observed in the inferior parietal lobule consisted of a reversal of the normal left-greater-than-right asymmetry that was localized to the angular gyrus and further confirmed with a measure of asymmetry. The specificity of this asymmetry finding was underscored by the fact that no differences were observed between schizophrenic and comparison subjects for total parietal lobe, superior parietal gyrus, or postcentral gyrus. Further, there was also an absence of group differences in the lateralization patterns for superior parietal gyrus and postcentral gyrus. This finding supports previous reports of abnormality within the parietal lobe (34–37) and localizes the abnormality to the angular gyrus. Of note, schizophrenic patients did not show leftward lateralization of the total parietal lobe, which was present in the comparison subjects. This result was driven by the reversal of the normal left-greater-than-right asymmetry within the angular gyrus. This finding further underscores the utility of separate volumetric analyses for structures comprising the parietal lobe.
The present study is, to our knowledge, the first to report a reversal of normal asymmetry in the angular gyrus in schizophrenic patients, a brain region belonging to the neural circuitry that supports semantic aspects of language processing. As noted in our introduction, recent fMRI data suggest the involvement of the inferior parietal lobule, and especially the angular gyrus, in semantic processing. This region, in fact, is regarded as part of a semantic-lexical network that includes the planum temporale and is involved in both assigning meaning to strings of sounds and, at its output stage, in generating associative links responsible for constructing complex meanings and thought processes. Previous studies in schizophrenia have reported abnormal cortical asymmetry in superior temporal gyrus, especially in the region of the planum temporale (38). Thus, the present finding, which extends the finding of abnormal asymmetry to the angular gyrus, enhances our understanding of the neural underpinnings of a core feature of schizophrenic syndrome: disordered thought and language processes.
The relationship between asymmetry of the planum temporale and that of the angular gyrus, the two structures belonging to the language network, has also been previously noted by Eidelberg and Galaburda (13). These investigators reported a correlation between the degree of lateralization of the planum temporale and the angular gyrus. Thus, there appears to be a leftward lateralization for structures specialized for language (7, 13, 18, 19). The presence of such asymmetries might have an evolutionary advantage in developing and supporting language in the human species, in which the specialized function of one hemisphere might confer additional advantage (18–20).
The reversal of normal asymmetry in the angular gyrus, in addition to previous reports of the reversed asymmetry in superior temporal gyrus, provides further support for the relationship between abnormal laterality patterns and the origins of schizophrenic pathology (18–20). Since cortical asymmetries are present during fetal development (13, 21), it is thus quite possible that abnormal asymmetries in these two regions in schizophrenia may have a common neurodevelopmental origin.
The affected structures might be abnormal as a result of faulty developmental mechanisms such as gliosis or pruning, the disease process itself, or both. In fact, striking correlations between the inferior parietal lobule and neuroanatomically connected cortical regions of the prefrontal cortex and the temporal lobe, which were observed only in the schizophrenic subjects, provide support for the existence of a pathologic process (such as excitoxicity) that affects multiple, functionally interconnected brain regions in schizophrenia (39–41). The high correlations between regions that constitute primarily heteromodal association cortex regions, and observed only in the patient group, support the notion that schizophrenia might preferentially affect the association cortex.
Also of interest is the finding that in comparison subjects, the asymmetry coefficient was inversely correlated with frontal lobe volumes, which indicates that a normal brain’s development entails leftward lateralization of language structures (13, 18, 19), and that this lateralization pattern is correlated in a healthy brain with the frontal lobes, which are intimately involved in mediating processes in parietal language areas.
A limitation of this study is the use of multiple tests, which elevates the risk of type I errors. However, we focused our attention on the inferior parietal lobule, where we had an a priori rationale for evaluating both the angular and supramarginal gyri. Furthermore, we used more conservative criteria for the significance level (i.e., p≤0.01) of the exploratory analyses as a compromise for the multiple correlations performed.
In summary, this study, which employed improved methods of parcellation and measurement of the gray matter of the parietal lobe, provides evidence for the absence of normal left-greater-than-right asymmetry in the angular gyrus, a brain region categorized functionally as part of the heteromodal association cortex and, importantly, linked to aspects of semantic processing. These findings, taken together, provide support for localized volumetric changes in schizophrenia associated with selective cognitive impairments and a possible neurodevelopmental component to schizophrenic pathogenesis. These findings also afford a more comprehensive understanding of schizophrenic pathology by demonstrating similar pathologic processes that affect functionally related brain areas: the inferior parietal lobule region and the temporal and frontal areas.
APPENDIX 1. Delineating Regions Within the Parietal Lobe
For parcellating the parietal lobe we first used a reslice editing algorithm to reformat images into the sagittal and axial plane. Specific markings were then made on the sagittal slices in order to define specific neuroanatomical boundaries for delineating the gyri. These markings were then recreated on the original three-dimensional Fourier transform spoiled gradient recall acquisition coronal images, where the regions of interest were completed by using manually guided tracing. The final editing step was a surface-rendering algorithm that was used to create three-dimensional views of the relevant structures (42). The three-dimensional images of the regions of interest could then be viewed individually or within the context of the entire cortex, whereby images could be rotated around x, y, and z axes in order to achieve the best possible visualization of each region of interest. After examining the three-dimensional images, any necessary corrections were made on the original region of interest tracing. Once editing was complete, volumetric measures were derived for each region of interest, as was done for the whole brain volume, by summing the voxels for each region of interest across all relevant slices.
Defining Regions of Interest
The boundaries for the regions of interest in this study were determined with the assistance of an anatomical atlas (43). For all boundaries that involved cutting planes, we corrected for head rotation (tilt) around all three axes. Head rotation about the fronto-occipital axis was measured by a line drawn perpendicular to the interhemispheric fissure on a coronal slice at the level of the parietal lobe. Head rotation about the vertical (z) axis was measured by a line drawn perpendicular to the interhemispheric fissure on an axial slice at the level of the parietal lobe. Head rotation about the bitemporal axis was measured by a line drawn from the most anterior point of the corpus callosum to the most posterior point (illustrated in figure 5) on a midsagittal slice. This reference line was more reliably determined than an anterior to posterior commissure line, which was verified as being virtually parallel with the callosal line (mean difference angle less than 3°) for the 30 cases reported here. After correction for rotation about the fronto-occipital and vertical axes, all brain rotation about the bitemporal axis was corrected to match the brain with the least rotation (brain with the callosal line most nearly horizontal).
Parietal Lobe
Medial surface.
The parietal lobe is bounded by the frontal lobe and occipital lobe and by the cingulate gyrus (figure 6). We defined the frontoparietal border by the central sulcus (label 1) and the marginal ramus of the cingulate sulcus (label 2) and, since the sulci do not intersect, by a vertical line that extended from the most posterior portion of the central sulcus to the cingulate sulcus (line A). This line was extended laterally in the coronal plane (perpendicular to the sagittal plane). The parieto-occipital fissure (label 4) was a clear anatomical boundary that separated the parietal and occipital lobes. The parietal lobe and cingulate gyrus were bounded anteriorly by the subparietal sulcus (label 3). In the absence of a clear anatomical division, we defined the posterior and ventral parietocingulate border by a vertical line (line B) that extended from the subparietal sulcus to the occipitoparietal fissure. This line was drawn on the coronal slice 13.5 mm (nine slices) posterior to the most posterior point of the callosum. This line was extended laterally in the coronal plane (perpendicular to the sagittal plane).
Lateral surface.
The lateral boundaries between the parietal lobe and the frontal, temporal, and occipital lobes can be seen in the three-dimensional surface renderings of the cortex in figure 7.
Anteriorly, the central sulcus (label 1) is seen as the parietofrontal lobe boundary. The Sylvian fissure (label 4) bounded the parietal and temporal lobes anteriorly. More posteriorly, the ventral boundary of the parietal lobe was defined by the three planes (labeled A, B, and C), which are all perpendicular to the sagittal plane. Plane A began at the dorsal level of the Sylvian fissure on the most posterior coronal slice of the postcentral gyrus and continued posteriorly by using the same vertical (z) position for 15 mm (10 coronal slices).
Plane B of the ventral parietal boundary was defined by two parallel lines. The first line was drawn on a midsagittal slice from the most anterior point of the corpus callosum and extending posteriorly at a 9° angle to the callosal reference line from figure 5 (figure 8, left image). The second line that defined this plane was drawn on a more lateral sagittal slice and used the same coordinates as the first boundary line (figure 8, right image). The 9° angle between the reference line and the boundary line was selected so as to include the maximum amount of parietal lobe gray matter without including any (or at least only minimal amounts of) temporal or occipital lobe tissue. The posterior boundary of the parietal lobe was the plane C in figure 7, defined by two parallel lines. The first line was drawn through the parieto-occipital fissure on a midsagittal slice (figure 2, left image). The second line was drawn on a more lateral sagittal slice by using the same coordinates as the first line (figure 9, right image).
Left and right parietal hemispheres were separated by the interhemispheric fissure.
Parcellation of the Parietal Lobe
The regions of interest within the parietal lobe include the postcentral gyrus, superior parietal gyrus, and inferior parietal lobule. The inferior parietal lobule consists of the angular gyrus and the supramarginal gyrus. As can be seen in figure 7, the postcentral gyrus was separated from the superior parietal gyrus and the inferior parietal lobule by the postcentral sulcus (label 2). The inferior parietal lobule was separated from the superior parietal gyrus by the intraparietal sulcus (label 3). The inferior parietal lobule was further subdivided into the angular gyrus and the supramarginal gyrus. In the absence of a clear, consistent anatomical boundary between the angular gyrus and the supramarginal gyrus, the bound between these two inferior parietal lobule structures was defined by the coronal slice midway between the most posterior and most anterior coronal slices of the inferior parietal lobule. For this reason it is possible that some volumetric data points categorized as belonging to the angular gyrus might be a part of the supramarginal gyrus and conversely, some supramarginal gyrus volumetric data could have been categorized as belonging to the angular gyrus. Subtle bias in the final volumetric data is thus conceivable. Data from other laboratories as well as data coming from this laboratory from a different sample should help resolve this issue.
Received Feb. 8, 1999; revisions received June 28, Aug. 16, and Sept. 20, 1999; accepted Sept. 20, 1999From the Laboratory of Neuroscience, Clinical Neuroscience Division, Department of Psychiatry, VA Medical Center, Harvard Medical School; and the Surgical Planning Laboratory, MRI Division, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston. Address reprint requests to Dr. Shenton, Department of Psychiatry-116A, VA Medical Center, Harvard Medical School, 940 Belmont St., Brockton, MA 02301. Supported by NIMH grants MH-50740 and MH-01110 (to Dr. Shenton) and MH-40799 (to Dr. McCarley), and grants from the Department of Veterans Affairs Center for Clinical and Basic Neuroscience Studies of Schizophrenia and a Department of Veterans Affairs Merit Application (Dr. McCarley). The authors thank Chris Dodd, B.A., Alaka Pellock, A.B., and Marie Fairbanks for their administrative assistance.
 |
 |
 |
 |
 |
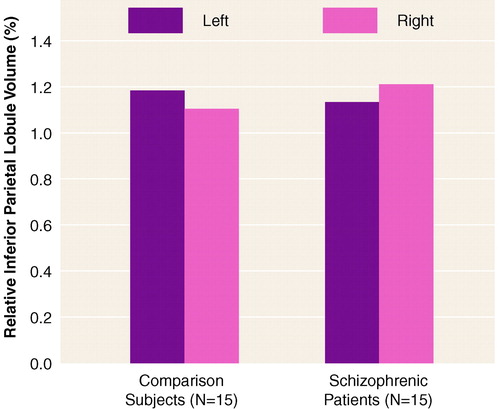
FIGURE 1. Differential Left-Versus-Right Asymmetry in Inferior Parietal Lobule Volume in Male Patients With Schizophrenia and Healthy Male Comparison Subjects
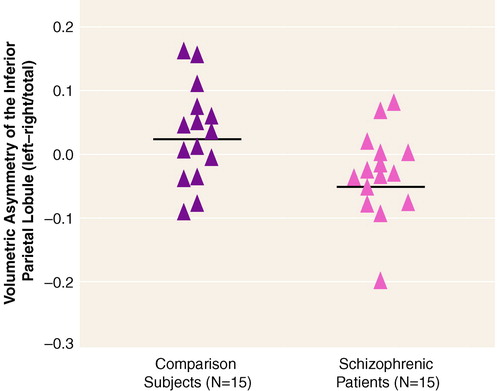
FIGURE 2. Asymmetry Coefficients for the Inferior Parietal Lobule in Male Patients With Schizophrenia and Healthy Male Comparison Subjects
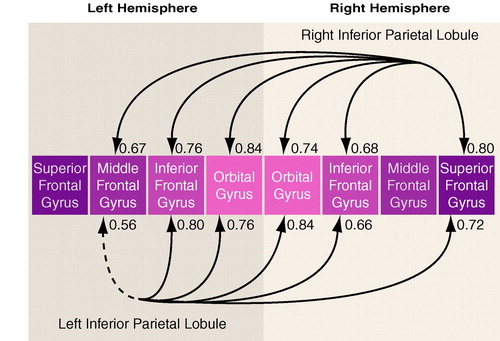
FIGURE 3. Gray Matter Volume Correlations Between the Inferior Parietal Lobule and Regions of the Prefrontal Cortex in 14 Male Schizophrenic Subjectsa
aSolid lines indicate significance at p<0.01; dotted line indicates significance at p<0.05.
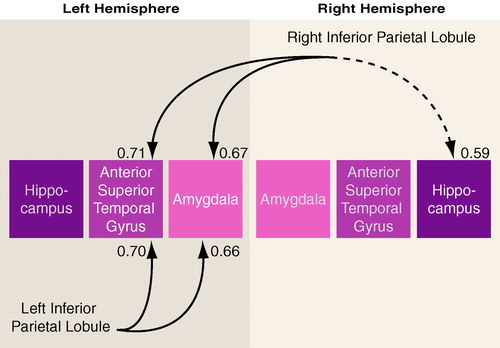
FIGURE 4. Gray Matter Volume Correlations Between the Inferior Parietal Lobule and Regions of the Temporal Lobe in 15 Male Schizophrenic Subjectsa
aSolid lines indicate significance at p<0.01; dotted line indicates significance at p<0.05.
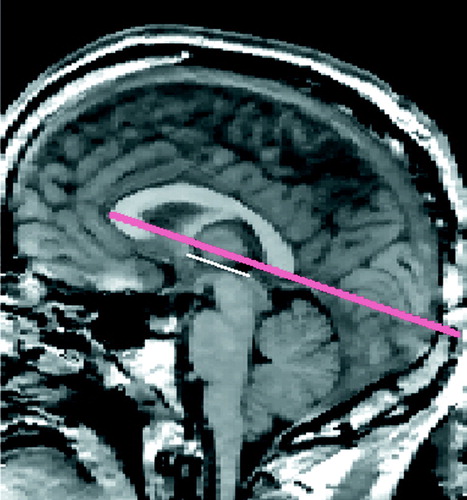
FIGURE 5. Midsagittal MRI Slice Depicting Measurement of Head Rotation (Tilt) About the Bitemporal Axisa
aThe reference line (in red), used to measure head tilt, was drawn from the most anterior point of the corpus callosum to the most posterior point. This line was found to be virtually parallel to the bicommissural line (i.e., the white line drawn between the anterior commissure and the posterior commissure).
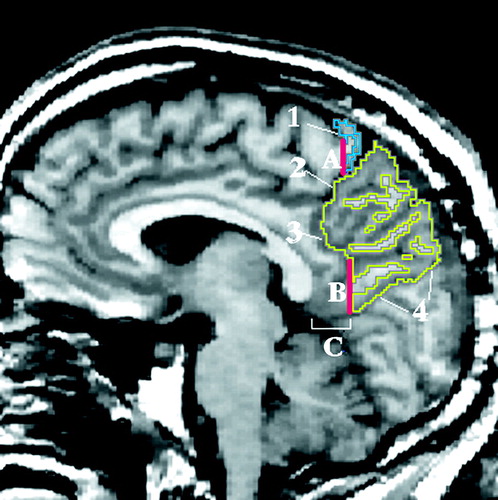
FIGURE 6. Regions of the Parietal Lobea
aImage depicts a sagittal MRI slice approximately 5 mm lateral to the midsagittal slice, with the superior parietal gyrus traced in yellow and the postcentral gyrus in blue.
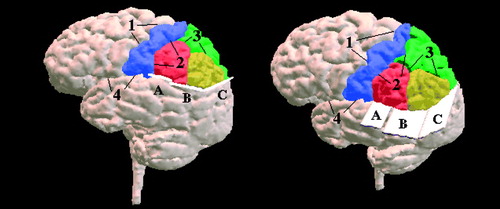
FIGURE 7. Three-Dimensional Surface Rendering of the Cortexa
aGyri of the parietal lobe are color-coded as follows: postcentral gyrus (blue), superior parietal gyrus (green), supramarginal gyrus (red), and angular gyrus (yellow). See text and subsequent figures for detailed descriptions of the numerically designated boundaries and the alphabetically labeled planes.
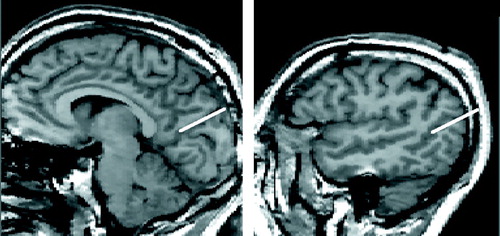
FIGURE 8. Boundaries Defining Plane B (from figure 7) of the Cortexa
aThe left side of the figure shows a midsagittal MRI slice with the reference line from figure 5 in red. The white line was drawn from the most anterior to the most posterior point of the corpus callosum, which formed a 9° angle with the reference line. The right side of the figure depicts the second line that defined the boundary of plane B, which was drawn on a lateral (sagittal) MRI slice with the same x, y coordinates as the red reference line.
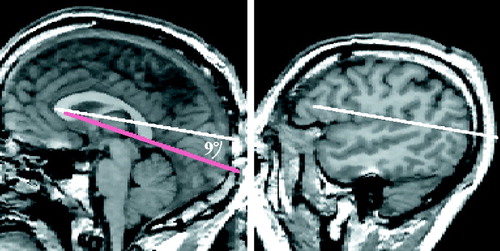
FIGURE 1. Boundaries Defining Plane C (from figure 7) of the Cortexa
aThe plane was defined by two parallel lines, the first of which is depicted in the midsagittal MRI slice on the left as a straight line drawn through the parieto-occipital fissure. The second line was drawn with identical x, y coordinates as the first line and is shown on the more lateral sagittal MRI slice on the right.
1. Mesulam MM: Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 1990; 28:597–613Crossref, Medline, Google Scholar
2. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
3. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160–203Crossref, Medline, Google Scholar
4. Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF: Measurement of visual sustained attention in schizophrenia using signal detection analysis and a newly developed computerized CPT task. Schizophr Res 1990; 3:329–332Crossref, Medline, Google Scholar
5. Shenton ME, Wible CG, McCarley RW: A review of magnetic resonance imaging studies of brain abnormalities in schizophrenia, in Brain Imaging in Clinical Psychiatry. Edited by Krishnan KRR, Doraiswamy PM. New York, Marcel Dekker, 1997, pp 297–380Google Scholar
6. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Google Scholar
7. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–17Google Scholar
8. Binder J: Functional magnetic resonance imaging: language mapping. Neurosurg Clin N Am 1997; 8:383–392Crossref, Medline, Google Scholar
9. Frackowiak RS: Functional mapping of verbal memory and language. Trends Neurosci 1994; 17:109–115Crossref, Medline, Google Scholar
10. Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R: The cortical localization of the lexicons: positron emission tomography evidence. Brain 1992; 115:1769–1782Google Scholar
11. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
12. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
13. Eidelberg D, Galaburda AM: Inferior parietal lobule: divergent architectonic asymmetries in the human brain. Arch Neurol 1984; 41:843–852Crossref, Medline, Google Scholar
14. Galaburda AM, Geschwind N: The human language areas and cerebral asymmetries. Rev Med Suisse Romande 1980; 100:119–128Medline, Google Scholar
15. Hier D, LeMay M, Rosenberger P: Autism associated with reversed cerebral asymmetry. Neurology 1978; 28:348–349Google Scholar
16. Hier DB, LeMay M, Rosenberger PB: Autism and unfavorable left-right asymmetries of the brain. J Autism Dev Disord 1979; 9:153–159Crossref, Medline, Google Scholar
17. Rosenberger PB, Hier DB: Cerebral asymmetry and verbal intellectual deficits. Ann Neurol 1980; 8:300–304Crossref, Medline, Google Scholar
18. Crow TJ: The continuum of psychosis and its genetic origins: the sixty-fifth Maudsley lecture. Br J Psychiatry 1990; 156:788–797Crossref, Medline, Google Scholar
19. Crow TJ: Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull 1990; 16:433–443Crossref, Medline, Google Scholar
20. Crow TJ: The nature of psychotic illness. Psychiatr Neurol Jpn 1992; 94:820–836Google Scholar
21. Chi JG, Dooling EC, Gilles FH: Gyral development of the human brain. Ann Neurol 1977; 1:86–93Crossref, Medline, Google Scholar
22. Cavada C, Goldman-Rakic PS: Posterior parietal cortex in rhesus monkey, I: parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol 1989; 287:393–421Crossref, Medline, Google Scholar
23. Seltzer B, Pandya DN: Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res 1984; 55:301–312Crossref, Medline, Google Scholar
24. Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW: Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry 1995; 52:279–288Crossref, Medline, Google Scholar
25. Wible CG, Shenton ME, Fischer IA, Allard JE, Kikinis R, Jolesz FA, Iosifescu DV, McCarley RW: Parcellation of the human prefrontal cortex using MRI. Psychiatry Res 1997; 76:29–40Crossref, Medline, Google Scholar
26. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
27. Wechsler D: Wechsler Memory Scale—Revised. New York, Harcourt Brace Jovanovich, 1981Google Scholar
28. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
29. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1981Google Scholar
30. Johnston MH, Holzman PS: Assessing Schizophrenic Thinking. San Francisco, Jossey-Bass, 1979Google Scholar
31. Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849–1855Google Scholar
32. Gerig G, Kikinis R, K�O: Significant Improvement of MR Image Data Quality Using Anisotropic Diffusion Filtering: Technical Report BIWI-TR-124. Zurich, Switzerland, Communication Technology Laboratory, Image Science Division, ETH Zurich, 1990Google Scholar
33. Cline HE, Lorensen WE, Ludke S, Crawford CR, Teeter BC: Two algorithms for the three-dimensional reconstruction of tomograms. Med Phys 1988; 15:320–327Crossref, Medline, Google Scholar
34. McGilchrist I, Goldstein LH, Jadresic D, Fenwick P: Thalamo-frontal psychosis. Br J Psychiatry 1993; 163:113–115Crossref, Medline, Google Scholar
35. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
36. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V II, O’Leary DS, Ehrhardt JC, Yuh WTC: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763–1769Google Scholar
37. Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, Snyder PJ, Lieberman JA: Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry 1994; 151:1437–1447Google Scholar
38. Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME: Left planum temporale volume reduction in schizophrenia. Arch Gen Psychiatry 1999; 56:142–148Crossref, Medline, Google Scholar
39. McCarley RW, Shenton ME, O’Donnell BF, Nestor PG: Uniting Kraepelin and Bleuler: the psychology of schizophrenia and the biology of temporal lobe abnormalities. Harv Rev Psychiatry 1993; 1:36–56Crossref, Medline, Google Scholar
40. Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW: NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci 1996; 16:2034–2043Google Scholar
41. Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW: Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283:70–74Crossref, Medline, Google Scholar
42. Cline HE, Lorensen WE, Souza SP, Jolesz FA, Kikinis R, Gerig G, Kennedy TE:3D surface rendered MR images of the brain and its vasculature. J Comput Assist Tomogr 1991; 15:344–351Google Scholar
43. Duvernoy HM: The Human Brain: Surface, Three-Dimensional Sectional Anatomy, and MRI. Vienna, Springer-Verlag, 1991Google Scholar



